Abstract
Purpose
To validate pathologic markers of response to preoperative chemotherapy as predictors of disease-free survival (DFS) after resection of colorectal liver metastases (CLM).
Patients and Methods
One hundred seventy one patients who underwent resection of CLM after preoperative chemotherapy at 4 centers were studied. Pathologic response defined as proportion of tumor cells remaining (categorized complete (0%), major (<50%) or minor (≥50%)) and tumor thickness at tumor–normal liver interface (TNI) (categorized <0.5 mm, 0.5 mm-<5 mm and ≥5 mm)—were assessed by a central pathology reviewer and local pathologists.
Results
Pathologic response was complete in 8%, major in 49% and minor in 43%. Tumor thickness at the TNI was <0.5 mm in 21%, 0.5 mm-<5 mm in 56% and ≥5 mm in 23%.In multivariate analyses, using either pathologic response or tumor thickness at TNI, pathologic response (P=.002,.009), tumor thickness at TNI (P=0.015, <.001), duration of preoperative chemotherapy(P=.028,.043), number of CLM (P=.038,.037) and margin (P=.011,.016) were associated with DFS. In a multivariate analysis using both parameters, tumor thickness at TNI (P=.004,.015), duration of preoperative chemotherapy(P=.025), number of nodules(P=.027) and margin(P=.014) were associated with DFS. Tumor size by pathology examination was the predictor of pathologic response. Predictors of tumor thickness at the TNI were tumor size and chemotherapy regimen. There was near perfect agreement for pathologic response (κ=.82) and substantial agreement (κ=.76) for tumor thickness between central reviewer and local pathologists.
Conclusion
Pathologic response and tumor thickness at the TNI are valid predictors of DFS after preoperative chemotherapy and surgery for CLM.
Introduction
Colorectal liver metastases affect 50% of patients with colorectal cancer and account for two-thirds of deaths from this disease.1 Neoadjuvant chemotherapy and liver resection is widely used to treat patients with colorectal liver metastases.2-5 Liver resection for colorectal liver metastases is supported by improved 5 year survival after surgery performed with curative intent.6,7 However, approximately 70% of patients develop disease recurrence after resection of colorectal liver metastases.8
Validated pathologic predictors of response to preoperative chemotherapy are of particular interest because in addition to predicting patient outcome, it contributes to evaluation of tumor biology and may be used to tailor postoperative treatment. These markers could be used as end points in studies of the efficacy of new drugs, an approach recently validated by the US Food and Drug Administration for studies in breast cancer,9 and could also be used as end points in studies of new biomarkers for response to chemotherapy.
Tumor regression after chemotherapy, measured on imaging and by pathologic examination of the colorectal liver metastases, has been tested as a prognostic factor for patient outcome in several studies.10-15 Two different pathologic markers of response to preoperative chemotherapy—pathologic response defined as the percentage of residual viable tumor cells and the tumor thickness at the tumor–normal liver interface—were shown to be associated with survival after resection of colorectal liver metastases in a large single-center cohort.13,14 These markers have also shown to be associated with preoperative imaging response described using the RECIST (Response Evaluation Criteria in Solid Tumors) criteria and morphologic criteria.14,16 However, the applicability of these 2 semiquantitative histopathologic markers in different patient populations has not been confirmed.
The aim of this study was to validate the 2 previously described pathologic markers of response to preoperative chemotherapy—pathologic response and tumor thickness at the tumor–normal liver interface—in a diverse patient population from several institutions and to test the agreement between local/peripheral pathologists from those institutions and central expert pathology review.
Patients and Methods
Patients
This retrospective study was approved by the institutional review boards of all the participating institutions. The study included patients who underwent preoperative chemotherapy followed by resection of colorectal liver metastases with curative intent (from 2001-2010), at 4 major hepatobiliary centers: Center Leon Bernard, Lyon, France; Ambroise Pare Hospital, Paris, France; The University of Texas MD Anderson Cancer Center, Houston, USA; and Medical University of Vienna, Austria. Inclusion criteria were resection of colorectal liver metastases with curative intent within 3 months after completion of preoperative chemotherapy; duration of preoperative chemotherapy less than 10 months; and preoperative chemotherapy consisting of either a fluoropyrimidine based, fluropyrimidine-and-irinotecan-based regimen or a fluoropyrimidine-and-oxaliplatin-based regimen with or without bevacizumab or cetuximab. Patients received preoperative chemotherapy either for initially unresectable or upfront resectable colorectal liver metastases. Adjuvant chemotherapy for primary colorectal cancer was not taken into account except for patients with synchronous colorectal liver metastases. Patients were excluded if they underwent staged liver resection, previous portal vein embolization, radiofrequency ablation concomitant to liver resection, or hepatic artery infusion. Patients with postoperative follow-up time less than 12 months and cause of death other than colorectal cancer were also excluded. Each center selected its patient population on the basis of these predefined criteria independently of the participating pathologists.
For each patient, the following demographic and clinicopathologic factors were collected by review of medical records: age, sex, site of primary tumor, primary tumor lymph node status, timing of detection of colorectal liver metastases in relation to detection of the primary tumor (synchronous or metachronous), type of liver resection (major [resection of ≥ 3 liver segments] or minor [all other procedures]), number of colorectal liver metastases, diameter of the largest metastasis, preoperative carcinoembryonic antigen (CEA) level, type and number of cycles of preoperative chemotherapy, surgical margin status, postoperative complications, and recurrence status.
