Abstract
Purpose
Our aim was to clarify the effective decrease in blood transfusion after primary total knee arthroplasty (TKA) from a multimodal blood-loss prevention approach (MBLPA) and the related risk factors of blood transfusion.
Methods
We retrospectively compared the rate of postoperative blood transfusion in 418 cases of primary TKA during 2010 from a single institution with two different groups of patients, allocating cases to the group with MBLPA (group 1, study group, N = 71) and controls to the group without MBLPA (group 2, standard group, N = 347). MBLPA procedure included pre-operative haemoglobin (Hb) optimisation; femoral canal obturation; limited incision and release; peri- and intra-articular use of saline with adrenalin, morpheic chloride, tobramycin, betamethasone and ropivacaine; tourniquet release after skin closure; 24 hour drain under atmospheric pressure; and two doses of tranexamic acid (TXA) IV. In the control group, surgeons followed the standard procedure without blood-saving techniques. Case–control comparison and blood transfusion risk factors were analysed.
Results
Group 1 had a zero transfusion rate (0/71), whereas 27.4 % of patients (95/347) in group 2 received allogenic blood transfusion. Significant transfusion risk factors were pre-operative Hb <12 g/dl), American Society of Anesthesiologists (ASA) status III and nonobese body mass index (BMI); Age and gender were not significant risk factors.
Conclusions
MBLPA in primary TKA was highly effective, with a zero transfusion rate. Risk factors for transfusion were determined, and eliminating them contributed to the avoidance of allogeneic blood transfusion in our study series.
Keywords: Blood-loss prevention, TKA, Transfusion risk factors, Tranexamic acid
Introduction
Total knee arthroplasty (TKA) is classically associated with blood loss, requiring allogenic blood transfusion at variable rates among different series: weighted average 44 %, range 9 –84 % in a systematic review [1]. Even higher rates were observed in pre-operative autologous blood donation [2]; this procedure has long been considered as not being cost effective [3]. Although some institutions still maintain pre-operative autologous donation to decrease allogenic transfusion [4], it did not appear to be effective in TKA, with a nonstatistically significant difference between autologous donors (21 % allogenic) and nondonors (27 %). Allogenic blood transfusion remains a standard treatment in case of postoperative anaemia after TKA, and two units packed red blood cells are routinely prepared pre-operatively for TKAs in many institutions. However, the risks of allogenic blood transfusions are well established [5], and careful transfusion indication, together with the use of antifibrinolytic medications such as tranexamic acid (TXA), can minimise the transfusion rate in TKA [6].
With currently available evidence, it is obvious that both autologous and allogenic blood transfusions in TKA are reducing and that both safety and cost-effectiveness criteria favour blood-saving strategies with the aim of avoiding transfusions secondary to TKA. Among those strategies, TXA administration has proved to effectively decrease transfusion rates, particularly in meta-analyses of trials [7–9] and with repeated dose [10]. Interest remains in clarifying how blood transfusion can even be effectively avoided after TKA.
The administration of TXA alone in TKA may not produce a clinically relevant decrease in transfusion rate when this rate is low. A recent, large cohort retrospective study [11] found a significant decrease, from 6.5 % to 0.3 %, in transfusion rate when TXA was used, further confirming the well-known effects of blood-loss management programmes in large populations. However, a recent clinical trial of TXA administered IV in TKA [12] compared with controls under contemporary blood-saving protocols without TXA could not prospectively confirm a significant difference in transfusion rate (7 % vs 1 %). The authors concluded that TXA administered IV reduced total blood loss but raised the issue that the effects on transfusion rate can differ among groups of surgical procedures and among patients, particularly if the other techniques are combined in a blood-saving protocol. On the other hand, patient characteristics influence the transfusion rate. Pre-operative haemoglobin (Hb) is a predictor of postoperative transfusion needs in retrospective [13] and prospective [14] studies and clinical trials [15]. No consensus exists about the threshold of Hb or haematocrit. The importance of patient age, weight and comorbidities are of variable significance among studies, and a recent systematic review stressed the low quality of available data and the need for additional analytical approaches in order to obtain a better understanding of transfusion requirements in orthopaedic procedures [1]. The two study questions addressed in our study were: (1) How much does a multimodal blood-loss prevention approach (MBLPA) minimise the blood transfusion rate after primary TKA? (2) What are the risks factors of blood transfusion?
