Abstract
Purpose
We evaluated the risk of hypersensitivity to metals in a population of consecutive subjects undergoing a total knee arthroplasty (TKA). We also proposed a diagnostic pathway to address any sensitivity to metals. We finally presented the mid-term outcomes of a full non allergenic knee implant.
Methods
We developed a protocol based on the medical history, patch testing, and on specific laboratory assays, in order to assess a sensitization to metals. Twenty-four patients (25 knees) with referred or suspected allergy to metals were found in more than 1,000 treated patients, with a mean age of 72.9 years. We proceeded to a radiologic study, a clinical evaluation by the visual analogic scale (VAS), and Knee Society rating system (KSS). In all cases a full anallergic cemented implant with an oxidized zirconium femoral component and an all-polyethylene tibial baseplate was chosen.
Results
Four (16.6%) of the 24 patients were considered to be hypersensitive to metals. The mean follow-up was 79.2 months. No patient reported any reaction related to hypersensitivity or complications after TKA. The VAS improved from a mean preoperative value of 7.2 to 1.8 postoperatively; the KSS and the functional score increased from 38 to 91 points and from 39 to 88 points, respectively.
Conclusions
We consider careful research of medical history for metals hypersensitivity crucial, and we perform patch testing and lab assays in case of doubtful sensitization. The choice of a modern hypoallergenic implant may prevent any kind of potential reactions.
Keywords: Hypersensitivity to metals, Total knee arthroplasty
Introduction
Hypersensitivity to orthopaedic implants is still a matter of controversy. Sensitivity to metals is very common, particularly to nickel, which is common in several objects and substances of daily and working life; the prevalence seems now to exceed the 10 % of the general population previously reported [1–3]. The clinical expression of this phenomenon typically includes local effects such as dermatitis, rash, erythema and rhinitis; rarely, general complications such as itching or asthma may occur [4–6]. Local articular events, such as persistent pain and swelling, and bone necrosis may also be induced as direct damage of the sensitivity to metals in patients after a joint replacement [4, 7–10]. Moreover, an early failure of an orthopaedic implant may occur related to the indirect activation of macrophages by metal ions released after contact with host fluids [4, 11]. This phenomenon was recently termed “aseptic lymphocyte-dominated vasculitis-associated lesion” (ALDVAL) by Willert et. al, and proposed as suggestive but not pathognomonic of an altered response to metals [12, 13]. During recent decades, several reports addressed the reactions to different orthopaedic implants, with a delayed-type 4 hypersensitivity pattern, sometimes persisting also after their removal [14]. On the other hand, no responses were reported in patients with known hypersensitivity to metals after a standard joint replacement with a low nickel content [15].
Given the increase of the number of total knee arthroplasties (TKAs) during recent years [16, 17], we would expect a corresponding rise of the cases of hypersensitivity to metal implants, which is still negligible [15, 18]. On the other hand, modern implants are made of alloys with less nickel content in contrast to past decades [18]. Nonetheless, many designs are now available with high-performance hypoallergenic materials. Few systems however present the features of a “full” non allergenic implant; they are characterized by an oxidized zirconium femoral component articulating with an all-polyethylene tibial component, implants with zirconium nitride femoral and tibial components, and systems with a ceramic femoral component. Encouraging outcomes comparable with standard Cr-Co alloys implants have been reported with a short to mid-term follow-up [19–22].
The currently available, most simple and reproducible test to detect a sensitivity to metals is patch testing. However, there is still debate on its predictive value, how its results may correspond to the response of the articular synovial tissue in contact with a metallic implant [3], and regarding the availability of appropriate challenge agents [23]. Several laboratory assays have been proposed over the years. The lymphocyte transformation testing (LTT) consists of a study of the proliferation of lymphocytes (obtained by a peripheral blood harvest) after contact with different metallic substances [23, 24]. The enzyme-linked immunosorbent assay (ELISA) allows study of the cytokines expression in supernatants produced by lymphocytes cultures [8, 23]. Confocal microscopy consists of the visualization of specific receptors (CD3, CD4) by fluorescent antibodies in a population of activated T-cells [25]. Migration inhibition assays measure the migration inhibition response of activated lymphocytes located in chambers separated by a membrane, through which cells previously incubated with radioactive [3H]-thymidine could pass only by an active migration [23].
The main purpose of the present study is the analysis of the outcomes of a full non allergenic implant at a mid-term follow-up. Further aims are the evaluation of the risk of hypersensitivity to metals in a population of consecutive subjects undergoing TKA at a single institution, and the results of the diagnostic pathways to address an actual risk or status of sensitivity to metals.