Assessment of Pathologic Response and Tumor Thickness at the Tumor–Normal Liver Interface
Pathologic response and tumor thickness at the tumor–normal liver interface (hereafter referred to as the tumor-normal interface) were assessed as previously described.13,14 All colorectal liver metastases were macroscopically localized in the surgical specimen after correlation with radiologic findings. In patients with multiple colorectal liver metastases, each lesion was sampled extensively from the center to the periphery to include multiple sections of tumor and nonneoplastic liver parenchyma. Hematoxylin-eosin-stained sections were reviewed independently by 2 pathologists: the corresponding institution's gastrointestinal pathologist (C.J., J.S., A.I.L., and A.A.) and the central pathology reviewer (D.M.M). The number of years of experience of these pathologists ranged from 4 to 15 years. All pathologists were blinded from other pathologists' interpretation, clinical information, treatment regimen, and study end point. The pathology parameters assessed by the central pathology reviewer were used for the analyses of predictors of survival and predictors of pathologic response and tumor thickness at the tumor-normal interface.
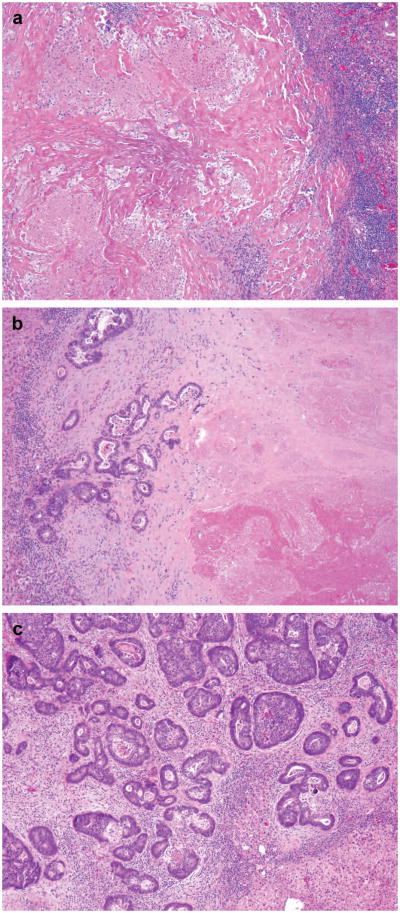
For the assessment of pathologic response, residual carcinoma was measured semiquantitatively by estimating the area of residual cancer cells as a proportion of the total tumor area as described in previous study.13 The tumor area included areas of acellular mucin, tumor necrosis, chemotherapy-related tissue injury, and other reparative changes. Pathologic response was categorized as follows: no residual cancer cells, complete response; 1% to 49% residual cancer cells, major response; and 50% or more residual cancer cells, minor response (Figure 1A). In patients with multiple tumor nodules, the mean of the values for the various tumor nodules was used.
Figure 1.
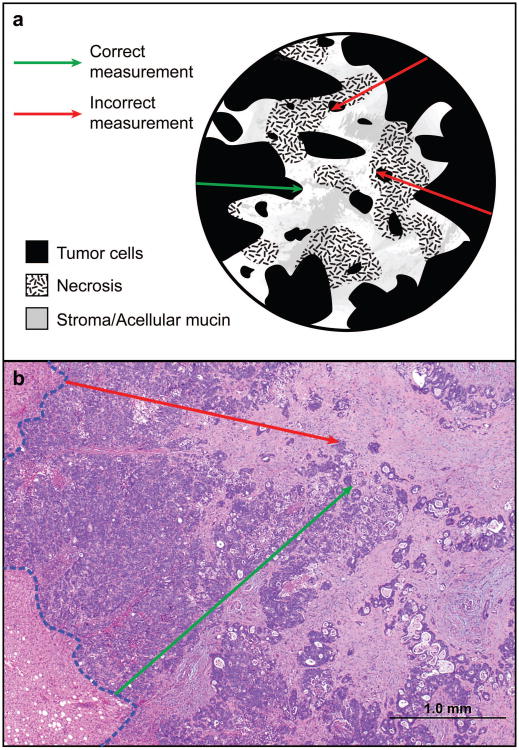
(A) Photomicrographs of representative examples of complete (a), major (b), and minor pathologic response (c). (a) shows tumor bed with necrotic debris, hyalinized/collagenized tissue and inflammatory cells with no tumor cells, (b) shows neoplastic glands occupying less than half of the tumor bed and (c) shows neoplastic glands occupying majority (>50%) of the tumor bed admixed with minor component of fibrocollagenous stroma with inflammation. (B) Cartoon (a) and photomicrograph (b) demonstrating correct and incorrect method of measuring tumor thickness at the tumor–normal liver interface. (a) shows rumor cells/neoplastic glands as homogenous dark area of the tumor bed. Different stromal components are shown with other symbols. The outer layer of the circle represents the tumor-normal liver interface. (b) Hematoxylin and eosin stained section showing normal liver parenchyma in the left lower corner, tumor normal liver interface highlighted with blue interrupted line. In both (a) and (b) The green arrow shows longest area of tumor cells without admixed stroma, necrosis or inflammation. Red arrow indicated focus with layer of tumor cells interrupted by fibrosis/necrosis.