Patients and methods
We conducted a retrospective case–control comparative study assessing all patients with unilateral primary TKA during 2010 at our institution, which has two orthopaedic surgical groups by which this procedure is performed. The surgical groups comprise four and ten senior orthopaedic surgeons, respectively. In the study period, 470 patients >18 years underwent primary TKA. Forty-two cases were dropped due to incomplete information. We created two comparative groups: group 1, study group using the MBLPA; and group 2, standard group in which the MBLPA was not used; 71 patients were allocated to group 1 and 347 to group 2. The difference was because four surgeons in group 1 adhered to the MBLPA whereas ten in group 2 did not. Patient demographics and clinical data were similar between groups, as shown in Table 1.
Table 1.
Patient characteristics in the two surgical groups at our institution, 2010
| Variable | Group 1 (MBLPA) | Group 2 (standard) | P value |
|---|---|---|---|
| Mean ± SD (min–max); N (%) | Mean ± SD (min–max); N (%) | ||
| Patient characteristics | |||
| Age | 73 ± 8.0 (52–89) | 72 ± 8.5 (34–88) | 0.521 |
| Gender | |||
| Male | 17 (25) | 94 (27) | 0.471 |
| Female | 50 (75) | 257 (73) | |
| BMI | 31 ± 4.5 (18–39) | 30 ± 4.8 (17–45) | 0.355 |
| BMI category | |||
| Normal–preobesity | 9 (13) | 66 (19) | 0.289 |
| Obesity I–II | 22 (30) | 83 (24) | |
| Obesity III | 40 (56) | 198 (57) | |
| ASA | 2.2 ± 0.5 (1–3) | 2.3 ± 0.5 (1–4) | 0.282 |
| ASA status | |||
| I | 2 (3) | 3 (1) | 0.382 |
| II | 49 (69) | 205 (65) | |
| III | 20 (28) | 107 (34) | |
| Comorbidities | |||
| Number | |||
| 0 | 37 (52) | 159 (46) | 0.307 |
| 1 | 30 (42) | 140 (40) | |
| 2 | 3 (4.5) | 38 (11) | |
| 3 | 1 (1.5) | 10 (3) | |
| Cardiopathy | 9 (16) | 60 (20) | 0.292 |
| High blood pressure | 31 (54) | 194 (66) | 0.067 |
| Diabetes mellitus | 8 (12) | 25 (7) | 0.138 |
| Dislipidemia | 10 (15) | 77 (22) | 0.082 |
| Venous insufficiency | 1 (1) | 11 (3) | 0.401 |
| Osteoporosis | 3 (4) | 1 (0.3) | 0.017 |
| Neoplasia | 1 (1.5) | 3 (0.8) | 0.504 |
| Hepatopathy | 2 (3) | 9 (3) | 0.549 |
| Vasculopathy | 1 (2) | 17 (5) | 0.185 |
| Pre-operative laboratories | |||
| Hb (g/dl) | 14.1 ± 1.24 (10–16.5) | 13.7 ± 1.31 (9.2–17.3) | 0.033 |
| Hb category | |||
| >13 | 59 (83) | 254 (73) | 0.337 |
| 12–13 | 9 (13) | 66 (19) | |
| <12 | 3 (4) | 27 (8) | |
| Haematocrit (%) | 43.1 ± 3.5 (30.9–50.2) | 41.8 ± 3.8 (28.8–52.0) | 0.001 |
| Discharge laboratories | |||
| Hb (g/dl) | 10.9 ± 0.9 (8.9–13.5) | 10.3 ± 1.2 (7.6–15.9) | 0.000 |
| Hb category | |||
| >13 | 3 (4) | 73 (51) | 0.001 |
| 12–13 | 7 (10) | 25 (7) | |
| <12 | 61 (86) | 249 (72) | |
| Haematocrit (%) | 34.0 ± 3.6 (27.9–49.9) | 31.7 ± 3.6 (23.9–47) | 0.001 |
MBLPA multimodal blood-loss prevention approach; Hb hemoglobin; BMI body mass index, ASA American Society of Anesthesiology, Hb haemoglobin, SD standard deviation