Patients and methods
Twenty-four patients (25 knees) with referred or suspect allergy to metals, or familiarity for hypersensitivity, were treated with a Genesis II® (Smith & Nephew, Warsaw, IN) with Oxinium® femoral and All-Poly® tibial components. Oxinium is a well-known material, consisting in a nickel-free metal alloy with an oxide layer conferring bearing properties of the ceramic without the fracture risk related to brittleness [26–28]. The tibial component was made entirely of polyethylene (PE) without metal-back; several papers during the last decades reported satisfactory results of all-polyethylene components in terms of tribologic behaviour and survivorship [29–32]. All the implants, available as cruciate retaining (CR) or posterior stabilized (PS) were cemented (Fig. 1).
Fig. 1.
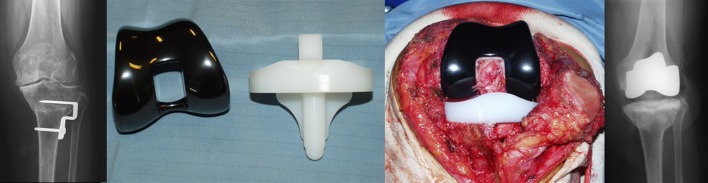
An anallergic total knee arthroplasty (TKA) with an oxidized zirconium femoral component and an all-polyethylene tibial component. Preoperative, intraoperative, and radiographic aspects after implantation
These patients were found in a series of 1,007 TKAs in 915 consecutive patients affected by knee arthritis at the authors’ institution, from June 2002 to January 2008, and representing 2.6 % of the group.
Seventeen were female subjects. The mean age was 72.9 (range, 54–86). One patient had a bilateral involvement. For all the other subjects, we proceeded to a radiologic study (standing X-rays, lateral and patellar views) via a pain and clinical evaluation with the visual analogic scale (VAS) and Knee Society rating system (KSS), respectively.
Since our early interest in hypersensitivity to metals, we proposed to all 1,007 patients a specific questionnaire to find any state of intolerance to metal ions. The medical history was focused on previous contacts with any substances for professional or hobby-related activities, and familiarity for immunological diseases. Attention was paid to any allergy to drugs, comorbidities, and related medical therapies.
In patients with a positive medical history, after adequate information and written consent, we suggested patch testing; we thus developed a protocol based on the medical history and patch testing. The patch testing was conducted to evaluate any reactivity to metals by an adhesive patch loaded with known concentrations of specific allergens as reported in Table 1, and compared with Vaseline. After 48 hours, the first inspection was done to assess any skin reaction, or to remove the patch in case of intense response. After 72 hours, the patch was finally removed and the final inspection was done.
Table 1.
Composition of the patch testing, with metal and cement allergens
| Cement components | Metal substances |
|---|---|
| Hydroxyethyl methacrylate | Cobalt chloride 1 % |
| Dimethyl-p-toluidine | Nickel sulfate 5 % |
| Benzoyl peroxide | Potassium dichromate 0.5 % |
| Vaseline | Chromium III |
| Vaseline |
Since 2006, we also introduced some specific laboratory assays such as LTT, ELISA, and confocal microscopy; all the patients suspected as candidates for TKA were submitted to this protocol before the patch testing. LTT was made by the evaluation of [3H]-thymidine uptake (3H-TdR) in lymphocytes. ELISA testing was conducted by the Luminex LabMAP system (Luminex Corporation, Austin, TX). Confocal microscopy was performed to evaluate any intracellular abnormalities after contact with metals by 3D images of the stimulated cells, obtaining 0.5 nm optical slides directly acquired and CT reconstructed.
A hypersensitivity to metals was strongly considered when the medical history and the patch testing (before 2006), and the history, patch testing, and laboratory findings (after 2006) were positive. It was suggested as “possible” when the medical history and the patch testing were positive, but the blood assessments were not performed (as before 2006) or negative. The follow-up was conducted at one, three, and six months after surgery, and then every year. The clinical evaluation consisted of the assessment of VAS, range of motion (ROM), and KSS; a radiographic study was done to calculate the femorotibial angle, the positioning of the components, and to monitor any radiolucency by the criteria of the Knee Society roentgenographic evaluation and scoring system [33].
Results
Five (20.8 %) of the 24 patients (five knees) were considered to be hypersensitive to metals, given the positive response to medical history, patch testing, and lab assays. In three subjects, a simultaneous sensitivity to two metals was demonstrated. Sixteen patients included before 2006 did not undergo any lab analysis, and were considered to be “possible” hypersensitive (positivity to both medical history and patch testing). A single patient, negative to the patch testing, was actually found to be positive to chrome after the lab tests. The results of the diagnostic evaluation for hypersensitivity to metals is shown in Table 2.