The tumor thickness at the tumor-normal interface was measured perpendicular to the tumor-normal interface at the focus with the maximum number of contiguous tumor cells in millimeters using a ruler or ocular micrometer (Figure 1B). In all tumors, the thickness was measured at multiple foci, and the greatest thickness was used in the analysis. In specimens with multiple tumors, tumor thickness was measured separately for each tumor nodule, and the average thickness was used for analysis, as described in prior study.14
A positive surgical margin was defined as the presence of tumor cells at or within 1mm of the line of transection.
Statistical analysis
Quantitative variables were expressed as median (range) and frequency. Comparisons between groups were analyzed with the chi-square or Fisher exact test for proportions and the Mann-Whitney U test or Kruskal-Wallis H test for continuous variables as appropriate.
Disease-free survival rates were calculated from the date of liver resection to the date of last follow-up or recurrence using the Kaplan-Meier method and compared using log-rank tests. Disease-free survival was preferred over overall survival as the end point because of its specificity for the disease-related outcome and insufficient maturity of the overall survival data from the institutions participating in this study. Univariate and multivariate analyses were used to examine the relationship between disease-free survival and various clinical and pathologic factors. All variables associated with disease-free survival with P ≤ 0.1 in a univariate proportional hazards model were subsequently entered into a Cox multivariate regression model with backward elimination (conditional logistic regression). Statistical significance was defined as P < 0.05.
The pathologic response and tumor thickness at the tumor-normal interface both reflect tumor regression after preoperative chemotherapy and have shown to strongly correlate with each other in our previous study14. Due to this interdependence of these two parameters, three models of multivariate analysis were created to appropriately assess the impact of these two parameters. Model 1 included all factors with P ≤ 0.1 in the univariate analysis except tumor thickness at tumor-normal interface. Model 2 included all factors with P ≤ 0.1 in univariate analysis except pathologic response. Model 3 included both pathologic response and tumor thickness at tumor-normal interface with other parameters with P ≤ 0.1 in univariate model. As these pathology markers were first described by pathologists at The University of Texas MD Anderson Cancer Center, level of agreement was assessed between a central pathology reviewer at MD Anderson and local pathologist at 4 institutions using Spearman correlation and κ statistics with quadratic weighting.
Analyses were performed with SPSS software (version 19.0, SPSS Inc, Chicago, Illinois).
Results
Study Population
The patient population included 88 men and 83 women with mean age of 60 years (range, 26-85 years). The patients' clinicopathologic characteristics by treating center are summarized in Table 1. Primary tumor characteristics did not significantly differ between the 4 centers. However, there were differences between the 4 centers with respect to age, gender, type of liver resection, diameter of the largest colorectal liver metastasis, preoperative CEA level, and type and median months of duration of preoperative chemotherapy regimen. Seventy of 106 patients with synchronous primary and liver metastases underwent colon resection followed by preoperative chemotherapy for liver metastases which was followed by liver resection. Eighteen patients underwent preoperative chemotherapy followed by synchronous colon and liver resection. Eight patients underwent preoperative chemotherapy followed by liver resection which was followed by resection of colon tumor. Adjuvant therapy for primary colon cancer was taken in to account in ten patients.
Table 1.
Clinicopathologic Characteristics of patients by treating center. *
| Characteristic | Center 1 (N=49) | Center 2 (N=34) | Center 3 (N=43) | Center 4 (N=45) | P Value |
|---|---|---|---|---|---|
| Median age (range), years | 57 (26-85) | 63 (37-83) | 59 (35-80) | 61 (30-78) | .016 |
| Male | |||||
| Yes | 31 | 10 | 23 | 24 | .023 |
| No | 18 | 24 | 20 | 21 | |
| Primary tumor site | |||||
| Colon | 40 | 22 | 32 | 29 | .21 |
| Rectum | 9 | 12 | 11 | 16 | |
| Positive lymph node primary | |||||
| Yes | 29 | 23 | 24 | 23 | .52 |
| No | 20 | 11 | 19 | 22 | |
| CLM synchronous to primary | |||||
| Yes | 36 | 20 | 26 | 24 | .23 |
| No | 13 | 14 | 17 | 21 | |
| Type of liver resection | |||||
| Major | 26 | 27 | 26 | 14 | <.001 |
| Minor | 23 | 7 | 17 | 31 | |
| Median no. of CLM (range) | 2 (1-11) | 2 (1-4) | 2 (1-13) | 1 (1-7) | .067 |
| Median diameter of the largest CLM (range), cm | 2.5 (0.5-13) | 3 (1-7) | 3 (1-12) | 1.8 (0.3-10) | <.001 |
| Median preoperative CEA level (range), ng/mL | 2.6 (0-859) | 3 (0 - 387) | 17 (0-1640) | 5 (0-153) | <.001 |
| Chemotherapy type† | |||||
| Fluoropyrimidine + oxaliplatin | 5 | 17 | 16 | 0 | <.001 |
| Fluoropyrimidine + irinotecan | 16 | 15 | 9 | 0 | |
| Fluoropyrimidine + oxaliplatin + bev | 24 | 0 | 2 | 40 | |
| Fluoropyrimidine + irinotecan + bev | 2 | 0 | 4 | 3 | |
| Median duration of preoperative CHT (range), months | 2 (1-9) | 3 (1.5-10) | 3 (1-10) | 4.5 (1-10) | <0.001 |
| Recurrence | |||||
| Yes | 33 | 24 | 29 | 28 | .88 |
| No | 16 | 10 | 14 | 17 | |
| Pathologic response | |||||
| Complete | 7 | 1 | 2 | 4 | .08 |
| Major | 19 | 15 | 21 | 29 | |
| Minor | 23 | 18 | 20 | 12 | |
| Tumor thickness at TNI | |||||
| <0.5 mm | 15 | 2 | 9 | 9 | <.001 |
| ≥0.5 mm and <5 mm | 17 | 25 | 20 | 35 | |
| ≥5 mm | 17 | 7 | 14 | 1 |
Abbreviations: CEA, carcinoembryonic antigen; CLM, colorectal liver metastases; TNI, tumor–normal tissue interface; bev, bevacizumab
Values in the table are numbers of patients (percentage) unless otherwise indicated.