In group 1, surgeons adhered to an MBLPA that included tourniquet with 100 mmHg above systolic pressure, released after skin closure; pre-operative Hb evaluation and, if <g/dl, pre-operative treatment IV with iron; surgical blood-saving protocol, including femoral canal obturation with bone graft, 24 hour drain without vacuum and opened two hours after closure and intra-articular infiltration of posterior, medial and lateral capsule and ligaments, before closure, with 80 cc saline with adrenalin 300 µg, morpheic chloride 10 mg, tobramycin 100 mg, betamethasone sodic phosphate 6 mg, betamethasone acetate 6 mg, and ropivacaine 200 mg; IV-administered TXA in two doses: 15 mg/kg in 100 cc saline slowly infused over 15–20 minutes before tourniquet release, and a second identical dose after three hours.
In group 2, surgeons followed the standard procedure without a particular blood-saving technique (tourniquet 350 mmHg, released before skin closure for electrocoagulation of bleeding; no limits to or treatment of pre-operative Hb; no femoral canal obturation, 24- to 48 hours vacuum drain (opened with skin closure, no intra-articular infiltration); no TXA administration.
All patients followed a thromboprophylaxis protocol consisting of a daily dose of low molecular weight heparin. This was adjusted to treatment dosage, if necessary, in patients already taking anticoagulants orally and discontinued 5 days before surgery. Acetylsalicylic acid was interrupted by the patient before surgery if taking >300 mg daily; patients taking <100 mg daily were permitted to continue.
The retrieved information included demographic data (sex, age, weight, height); clinical data [date of diagnosis, surgery and discharge, comorbidities, drug intake, American Society of Anesthesiologists (ASA) pre-operative risk status, pre-operative Hb optimisation]; transfusion records (number of packed red blood cell units infused; transfusion date); presurgery and discharge laboratory records (Hb, hematocrit); and the hospital healthcare team the patient was allocated to.
Body mass index (BMI) was calculated and classified as BMI III (>40), BMI II–I (30–40) and normal-preobesity (18.5–30). ASA status was further categorised into ASA I-II and ASA III. Hb was categorised in three intervals (<12 g/dl, 12–13 g/dl, >13 g/dl) to clarify the possible thresholds of 12 and 13 g/dl, respectively. Repeated laboratory tests, including haematocrit and Hb determinations, were performed postoperatively in the recovery unit day one and at discharge.
The primary endpoint of the study was the administration of allogenic blood transfusion at any point during the postoperative course before discharge from either orthopaedic unit. The need for transfusion was determined in both groups by the orthopaedic surgeon on call, if patient Hb was <8.0 g/dl or if symptoms of acute anaemia (dizziness, nausea, asthenia, mucocutaneous pallor) were present with Hb between 8.0 and 10.0 g/dl. In patients with cardiovascular or pulmonary comorbidities, the transfusion threshold was set at 9.0 g/dl. Study size was determined by the data from available procedures, performed over the course of one year, in each of the two groups; this produced 71 and 347 cases in groups 1 and 2, respectively. The power of the analysis was statistically adequate [pre-Hb power (α = 0.05) = 0.80; discharge Hb power (α = 0.05) = 0.99].