Table 2.
Overall results of patch and lab testings
| Result | Medical history for metal sensitivity | Patch testing | Lab testing (evaluated from 2006) |
|---|---|---|---|
| Positivity | Ascertained: 9 / 24 | Nickel: 21 / 24 | Nickel: 5 / 6 |
| Chrome: 6a / 24 | Chrome: 4a / 5 | ||
| Suspected: 13 / 24 | Cobalt: 4 / 24 | Cobalt: 1 / 1 |
a One subject was negative to chrome at the patch testing, but was found positive to LTT
The overall incidence of an actual sensitivity was of 0.49 % (five patients over 1,007 TKA procedures).
All patients underwent TKA with the abovementioned implant: in one female patient we performed a bilateral TKA with a yearly interval. Seven patients received a CR implant, while in the remainders a PS prosthesis was chosen. Patella resurfacing was performed in five patients. All the patients received a posterior stabilized TKA.
The mean follow-up was 79.2 months (range, 61–90). No patient was lost at the follow-up. No patient reported any reaction related to hypersensitivity, no case of anterior knee pain was referred, and no implant failed at the latest follow-up.
The mean femorotibial angle in the coronal plane averaged 7.0° varus preoperatively (range, 1°–10°) in 18 patients and 11.2° valgus (range, 9°–14°) in six patients. After surgery, the mean position of the femoral component was 4.2° valgus (range, 1.9°–7.7°) relative to the anatomic axis of the femur on the standing AP radiograms, and 0.7° flexion (range, 0.9° extension to 3.3° flexion) on the lateral radiograms. The mean alignment of the tibial component was 90.9° (range, 88.5°–92.3°) relative to the mechanical axis of the tibia on the standing AP radiograms and 4.4° posterior slope (range, 1.4°–8.1°) on the lateral radiograms.
At the final followup, VAS improved from a mean pre-operative value of 7.2 (range, 6–9) to 1.8 (range, 0–3). The KSS increased from an average of 38 points (range, 18–59) pre-operatively to 91 points (range, 65–100) at the latest evaluation. The functional score improved from 39 points (range, 5–55) to 88 points (range, 55–100). The mean total flexion arc improved from 90° (range, 20°–130°) preoperatively to 115° (range, 85°–145°) at the time of the latest follow-up.
Radiolucencies were present in two implants (one on the femoral side—anterior cortex and one on the medial tibial plateau), but according to the Knee Society roentgenographic evaluation, none of these was >2 mm, and there was no significant progression at the latest radiologic follow-up.
Discussion
Many TKAs are performed worldwide every day, but few are the cases of actual hypersensitivity to metal implants, where the incidence is estimated to be lower than 1 % [18]. Hypersensitivity to orthopaedic implants is a still debated issue, since it has to be considered as a diagnosis of exclusion. The main causes of a painful TKA are, generally, infection, aseptic loosening, and instability [34]. Critical points are the tools to confirm this suspect, once the most frequent causes have been excluded [9, 10]. The patch testing has several limits, due to the skin reactivity being different from the response of the joint tissues [3, 23]. Cellular reactions to a contact with metals may be analysed in different ways: LTT, ELISA, MIF, and confocal microscopy are the most addressed [18, 35]. It is now reasonable to consider that these methodologies may together give a comprehensive determination of a hypersensitivity to metals [23].
Hallab and his group have been the most interested in this topic, proposing several methods over the years to assess a sensitivity to orthopaedic implants [3, 8, 11, 13, 23, 25]. An in vitro evaluation has been proposed by Merritt and Rodrigo, showing a significative percentage of sensitization in a population of asymptomatic patients after a joint replacement, but a low risk of severe reactions to metal components [18]. A histomorphological study was conducted by Willert et al. in 19 specimens of periprosthetic tissues coming from patients with failed metal-on-metal hip arthroplasties; a specific hystologic pattern was found at light microscopy (ALDVAL), probably related to a periprosthetic bone loss initiated by a sensitization and immunological response after continuous release of metal ions by the prosthetic components [12]. However, the low frequency of this phenomenon and the lack of a study at electron microscopy makes this hystopathologic pattern not pathognomonic of a metal hypersensitivity.
In the present study, we confirmed the low prevalence of the risk for sensitivity to metals in a cohort of patients undergoing a TKA (20.8 % in more than 1,000 procedures in about seven years). Moreover, only a part of these subjects showed an actual sensitivity (0.49 %) based on a diagnostic protocol consisting of a multiple evaluation. Before 2006, all patients with positive history and patch testing were suspected to be possible hypersensitive. After development of the lab protocol (2006), all the patients with positive medical history and negative patch testing presented negative results in the blood assays. Furthermore, not all the patients positive to the clinical history and patch testing were found to be positive to lab tests (Table 2).