Patients receiving multiple regimens of preoperative chemotherapy (n=13), only fluropyrimidines (n=4) and cetuximab (n=1) were excluded from this analysis, finally including 153 patients.
Among the 65 patients with metachronous colorectal liver metastases, 32 stage III patients received adjuvant chemotherapy for the primary tumor and, at the diagnosis of liver metastases, preoperative chemotherapy for liver metastases. Remaining 33 stage I-II patients with metachronous colorectal liver metastases, received preoperative chemotherapy for either multiple, or larger than 5 cm, or initially unresectable liver metastases. Remaining 33 stage I-II patients with metachronous colorectal liver metastases, received preoperative chemotherapy for either multiple, or larger than 5 cm, or initially unresectable liver metastases.
One hundred and four patients (61%) received single line oxaliplatin-based chemotherapy with or without bevacizumab, 49 patients (28%) received single line irinotecan-based chemotherapy with or without bevacizumab, and remaining 18 patients (11%) received multiple preoperative chemotherapy lines regimens containing both oxaliplatin and irinotecan (n=13), only fluropyrimidines (n=4) or cetuximab (n=1). Preoperative chemotherapy regimen from one center included bevacizumab for all patients while other center included all patients without bevacizumab. Other two centers had patients with and without bevacizumab. The majority of the patients had multiple liver metastases (104, 60%). The median diameter of the largest tumor nodule was 3.1 cm (range, 0.3-13 cm). Most patients (93, 54%) had major hepatectomy. One hundred thirty-three patients (78%) received postoperative chemotherapy. Postoperative reversible complications occurred in 48 (28%) patients, and tumor recurrence occurred in 124 (72%) patients.
Pathologic examination showed complete response in 14 (8%) patients, major response in 84 (49%), and minor response in 73 (43%). Tumor thickness at the tumor-normal interface was <0.5 mm in 35 (21%) patients, 0.5 to <5 mm in 97 (56%), and ≥5 mm in 39 (23%). Eight (5%) patients had positive surgical resection margin.
Predictors of Disease-Free Survival
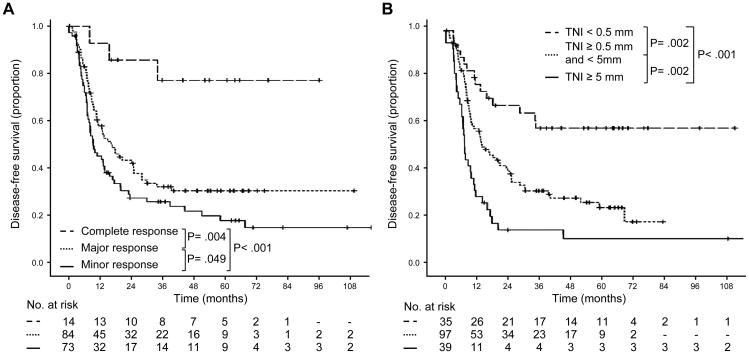
Median duration of follow up for disease-free survival was 42 months (range, 3-121 months). Pathologic response and tumor thickness at the tumor-normal interface were both associated with disease-free survival as continuous variables (P = .002 and P = .001, respectively). Disease-free survival curves for each category of pathologic response and tumor thickness at the tumor-normal interface are shown in Figure 2. By log-rank test, the survival differences between complete and major response (P = .004) and between major and minor response (P = .049) were statistically significant. For the entire study population, the cumulative 3-year and 5-year disease-free survival rates were 33% and 28%, respectively. The cumulative 3-year and 5-year disease-free survival rates, respectively, by categories of response were as follows: complete response, 77% and 77%; major response, 32% and 31%; and minor response, 26% and 18%.
Figure 2.
Disease-free survival in patients undergoing resection of colorectal liver metastases stratified by (A) categories of pathologic response and (B) categories of tumor thickness at the tumor–normal liver interface.