Statistical analysis
Parametric and nonparametric statistics, comprising Fisher’s exact, Mann–Whitney and chi-square tests were used to compare variables, with a confidence level (CL) of 95 %. A logistic regression was modeled to identify risk factors, and odds ratios (ORs) were obtained. Statistical analysis was performed using STATA software (StataCorp., College Station, TX, USA)
Results
In 2010, the mean prevalence of blood transfusion after primary TKA at our institution was 23 % (n = 95/418), with a mean of ±1.35 packed red blood cells per patient (minimum one - maximum four). When transfusions were compared for surgeries performed in each of the two surgical groups with different strategies toward blood-loss management, significantly different transfusions rates were found: mean in group 2 was 27 % (n = 95/347), and group 1 patients required zero transfusions (n = 0/71) (Table 2). Patient characteristics in both groups were statistically similar, except for pre-operative Hb and haematocrit, as described in Table 1, although the average in both was >13.5 mg/dl. The reported intake of acetylsalicylic acid was 11 % higher in group 2 (discontinued one week before surgery), but this variable was not identified as a transfusion risk factor in the logistic regression (OR = 1.2; p = 0.508) (Table 3). Data indicate that surgical-group allocation played an important role in the probability of patients requiring blood transfusion after TKA. As patient, surgeon and general setting characteristics did not differ, this finding is explained by the blood-loss management technique and the prevention protocol used in group 1, as described above.
Table 2.
Number of patients and transfusions in each surgical group at our institution, 2010
| Transfusion | No transfusion | Total number | |
|---|---|---|---|
| Group 1 (MBLPA) | 0 | 71 | 71 |
| Group 2 (standard) | 95 | 252 | 347 |
| 95 | 323 | 418 |
MBLPA multimodal blood-loss prevention approach
Table 3.
Risk factors for transfusion after primary total knee arthroplasty at our institution, 2010
| Variable | OR | 95 % CI | P value |
|---|---|---|---|
| ASA | |||
| I–II | 1 | ||
| III | 1.7 | 1.0–2.9 | 0.031 |
| Hb preop | |||
| >13 | 1 | ||
| 12–13 | 2.9 | 1.6–5.4 | 0.001 |
| <12 | 4.7 | 2.0–10.6 | 0.000 |
| Obesity | |||
| Obesity III | 1 | ||
| Obesity I–II | 0.7 | 0.3–1.5 | 0.354 |
| Normal–preobesity | 1.9 | 1.0–3.6 | 0.035 |
| Gender | |||
| Male | 1 | ||
| Female | 1.4 | 0.7–2.6 | 0.300 |
| Age | 0.98 | 0.9–1.0 | 0.501 |
ASA American Society of Anesthesiologists, preop. pre-operatively, OR odds ration; CI confidence interval; Hb haemoglobin
Adjusted model: pseudo-R 2 (pR2) = 0.09, p < 0.001
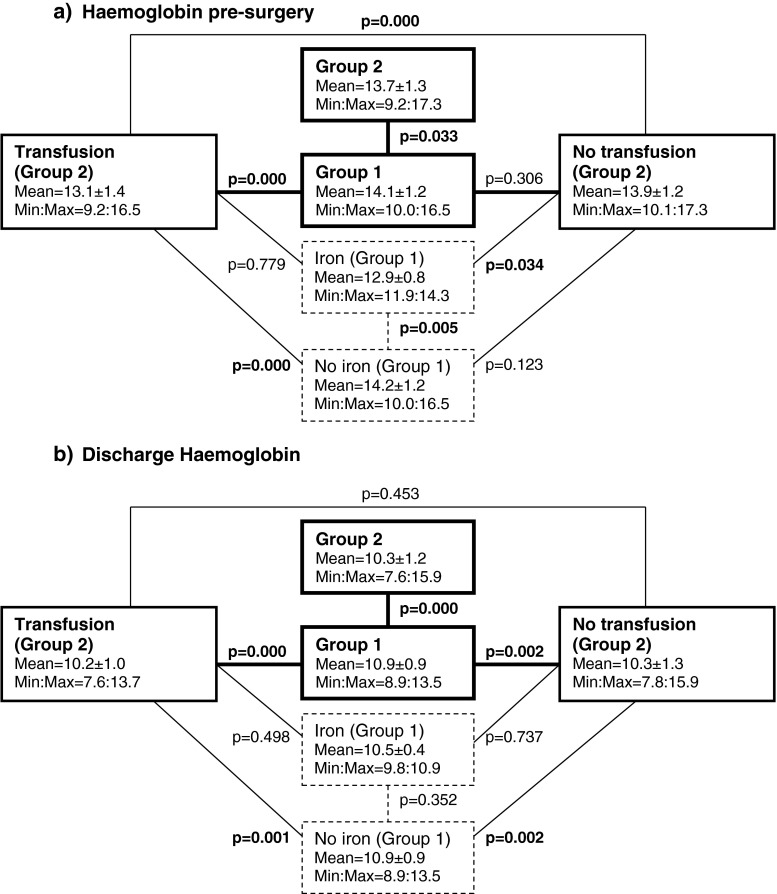
