Abstract
Purpose
During total knee arthroplasty (TKA) blood loss can be significant and in spite of all techniques for reducing blood loss there is still a significant possibility for blood transfusions. For blood loss management during TKA, pre-operative autologous blood donation (PABD) is still a standard of care. In this prospective randomised study we have evaluated the efficacy of PABD in patients undergoing TKA to answer the question whether there is any need for autologous blood donations during TKA and, if yes, for which group of patients.
Methods
Patients were randomised to three groups. In group 1 patients did not donate autologous blood, in group 2 patients donated 1 dose 72 hours prior to TKA and in group 3 patients donated autologous blood 14 days prior to TKA. In all patients haemoglobin, haematocrit, thrombocyte and reticulocyte values, iron concentrations (Fe, unsaturated iron binding capacity, total iron binding capacity), activated partial thromboplastin time, prothrombin time, and intra-operative and post-operative blood loss were measured and compared.
Results
With PABD there was no reduction in allogeneic blood transfusions and a large number of taken doses of autologous blood was discarded, which significantly increased the cost of treatment for these patients. For patients undergoing TKA, PABD can provoke iatrogenic anaemia and thereby increase the likelihood of the need for allogeneic blood transfusion.
Conclusions
Results of our study showed that PABD in non-anaemic patients is not justified and is not economically feasible.
Keywords: Total knee arthroplasty, Blood loss, Transfusion, Blood donation, Cost of treatment
Introduction
During total knee arthroplasty (TKA) blood loss can be significant and in spite of all the techniques for reducing blood loss there is still a significant probability for blood transfusions [1–3]. For blood loss management during TKA, pre-operative autologous blood donation (PABD) is still a standard of care. Although improvement of screening techniques has almost eliminated the risk of blood-transmitted diseases, there is still a risk during transfusions of allergic reactions, post-operative infections and transfusion reactions when allogeneic blood transfusions are used [4, 5]. Complications such as bacterial contamination, febrile non-haemolytic transfusion reaction or iatrogenic anaemia can be seen when autologous blood is used. This could also increase the need for allogeneic transfusions [1, 4, 6]. Usually TKA is performed under tourniquet and intra-operative blood losses are low. In such situations, post-operative autotransfusion systems are beneficial and can return autologous blood for the first six hours after the operation [7–9]. The majority of studies, mostly retrospective, have shown that a significant number of pre-operatively collected blood units is thrown away [10], and the use of PABD does not significantly reduce the use of post-operative allogeneic blood transfusions, which in turn increases the cost of treatment [1, 6, 10–14]. In regards to this, the question is whether there is any real need for autologous blood donations during TKA and, if yes, for which group of patients [15–17].
Materials and methods
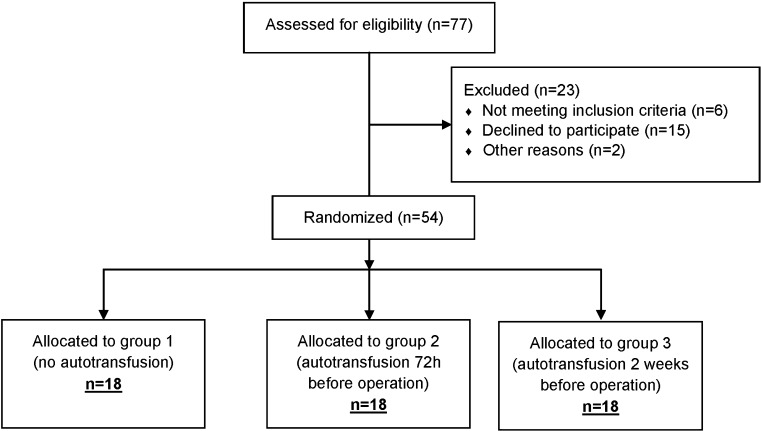
This was a prospective, randomised open type study since blinding due to type of surgery was not possible. It was performed between January 2012 and January 2013 at our department. Two senior surgeons performed TKA in the standard way, with tourniquet application before incision, standard medial parapatellar approach, measured resection technique and cemented Lima Multigen Plus Total Knee System (Lima-Lto, Udine, Italy). All consecutive patients were included who met the following criteria: primary knee arthritis, haemoglobin (Hb) level > 120 g/l, age between 40 and 80 years and American Society of Anesthesiologists (ASA) I, II and III. Exclusion criteria were: secondary knee arthritis, infection of any kind, seizures and uncontrolled hypertension, myocardial infarction or gastrointestinal bleeding within six months prior to surgery, bleeding disorders, malignancy and cytostatic or immunosuppressive therapy. Prior to surgery, all patients willing to