By log-rank test, the survival differences between patients with tumor thickness at the tumor-normal interface <0.5 mm and 0.5 to <5 mm and the survival difference between patients with tumor thickness at the tumor-normal interface 0.5 to <5 mm and ≥5 mm were statistically significant (P = 0.002). The cumulative 3-year and 5-year disease-free survival rates, respectively, by categories of tumor thickness at the tumor-normal interface were as follows: <0.5 mm, 58% and 58%; 0.5 to <5 mm, 31% and 24%; and ≥5 mm, 15% and 11%.
Results of univariate and multivariate analyses of the predictors of disease-free survival are shown in Table 2 and 3 respectively. In univariate analysis, factors associated with worse 3- and 5-year survival were high preoperative CEA level, duration of preoperative chemotherapy >13 months, minor vs. major or complete pathologic response, major vs. complete pathologic response, tumor thickness at the tumor-normal interface ≥0.5 mm, and positive resection margin. In model 1 of multivariate analysis, minor and major pathologic response were associated with shorter DFS (for minor pathologic response, P=.002 and HR=6.33; for major pathologic response, P=.009 and HR=4.72). In model 2, a higher tumor thickness at TNI independently predicted a shorter DFS (for tumor thickness ≥5mm, P<.001 and HR=3.89; for tumor thickness from 0.5mm to 5 mm, P=.015 and HR=2.07). Finally, in model 3, tumor thickness at TNI was independently associated with DFS (P=.004 and HR=2.71 for tumor thickness ≥5mm; P=.015 and HR=2.03 for tumor thickness from 0.5mm to 5 mm). In all three models of multivariate analysis, duration of chemotherapy > 3months, number of tumor nodules ≥ 3, and positive resection margin were independent predictors of worse 3- and 5-year survival.
Table 2.
Univariate Analysis of Factors Associated with Disease-free Survival.
| Characteristic | No. of Patients | 3-year DFS, % | 5-year DFS, % | P Value | HR | 95% CI |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 88 | 34 | 30 | .82 | 1.04 | .72 – 1.50 |
| Female | 83 | 32 | 27 | |||
| Age | ||||||
| <60 years | 88 | 32 | 30 | .68 | .92 | .64 – 1.34 |
| ≥60 years | 83 | 34 | 26 | |||
| Synchronous CLM | ||||||
| Yes | 106 | 37 | 30 | .56 | .92 | .63 – 1.34 |
| No | 65 | 26 | 26 | |||
| Primary tumor site | ||||||
| Colon | 123 | 31 | 30 | .71 | .93 | .64 – 1.35 |
| Rectum | 48 | 36 | 27 | |||
| Primary tumor lymph node status | ||||||
| Positive | 99 | 30 | 26 | .25 | 1.24 | .85 – 1.81 |
| Negative | 72 | 37 | 32 | |||
| Chemotherapy | ||||||
| Irinotecan | 60 | 25 | 21 | .088 | 1.39 | .95 – 2.02 |
| Oxaliplatin | 111 | 37 | 33 | |||
| Bevacizumab | ||||||
| Yes | 75 | 37 | 35 | .38 | .85 | .59 – 1.23 |
| No | 96 | 29 | 24 | |||
| Months of chemotherapy | ||||||
| ≤3 | 85 | 39 | 35 | .056 | 1.43 | .99 – 2.07 |
| >3 | 86 | 28 | 21 | |||
| CEA level ≥5 ng/mLΩ | ||||||
| Yes | 73 | 21 | 17 | .005 | 1.69 | 1.17 – 2.44 |
| No | 70 | 44 | 38 | |||
| No. of CLM | ||||||
| <3 | 111 | 35 | 30 | .09 | 1.46 | .94 – 2.26 |
| ≥3 | 60 | 30 | 25 | |||
| Diameter of largest CLM ≥3 cm | ||||||
| Yes | 82 | 29 | 25 | .17 | 1.28 | .89 – 1.85 |
| No | 89 | 37 | 31 | |||
| Pathologic response | ||||||
| Complete | 14 | 77 | 77 | |||
| Major | 84 | 32 | 31 | .001† | 6.81 | 2.13– 21.8 |
| Minor | 73 | 26 | 18 | .049‡ | 4.69 | 1.47 – 15 |
| Tumor thickness at TNI | ||||||
| <0.5 | 35 | 58 | 58 | |||
| ≥0.5 mm and <5 mm | 97 | 31 | 24 | <.001§ | 4.54 | 2.41 – 8.45 |
| ≥5 mm mm | 39 | 15 | 11 | .003** | 2.39 | 1.34 – 4.27 |
| Positive margin | ||||||
| No | 163 | 35 | 30 | |||
| Yes | 8 | 0 | 0 | .003 | 2.86 | 1.37 – 5.94 |
Abbreviations: HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; CLM, colorectal liver metastases; HR, hazard ratio; TNI, tumor–normal tissue interface.
Patients with complete vs major vs minor response.
Patients with major vs minor response.
Patients with TNI thickness <0.5 mm vs ≥0.5 mm and <5 mm vs ≥5 mm.
Patients with TNI thickness ≥0.5 mm and <5 mm vs ≥5 mm.
Missing data for 28 patients.
Table 3.