Almost 10 % of patients in group 1 (n = 7/71) received pre-operative Hb optimisation (based on iron administration IV) as a preparation for TKA surgery. Their mean Hb concentration on the day of surgery was 12.9 ± 0.8 g/dl (minimum/maximum range 11.9–14.3), which was still significantly lower than patients who did not receive iron treatment (14.2 ± 1.2 g/dl) in the same surgical group (p = 0.005) (Fig. 1a). When addressing differences in pre-operative Hb between groups, patients with and without transfusion and those with and without iron treatment, it became evident that patients with iron treatment IV in group 1 were not statistically different from those requiring transfusion in group 2 (p = 0.779). Also, patients who did not receive iron treatment in group 1 were not statistically different from those not requiring transfusion in group 2 (p = 0.123).
Fig. 1.
Differences in mean concentration of haemoglobin (g/dl) among patient groups. a Pre-operative haemoglobin (Hb) level. b Hb at discharge. Block 1 patients operated at the surgical block where pre-operative Hb optimisation, surgical technique to decrease blood loss and tranexamic acid (TXA) administration IV were performed. Block 2 patients operated without blood-loss prevention protocol. Iron subset of patients in Block 1 who received pre-operative Hb optimisation based on iron administration. No iron subset of patients in Block 1 who received no iron treatment pre-operatively
When comparing laboratory data at discharge, group 1 with zero transfusions showed a statistically higher Hb concentration (10.9 ± 0.9 g/dl, range 8.9–13.5) than group 2 (10.3 ± 7.6 g/dl, range 7.6–15.9) despite including patients in group 2 who received transfusion (Fig. 1b). At discharge, there were no statistically significant differences (p = 0.387) in Hb in group 1 between subgroups pretreated and not pretreated with iron (mean 10.5 ± 0.4 g/dl; minimum/maximum 9.8–10.9 vs 10.9 ± 0.9 g/dl; minimum/maximum 8.9–13.5, respectively).
The main risks factors found to influence a significant logistic regression were pre-operative Hb concentration, ASA status and BMI adjusted for age and sex, as displayed in Fig. 2 and numerically described in Table 3. Patients with a pre-operative Hb determination <12 g/dl had five times more risk of been transfused (OR = 4.7) than those with Hb level >13 g/dl, adjusted for the other variables in the model. Also, patients with Hb between 12 and 13 g/dl had three times more risk than those >13 g/dl. An ASA status of III increased the probability of being transfused (OR = 1.7) by almost two times compared with ASA I–II. A nonobese BMI was also a risk factor for transfusion compared with BMI I–III (correlation between Hb and BMI was not statistically significant, with rho = 0.10 (p = 0.14). A combination of those three factors estimated a probability of blood transfusion of >70 %.