We did consider it reasonable to use a non allergenic implant in all cases (possible or ascertain sensitivity) independently by the opportunity to perform any laboratory assay. By using the abovementioned type of implant, we had no complications, and we did not observe critical situations at mid-term follow-up also regarding the use of an all-polyethylene tibial component. The clinical outcomes were substantially comparable to our previously reported data regarding the same implant with standard Cr-Co femoral components and metalback tibial baseplate in the same period [20], and in line with the most recent series [31, 32].
The availability of the assays as mentioned surely will clarify by a scientific point of view the status of hypersensitivity; however, as demonstrated by our experience before 2006, we feel that an implant with high-performance materials and hypoallergenic properties is to be reasonably considered in any case of suspected hypersensitivity to metals.
This study has several limits. The study population is small; however, the prevalence of an actual hypersensitivity in our series needing a TKA was substantially low. The diagnosis of hypersensitivity to metals is still debated, and this clinical issue has to be considered only by exclusion criteria, using a combination of the mentioned assays. We used only a single implant for TKA with respect to several available, given the confidence with this modern and highly modular system.
However, this study shows an easy and reproducible approach for the patients with any risk factor for hypersensitivity to metals undergoing a knee replacement. We consider of paramount importance a careful research of any previous altered contact with metals in the medical history of the patients who are candidates for a TKA. The choice of a modern hypoallergenic implant prevents any kind of potential reactions, ensuring on the other hand a mid-term survivorship comparable to standard prostheses, given the high tribologic performances of these materials.
Acknowledgments
The authors state that they had no funding or any conflict of interests, which may have produced a bias in the preparation of the manuscript.
References
- 1.Möller H. Nickel dermatitis: problems solved and unsolved. Contact Dermatitis. 1990;23:217–220. doi: 10.1111/j.1600-0536.1990.tb05001.x. [DOI] [PubMed] [Google Scholar]
- 2.Goh CL. Prevalence of contact allergy by sex, race and age. Contact Dermatitis. 1986;14:237–240. doi: 10.1111/j.1600-0536.1986.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 3.Hallab NJ, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. doi: 10.1302/0301-620X.83B3.9674. [DOI] [PubMed] [Google Scholar]
- 4.Elves MW, Wilson JN, Scales JT, Kemp HBS. Incidence of metal sensitivity in patients with total joint replacements. Br Med J. 1975;4:376–378. doi: 10.1136/bmj.4.5993.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menné T, Nieboer E. Metal contact dermatitis: a common and potentially debilitating disease. Endeavour. 1989;13:117–122. doi: 10.1016/0160-9327(89)90085-9. [DOI] [PubMed] [Google Scholar]
- 6.Balato N, Lembo G, Patruno C, Ayala F. Generalized dermatitis due to an osteosynthesis screw. Contact Dermatitis. 1991;24:310. doi: 10.1111/j.1600-0536.1991.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 7.Deutman R, Mulder TJ, Brian R, Nater JP. Metal sensitivity before and after total hip arthroplasties. J Bone Joint Surg Am. 1977;59:862–865. [PubMed] [Google Scholar]
- 8.Hallab NJ, Caicedo M, Finnegan A, Jacobs JJ. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg Res. 2008;3:6. doi: 10.1186/1749-799X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carulli C, Villano M, Bucciarelli G, Martini C, Innocenti M. Painful knee arthroplasty: definition and overview. Clin Cases Miner Bone Metab. 2011;8:23–25. [PMC free article] [PubMed] [Google Scholar]
- 10.Villano M, Carulli C, Puccini S, Soderi S, Innocenti M. Painful knee arthroplasty: surgical approach. Clin Case Bone Miner Metab. 2011;8:26–28. [PMC free article] [PubMed] [Google Scholar]
- 11.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67:182–188. [PubMed] [Google Scholar]