Multivariate Analysis of Factors Associated with Disease-Free Survival.
| Characteristics | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | (95% CI) | P | HR | (95% CI) | P | HR | (95% CI) | |
| Irinotecan | |||||||||
| Yes | .45 | 1.17 | .78 – 1.74 | .87 | 1.03 | .68 – 1.57 | .86 | 1.03 | .68 – 1.58 |
| No | |||||||||
| Months of chemotherapy | |||||||||
| ≤3 | |||||||||
| >3 | .028 | 1.52 | 1.05 – 2.22 | .043 | 1.48 | 1.01 – 2.17 | .025 | 1.55 | 1.06 – 2.28 |
| Pathologic response | |||||||||
| Complete | |||||||||
| Major | .009 | 4.72 | 1.47 – 15.14 | - | - | - | 1 | 1 | .63 – 1.57 |
| Minor | .002 | 6.33 | 1.97 – 20.31 | - | - | - | .064 | 3.61 | .93 – 13.88 |
| Tumor thickness at TNI | |||||||||
| <0.5 mm | |||||||||
| ≥0.5 mm and <5 mm | - | - | - | .015 | 2.07 | 1.15 – 3.71 | .015 | 2.03 | 1.21 – 3.32 |
| ≥5 mm | - | - | - | <.001 | 3.89 | 2.06 – 7.37 | .004 | 2.71 | 1.25 – 6.06 |
| No. of CLM | |||||||||
| <3 | |||||||||
| ≥3 | .038 | 1.60 | 1.03 – 2.50 | .037 | 1.61 | 1.03 – 2.51 | .027 | 1.55 | 1.05 – 2.28 |
| Positive margin | |||||||||
| No | |||||||||
| Yes | .011 | 2.66 | 1.25 – 5.64 | .016 | 2.50 | 1.18 – 5.30 | .014 | 2.57 | 1.21 – 5.49 |
Abbreviations: HR, hazard ratio; CI, confidence interval; TNI, tumor–normal liver interface CLM, colorectal liver metastases.
CEA was not included in the multivariate analyses because of missing data for 28 patients.
Model 1 and model 2 included all factors significant in univariate analysis except tumor thickness at TNI and pathologic response, respectively.
Model 3 included all factors significant in univariate analysis.
Predictors of Pathologic Response and Tumor Thickness at the Tumor-Normal Interface
Results of univariate analysis of predictors of pathologic response and tumor thickness at the tumor-normal interface are shown in Table 4. Tumor size was the only predictor of pathologic response. Predictors of smaller tumor thickness at the tumor-normal interface were smaller tumor size, oxaliplatin-based chemotherapy, and use of bevacizumab.
Table 4.
Univariate Analysis of Predictors of Pathologic Response and Tumor Thickness at TNI*.
| Pathologic Response | Tumor Thickness at TNI | |||||||
|---|---|---|---|---|---|---|---|---|
| Complete (N=14) | Major (N=84) | Minor (N=73) | P | <0.5 mm (N=35) | ≤0.5 mm and <5 mm (N=97) | ≥5 mm (N=39) | P | |
| Male sex | 7 | 41 | 40 | .75 | 20 | 44 | 24 | .18 |
| Median age (range), yr | 60 (36-71) | 60 (26-83) | 60 (34-85) | .72 | 61 (26-85) | 60 (30-83) | 58 (34-83) | .48 |
| Synchronous CLM | 8 | 54 | 44 | .81 | 24 | 60 | 22 | .56 |
| Rectal primary | 6 | 36 | 36 | .70 | 16 | 40 | 22 | .28 |
| Positive lymphnode primary | 6 | 50 | 43 | .49 | 17 | 60 | 22 | .39 |
| Median CEA (range), ng/mL | 2.5 (1-50) | 4 (0-1640) | 5 (0-963) | .93 | 2.7 (0-113) | 4 (0-1640) | 6 (0-859) | .22 |
| Median duration of preoperative chemotherapy (range), months | 3 (2-12) | 3 (0-10) | 3 (0-11) | .68 | 3 (0-12) | 4 (0-11) | 3 (0-6.5) | .08 |
| Chemotherapy regimens† | ||||||||
| Oxaliplatin±bevacizumab | 10 | 56 | 40 | .31 | 23 | 68 | 15 | .018 |
| Irinotecan±bevacizumab | 2 | 22 | 23 | 7 | 24 | 16 | ||
| Bevacizumab† | ||||||||
| Yes | 5 | 41 | 25 | .3 | 15 | 48 | 8 | .036 |
| No | 7 | 37 | 38 | 15 | 44 | 23 | ||
| Number of CLM, median (range) | 2 (1-7) | 2 (1-13) | 2 (1-13) | .84 | 2 (1-13) | 2 (1-13) | 2 (1-11) | .996 |
| Median diameter of the largest CLM (range), cm | 1.1 (0.3-4.5) | 2.5 (0.5-9.5) | 3 (0.3-13) | .002 | 2 (0.3-8) | 3 (0.3-9.5) | 3.5 (1-13) | <.001 |
| Tumor thickness at TNI <0.5 mm | 14 (100) | 20 (23.8) | 1 (1.4) | <.001 | - | - | - | - |
| Complete pathologic response | - | - | - | - | 14 (40) | 0 | 0 | <.001 |
Abbreviations: CEA, carcinoembryonic antigen; CLM, colorectal liver metastases; TNI, tumor–normal tissue interface.
Values in table are number of patients unless otherwise indicated.