Fig. 2.
Estimated probability of being transfused after primary total knee arthroplasty (TKA) at La Paz Hospital in 2010, related to pre-operative haemoglobin (Hb) categories, obesity and American Society of Anesthesiologists (ASA)
Discussion
Transfusion rates for unilateral primary TKA reports in the past decade have been high. Most hospitals have now adopted protocols to decrease the allogenic blood transfusion rate, which is now <10 %. This is why a zero transfusion rate after TKA can be a realistic goal. This goal was achieved in one of our surgical groups, which impelled us to scrutinise the risk factors for transfusion and the differences in patient blood management.
This study has several limitations. A prospective, randomised study would be the best option to compare blood-loss management techniques, but it is doubtful whether comparison with placebo or surgical technique with no blood-loss prevention is ethically acceptable after the available meta-analysis and systematic reviews confirming the benefits of blood-loss management. Also, the number of patients in this study was limited because after the results seen at the end of 2010, surgeons in group 2 modified their blood-loss management approach, incorporating techniques to decrease blood loss in TKA procedures. Again, it is probably not ethical to prospectively reproduce this study in view of the overwhelming amount of literature supporting the use of blood-loss-control protocols. The number of patients studied per surgical team was also different (71 vs 347) due to the number of surgeons in each group. However, the power of the analysis was statistically adequate (0.80 for pre-operative Hb and 0.99 for Hb at discharge). Although surgeons were grouped based in their preferences regarding blood-loss prevention in 2010 (groups 1 and 2), all covered TKA surgery as well as other reconstructive procedures, had a standardised technique and their qualifications were deemed equivalent between groups; the only detected difference was their approach to blood-loss management. Regarding the logistic regression model, the pseudo-R2 was low, but the model was found to be significant (pR2 = 0.009, p < 0.001). Finally, as is common in retrospective studies, we encountered lost information that forced us reduce the number of analysed cases, particularly lost cases that were referred and the perioperative labs were not retrospectively available in the electronic file. However, lost cases were <9 %.
We attained a zero transfusion rate in group 1, was noted previously in our setting [1] as in recent studies (1/330 with TXA IV and 0/130 with TXA topically) [11]. The contribution of our study is that this zero rate (0/71) was obtained in a similar patient population as those in group 2, in whom the transfusion rate was 27.4 % (95/347), therefore confirming the effectiveness of the applied protocol. Furthermore, the subset of patients with pre-operative optimisation of Hb in group 1 had statistically similar pre-operative Hb than patients in group 2 requiring transfusion. This observation further confirms the role of the blood-loss prevention protocol to avoid transfusions in this set of patients. Again, Hb at discharge further confirmed that patients from group 1 lost significantly less blood than patients from group 2 who received no transfusion, although pre-operative Hb between these two sets of patients was statistically similar. We consider that these findings provide further proof about the role of surgical protocols—including many surgical gestures, not only TXA—independent of pre-operative Hb. We consider that if the aim is zero transfusion rate, all these factors are required in a global strategy, and this can be achieved, as we confirmed in our study. Other authors suggest that computer-assisted surgery could also decrease blood loss [16], but it is worthy to note that we achieved our goal without this technique.