- 12.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs JJ, Hallab NJ. Loosening and osteolysis associated with metal-on-metal bearings: a local effect of metal hypersensitivity? J Bone Joint Surg Am. 2006;88:1171–1172. doi: 10.2106/JBJS.F.00453. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie AW, Aitken CV, Ridsdill-Smith R. Urticaria after insertion of Smith-Petersen Vitallium nail. Br Med J. 1967;5570:36. doi: 10.1136/bmj.4.5570.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thienpont E, Berger Y. No allergic reaction after TKA in a chrome-cobalt-nickel-sensitive patient: case report and review of the literature. Knee Surg Sports Traumatol Arthrosc. 2013;21:636–640. doi: 10.1007/s00167-012-2000-z. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467:2606–2612. doi: 10.1007/s11999-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeney JA, Eunice S, Pashos G, Wright RW, Clohisy JC. What is the evidence for total knee arthroplasty in young patients? A systematic review of the literature. Clin Orthop Relat Res. 2011;469:574–583. doi: 10.1007/s11999-010-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merritt K, Rodrigo JJ. Immune response to synthetic materials. Sensitization of patients receiving orthopaedic implants. Clin Orthop Relat Res. 1996;326:71–79. doi: 10.1097/00003086-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Innocenti M, Civinini R, Carulli C, Villano M, Linari S, Morfini M. A modular total knee arthroplasty in haemophilic arthropathy. Knee. 2007;14:264–268. doi: 10.1016/j.knee.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Majima T, Yasuda K, Tago H, Aoki Y, Minami A. Clinical results of posterior cruciate ligament retaining TKA with alumina ceramic condylar prosthesis: comparison to Co-Cr alloy prosthesis. Knee Surg Sports Traumatol Arthrosc. 2008;16:152–156. doi: 10.1007/s00167-007-0435-4. [DOI] [PubMed] [Google Scholar]
- 21.Innocenti M, Civinini R, Carulli C, Matassi F, Villano M. The 5-year results of an oxidized zirconium femoral component for TKA. Clin Orthop Relat Res. 2010;468:1258–1263. doi: 10.1007/s11999-009-1109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iida T, Minoda Y, Kadoya Y, Matsui Y, Kobayashi A, Iwaki H, Ikebuchi M, Yoshida T, Nakamura H. Mid-term clinical results of alumina medial pivot total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20:1514–1519. doi: 10.1007/s00167-011-1734-3. [DOI] [PubMed] [Google Scholar]
- 23.Hallab NJ, Mikecz K, Jacobs JJ. A triple assay technique for the evaluation of metal-induced, delayed-type hypersensitivity responses in patients with or receiving total joint arthroplasty. J Biomed Mater Res. 2000;53:480–489. doi: 10.1002/1097-4636(200009)53:5<480::AID-JBM6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 24.Everness KM, Gawkrodger DJ, Botham PA, Hunter JA. The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermat. 1990;122:293–298. doi: 10.1111/j.1365-2133.1990.tb08276.x. [DOI] [PubMed] [Google Scholar]
- 25.Hallab NJ, Caicedo M, Epstein R, McAllister K, Jacobs JJ. In vitro reactivity to implant metals demonstrates a person-dependent association with both T-cell and B-cell activation. J Biomed Mater Res. 2010;92:667–682. doi: 10.1002/jbm.a.32368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White SE, Whiteside LA, McCarthy DS, Anthony M, Poggie RA. Simulated knee wear with cobalt chromium and oxidized zirconium knee femoral components. Clin Orthop Relat Res. 1994;309:176–184. [PubMed] [Google Scholar]
- 27.Ries MD, Salehi A, Widding K, Hunter G. Polyethylene wear performance of oxidized zirconium and cobalt-chromium knee components under abrasive conditions. J Bone Joint Surg Am. 2002;84(suppl 2):129–135. doi: 10.2106/00004623-200200002-00018. [DOI] [PubMed] [Google Scholar]
- 28.Hernigou P, Nogier A, Manicom O, Poignard A, De Abreu L, Filippini P. Alternative femoral bearing surface options for knee replacement in young patients. Knee. 2004;11:169–172. doi: 10.1016/j.knee.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Norgren B, Dalén T, Nilsson KG. All-poly tibial component better than metal-backed: a randomized RSA study. Knee. 2004;11:189–196. doi: 10.1016/S0968-0160(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 30.Dojcinovic S, Ait Si Selmi T, Servien E, Verdonk PC, Neyret P. A comparison of all-polyethylene and metal-backed tibial components in total knee arthroplasty. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:364–372. doi: 10.1016/S0035-1040(07)90278-0. [DOI] [PubMed] [Google Scholar]
- 31.Browne JA, Gall Sims SE, Giuseffi SA, Trousdale RT. All-polyethylene tibial components in modern total knee arthroplasty. J Am Acad Orthop Surg. 2011;19:527–535. doi: 10.5435/00124635-201109000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470:3549–3559. doi: 10.1007/s11999-012-2582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 34.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Merritt K. Detection of antibodies against corrosion products in patients after Co-Cr total joint replacements. Biomed Mater Res. 1994;28:1249–1258. doi: 10.1002/jbm.820281102. [DOI] [PubMed] [Google Scholar]