Patients receiving multiple regimens of preoperative chemotherapy (n=13), only fluropyrimidines (n=4) and cetuximab (n=1) were excluded from this analysis, finally including 153 patients.
Agreement between Local Pathologists and Central Pathology Reviewer for the Measurement Pathologic Markers of Response to Chemotherapy
The local pathologist and the central pathology reviewer agreed with respect to categorization of both pathologic response and tumor thickness at the tumor-normal interface in 116 (68%) patients. In 167 (98%) patients, there was agreement for 1 of the 2 parameters. Disagreement for both parameters was observed in only 4 (2%) patients.
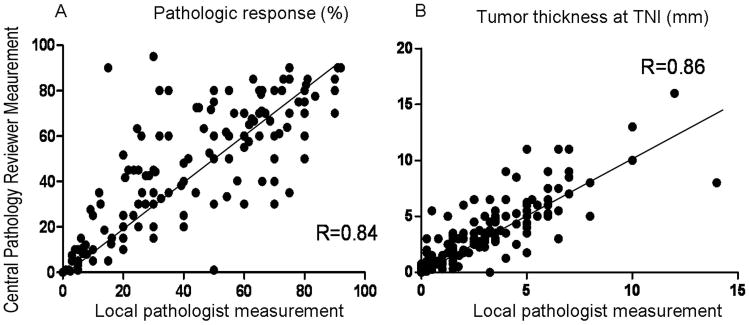
The median difference for pathologic response between the measurements by the local pathologists and the measurements by the central reviewer was 5%. There was complete agreement in assessment of response between the central pathology reviewer and local pathologists in complete response category. Of the 84 patients classified by the central pathology reviewer as having a major response, 9 (11%) were classified as minor responders by a local pathologist. Of the 69 patients classified by the central pathology reviewer as having a minor response, 2 (3%) were classified as major responders by a local pathologist. Figure 3A shows a strong linear correlation between pathologic response measured by local pathologists and pathologic response measured by the central pathology reviewer. The overall κ between the central pathology reviewer and local pathologists for the three categories of pathology response was 0.82 (almost perfect agreement).
Figure 3.
Scatter plot correlating measurements of pathologic response (A) and tumor thickness at the tumor–normal liver interface (B) between the central pathology reviewer and local pathologists.
The median difference for tumor thickness at the tumor-normal interface between the measurements by the local pathologists and the measurements by the central pathology reviewer was 0.5 mm. Of the 35 patients classified by the central pathology reviewer as having tumor thickness of <0.5 mm, 7 (20%) were classified as having thickness ≥0.5 mm by a local pathologist. Of the 97 patients classified by the central pathology reviewer as having tumor thickness of 0.5 mm to <5 mm, 16 (16%) were classified in a different category by a local pathologist. Of the 39 patients classified by the central pathology reviewer as having tumor thickness of at least 5 mm, 12 (31%) were classified as having tumor thickness of 0.5 mm to <5 mm by a local pathologist. Figure 3B shows a strong linear correlation between tumor thickness measured by local pathologists and tumor thickness measured by the central pathology reviewer. The overall κ between central pathology reviewer and local pathologist was 0.76 (substantial agreement).
Discussion
This study validates pathologic response and tumor thickness at the tumor-normal interface as predictors of disease-free survival in an independent, multicenter series of patients with colorectal liver metastases who underwent resection after routinely used preoperative chemotherapy regimens. In addition, our findings indicate good interobserver agreement between the central pathology reviewer and local pathologists in the measured values of these 2 parameters.
Pathologic response to preoperative chemotherapy has been shown to be of prognostic significance in cancer of solid organs, including cancer of the breast, esophagus, and rectum.17-19 Given the approximate 2 year and 5 year median time to recurrence or death after hepatectomies for colorectal cancer, implementation of a pathologic response end point would substantially increase interest in novel drug development in this setting. In addition, studies designed to assess potential biomarkers for response to chemotherapy in cancer of solid organs frequently use pathologic response as a primary or secondary end point.20,21 Our findings suggest that pathologic response and tumor thickness at tumor-normal interface may also be suitable end points for studies of biomarkers assessment for response to chemotherapy in patients with colorectal liver metastases.
The pathologic markers validated in this study could also make it possible to tailor postoperative chemotherapy in patients with colorectal liver metastases on the basis of the response to preoperative chemotherapy. At present, patients with colorectal liver metastases usually receive the same chemotherapy regimen before and after surgery regardless of the response to preoperative chemotherapy.2 In the future, patients who do not respond adequately to preoperative chemotherapy as judged on the basis of measurement of our 2 validated prognostic markers could be switched to a different and hopefully more effective chemotherapy regimen after resection of the metastases. This concept has never been evaluated and could be tested prospectively.