A most interesting part of our study was the comparison between the two sets of patients with and without blood-loss prevention protocol. Patient allocation could be safely performed in retrospect because in 2010, surgeons in both groups were adopting standardised procedures with divergence in blood-loss management, as expressed above. Although preoperative Hb was slightly higher in the set of patients that required no transfusion (group 1), this was similar to the subset of patients that required no transfusion in group 2, although postoperative Hb was significantly higher in group 1 under the blood-loss-prevention protocol (femoral canal obturation with bone graft, limited incision and soft tissue release, peri- and intra-articular infiltration, tourniquet release after skin closure, 24 hours drain under atmospheric pressure without vacuum and opened only one hour after skin closure, and two doses of TXA administration IV, as described elsewhere) [17]. These results are in full agreement with the literature, highlighting the potent effects of TXA in reducing blood loss [4, 7, 11, 18]. Intra-articular administration of TXA [18] or platelet-rich plasma (PRP) [19] also decreases blood loss and even joint swelling or pain. These techniques, still under evaluation, should be carefully considered. However, IV administration of TXA alone in TKA may not produce a clinically relevant decrease in the transfusion rate. A recent, large-cohort, retrospective study [11] found a significant decrease, from 6.5 % to 0.3 %, in transfusion rate with TXA IV, further confirming the well-known effects of blood-loss management programmes. A recent clinical trial of TXA IV in TKA [12] with controls under contemporary blood-saving protocols without TXA observed a nonsignificant difference in transfusion rate (7 % versus 1 %), concluding that TXA IV reduces total blood loss, although the effects on transfusion rate can differ. We observed a 27.4 % transfusion rate (95/347) in group 2 patients, greater than in the above-mentioned studies. Indirectly, we conclude that without any blood-loss protocol, transfusion rate is much higher than with such a protocol without TXA, as shown in the above-mentioned studies, and this further reinforces the need for multimodal blood-loss-prevention protocols.
Focusing on a recent systematic review that requested more data on transfusion after TKA [1], our results confirmed that pre-operative Hb >13 g/dl is a safe threshold to avoid transfusion. The risk of transfusion rose three times when pre-operative Hb was <13 g/dl and five times when <12 g/dl. We found that the threshold of 12 g/dl, as supported by a recent study [20], can be elevated to 13 g/dl, and this may lead to a zero transfusion rate, particularly if all the other aspects of blood-loss prevention are incorporated in the protocol.
We also confirmed that gender and age were not major determinants of transfusion, in coincidence with other authors [14, 15] but in disagreement with authors who considered age [21]. The difference inf our study may be patient age—mostly between 70 and 80 years in our series—which could make our series less sensitive when studying the age influence. However, we determined that higher BMI was associated with reduced transfusion risk (adjusted for gender and age), even if there was no correlation between obesity and pre-operative Hb. We also found, as did other authors [1], that comorbidities (ASA III) were associated with higher transfusion risk compared with ASA I or II (OR = 1.7) .These are patient characteristics that cannot be avoided but should suggest caution regarding appropriate pre-operative evaluation and eventual treatment of the most important determinant, such as pre-operative Hb concentration. If this is above the safety threshold of 13 g/dl before TKA surgery, the case would be less prone to transfusion.
In conclusion, we found that MBLPA in primary TKA was effective, with a zero transfusion rate. Patients with pre-operative Hb <13 g/dl, nonobese patients and ASA III patients were at higher risk of blood transfusion. Patients with a pre-operative Hb determination <12 g/dl had five times more risk of being transfused than those with Hb >13 g/dl. Also, patients with Hb between 12 and 13 g/dl had three times more risk than those >13 g/dl. ASA III status increased the probability of being transfused almost two times compared with ASA I–II. A nonobese BMI was also a risk factor for transfusion compared with BMI I–III. A combination of those three factors estimated a probability of blood transfusion >70 %.
Acknowledgments
Conflict of interest
TNone.
Footnotes
An investigation performed at La Paz University Hospital, Madrid, Spain.