Several criteria have been described for categorization of regression of colorectal metastases after chemotherapy. In a single-institution study of patients undergoing resection after preoperative chemotherapy without inclusion of bevacizumab, tumor regression grade based on semiquantitative assessment of fibrosis and residual tumor cells in resected colorectal liver metastases was associated with survival outcome.11 In a large single-institution study, Adam et al12 demonstrated superior survivor outcome of patients with complete pathologic response. Blazer et al13 showed that pathologic response categorized as complete, major, or minor response was associated with overall survival in multivariate analysis. Subsequently, Maru et al14 showed that tumor thickness at the tumor-normal interface was associated with recurrence-free survival and preoperative imaging response. The later study showed that highest uninterrupted tumor thickness at TNI for one tumor nodule and mean of all maximum tumor thicknesses of multiple tumors was significantly associated with DFS. Since the response of preoperative chemotherapy is heterogenous in different tumor nodules, mean of maximum tumor thickness at TNI was utilized for the correlation in patients with multiple tumors in this study and prior study. Using only the maximum thickness in case of multiple tumors would likely to skew the data towards the tumors with poor response. The present study is validation of the studies by Blazer et al13 and Maru et al14 with a focus on disease-free survival. The tumor thickness at tumor normal interface was found to be a better predictor of disease free survival in a multivariate analysis which included both pathologic response and tumor thickness at tumor-normal interface. However, due to the interdependence of these two parameters, we propose that both the parameters should be included in the pathology report since they were significant in multivariate analyses when one of them was excluded. Although not included in this study, a scoring system which includes these two parameters and other parameters including tumor size, number of tumor nodules and margin status to predict patient outcome of colorectal liver metastases may conribute significantly in postoperative management of patients with colorectal liver metastases.
There were significant differences in clinical and treatment characteristics between the patients at the 4 centers. Validation of the 2 tested pathology markers in this heterogeneous patient population supports application of these markers in a wide population of patients with resected colorectal liver metastases. The significance of these pathology parameters as markers of chemotherapy response is enhanced by good agreement between central pathology review and local pathologists. In a very small percentage of cases (2%), there was disagreement in categorization of both parameters. The higher rate of agreement with respect to just 1 of the 2 pathologic markers as compared to agreement with respect to both markers favors including information about both markers in the surgical pathology report.
Present study also validated previously described predictors of pathology response and tumor thickness at the tumor-normal interface. Tumor size was the predictor of pathologic response in the present study and in previous studies by Adam et al. 12 and Blazer et al. 13 Similar to prior study by Maru et al. 14, oxaliplatin based chemotherapy regimen and bevasizumab were associated with thin tumor thickness at the tumor-normal interface in the present study.
In conclusion, this study is the first to validate the pathologic markers of response to chemotherapy as predictors of disease-free survival in patients undergoing resection of colorectal liver metastases. The pathologic response and tumor thickness at the tumor-normal interface are reliable criteria that may be used in routine clinical practice and are new end points for assessment of biomarkers of chemotherapy response.
Acknowledgments
The authors thank Stephanie Deming, The University of Texas M.D.Anderson Cancer Center for editing.
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA016672.
Footnotes
Financial disclosures: 1. Dipen Maru: Received research funding from Taiho Pharma Inc. USA. 2. JN Vauthey: Received honorarium from Roche and Sanofi Aventis and research funding from Rosche. 3. Prof. Gruenberger is a consultant and received honorarium from Rosche and Merck Serano. He is also a consultant for Sanofi Aventis and has received funding from Amgen. 4. Prof Benoist received honorarium from Rosche and Merck Serano.
Accepted for platform presentation at United States and Canadian Academy of Pathology annual meeting at Baltimore, MD on March 4, 2013
Precis: The pathologic response and tumor thickness at the tumor-normal interface are reproducible criteria that may be used in routine clinical practice and are new end points for assessment of biomarkers of chemotherapy response in colorectal liver metastases.
References
- 1.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 4.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreou A, Aloia TA, Brouquet, et al. Recent advances in treatment of colorectal liver metastases. Gastrointest Cancer Res. 2011;4(4 suppl1):S2–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 8.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 9.Prowell T, Pazdur R. Pathologic complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 10.Allen PJ, Kemeny N, Jarnagin W, et al. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7(1):109–115. doi: 10.1016/S1091-255X(02)00121-X. [DOI] [PubMed] [Google Scholar]
- 11.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, Wicherts DA, de Haas RJ, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 13.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 14.Maru DM, Kopetz S, Boonsirikamchai P, et al. Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]
- 15.Poultsides GA, Bao F, Servais EL, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012 Apr 3; doi: 10.1245/s10434-012-2335-1. Epub of ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Chun YS, Vauthey JN, Boonsirikamchi P, et al. Association of computed tomography criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302(21):2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant therapy. J Clin Oncol. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 18.Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103(7):1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 19.Rodel C, Martus P, Papdoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy in rectal cancer. J Clin Oncol. 2006;23(34):8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 20.Ghadimi BM, Grade M, Difilippantonio MJ, et al. Effectiveness of gene expression profiling for response prediction of rectal adenocarcinomas to preoperative chemoradiotherapy. J Clin Oncol. 2005;23(9):1826–1838. doi: 10.1200/JCO.2005.00.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuerigen O, Schneeweiss A, Toeds G, et al. Gene expression signature predicting pathologic complete response with gemcitabine, epirubicin, and docetaxel in primary breast cancer. J Clin Oncol. 2006;24(12):1839–1845. doi: 10.1200/JCO.2005.04.7019. [DOI] [PubMed] [Google Scholar]
- 22.Klinger M, Tamandi D, Eipeldauer S, et al. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17(8):2059–2065. doi: 10.1245/s10434-010-0972-9. [DOI] [PubMed] [Google Scholar]