References
- 1.Barr PJ, Donnelly M, Cardwell C, Alam SS, Morris K, Parker M, Bailie KE. Drivers of transfusion decision making and quality of the evidence in orthopedic surgery: a systematic review of the literature. Transfus Med Rev. 2011;25(4):304–316. doi: 10.1016/j.tmrv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Couvret C, Tricoche S, Baud A, Dabo B, Buchet S, Palud M, Fusciardi J. The reduction of preoperative autologous blood donation for primary total hip or knee arthroplasty: the effect on subsequent transfusion rates. Anesth Analg. 2002;94(4):815–823. doi: 10.1097/00000539-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Etchason J, Petz L, Keeler E, Calhoun L, Kleinman S, Snider C, Fink A, Brook R. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995;332(11):719–724. doi: 10.1056/NEJM199503163321106. [DOI] [PubMed] [Google Scholar]
- 4.Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013 doi: 10.1016/j.jss.2013.03.099. [DOI] [PubMed] [Google Scholar]
- 5.Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth. 2005;95(1):33–42. doi: 10.1093/bja/aeh290. [DOI] [PubMed] [Google Scholar]
- 6.Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 Suppl A):8–10. doi: 10.1302/0301-620X.94B11.30618. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6(1):184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153–1159. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742–1752. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 10.Iwai T, Tsuji S, Tomita T, Sugamoto K, Hideki Y, Hamada M. Repeat-dose intravenous tranexamic acid further decreases blood loss in total knee arthroplasty. Int Orthop. 2013;37(3):441–445. doi: 10.1007/s00264-013-1787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on blood loss and transfusion rate in primary total knee arthroplasty. J Arthroplasty. 2013 doi: 10.1016/j.arth.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Kim TK, Chang CB, Kang YG, Seo ES, Lee JH, Yun JH, Lee SH. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013 doi: 10.1007/s00167-013-2492-1. [DOI] [PubMed] [Google Scholar]
- 13.Evans S, O’Loughlin E, Bruce J. Retrospective audit of blood transfusion and comparison with haemoglobin concentration in patients undergoing elective primary and revision lower limb arthroplasty. Anaesth Intensive Care. 2011;39(3):480–485. doi: 10.1177/0310057X1103900322. [DOI] [PubMed] [Google Scholar]
- 14.Guerin S, Collins C, Kapoor H, McClean I, Collins D. Blood transfusion requirement prediction in patients undergoing primary total hip and knee arthroplasty. Transfus Med. 2007;17(1):37–43. doi: 10.1111/j.1365-3148.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 15.Mesa-Ramos F, Mesa-Ramos M, Maquieira-Canosa C, Carpintero P. Predictors for blood transfusion following total knee arthroplasty: a prospective randomised study. Acta Orthop Belg. 2008;74(1):83–89. [PubMed] [Google Scholar]
- 16.Conteduca F, Massai F, Iorio R, Zanzotto E, Luzon D, Ferretti A. Blood loss in computer-assisted mobile bearing total knee arthroplasty. A comparison of computer-assisted surgery with a conventional technique. Int Orthop. 2009;33(6):1609–1613. doi: 10.1007/s00264-008-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega-Andreu M, Perez-Chrzanowska H, Figueredo R, Gomez-Barrena E. Blood loss control with two doses of tranexamic Acid in a multimodal protocol for total knee arthroplasty. Open Orthop J. 2011;5:44–48. doi: 10.2174/1874325001105010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, Kubo S, Matsumoto T, Matsushita T, Chin T, Iguchi T, Kurosaka M, Kuroda R. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639–1645. doi: 10.1007/s00264-010-1205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal AK, Shashikanth VS, Marwaha N. Platelet-rich plasma prevents blood loss and pain and enhances early functional outcome after total knee arthroplasty: a prospective randomised controlled study. Int Orthop. 2013 doi: 10.1007/s00264-013-2136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvizi J, Chaudhry S, Rasouli MR, Pulido L, Joshi A, Herman JH, Rothman RH. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25–31. doi: 10.1055/s-0031-1275404. [DOI] [PubMed] [Google Scholar]
- 21.Noticewala MS, Nyce JD, Wang W, Geller JA, Macaulay W. Predicting need for allogeneic transfusion after total knee arthroplasty. J Arthroplasty. 2012;27(6):961–967. doi: 10.1016/j.arth.2011.10.008. [DOI] [PubMed] [Google Scholar]