participate signed informed consent which was approved together with the research by the local Ethics Committee. Sample size was calculated with NCSS/PASS [Hintze J (2011). PASS 11. NCSS, LLC. Kaysville, UT, USA] software package using data on post-operative Hb concentration after TKA from Cip et al. [18]. The null hypothesis was defined as equivalence in Hb concentration on post-operative day two between subjects without pre-operative blood donation and subjects who gave blood 72 hours prior to operation. Equivalence was defined as a difference in Hb levels of ± 10 g/l around the hypothesised mean value of 100 g/l, with standard deviation of Hb levels of 10 g/l [18]. With statistical power (β) set to 0.80 and type I error (α) to 0.05, the final sample size was 18 subjects per group. Patients were divided into three groups, group 1: no blood donation, group 2: autologous blood donation 72 hours before surgery and group 3: autologous blood donation 14 days before surgery. The number of included patients is shown in Fig. 1. Randomisation was performed by computer in accordance with previously published guidelines [19]. All patients were operated under spinal anaesthesia with 0.5 % Chirocaine (levobupivacaine, Abbott, AbbVie Limited, Maidenhead, UK) with the same thromboprophylaxis protocol (fondaparinux sodium 10 mg sc, GlaxoSmithKline) with the first dose ten hours after surgery followed by once daily 10 mg sc dose for the following eight days. After the fifth day, conversion to peroral Martefarin was started according to prothrombin time (PT) and international normalised ratio (INR) times. Martefarin was continued for five weeks. Before wound closure in all patients, one intra-articular drain was inserted (Ch 14) and was connected to REDAX (Drentech vacuum unit, Mirandola, Italy). In all patients Hb, haematocrit, thrombocyte and reticulocyte values were measured (pre-operatively in the outpatient clinic, on the day of surgery, three hours after surgery, on post-operative day one, post-operative day two, post-operative day five and on the day before discharge). In all patients, iron concentrations (Fe, unsaturated iron binding capacity, total iron binding capacity), activated partial thromboplastin time (APTT), PT (pre-operatively in the outpatient clinic, on post-operative day one, post-operative day two, post-operative day five and on the day before discharge) were measured. In all patients, intra-operative and post-operative blood loss was measured and blood transfusions were recorded (autologous and allogeneic). Within the first six hours, all autologous blood collected with REDAX was returned to patients. Additionally, if needed, autologous or allogeneic blood transfusion was indicated by an independent anaesthesiologist according to the patient’s general condition and the following criteria: acute blood loss of more than 25 % of total blood volume with or without haemorrhagic shock, tachycardia and hypoxia that do not respond to rehydration, Hb < 70 g/l in healthy subjects and Hb < 90 g/l in patients with concomitant cardiac or cerebrovascular disorders. Application of autologous blood had the advantage over the use of allogeneic blood. Within the first 24 hours after surgery patients recorded pain scores using a visual analogue scale (VAS) with a range from 0 (completely pain free) to 100 (worst possible pain). After the first 24 hours, VAS was recorded until discharge from hospital at different time intervals. Patients from group 2 (72 hours) and group 3 (14 days) immediately after autologous blood donations began taking oral iron (Ferrum Lek, dextriferron 100 mg 3×1 tablet) until the day before the surgery. Analysis of the data was performed using the software package STATISTICA [version 10, StatSoft (data analysis software system), www.statsoft.com]. The level of significance was set at 0.05 for all tests. After analysing distribution normality, the primary analysis was performed using Student’s t test, while the secondary analysis was conducted with one-way analysis of variance (ANOVA). Values of categorical variables are shown using contingency tables, while other variables are shown as mean values and standard deviation.
Fig. 1.
Study flow chart
Results
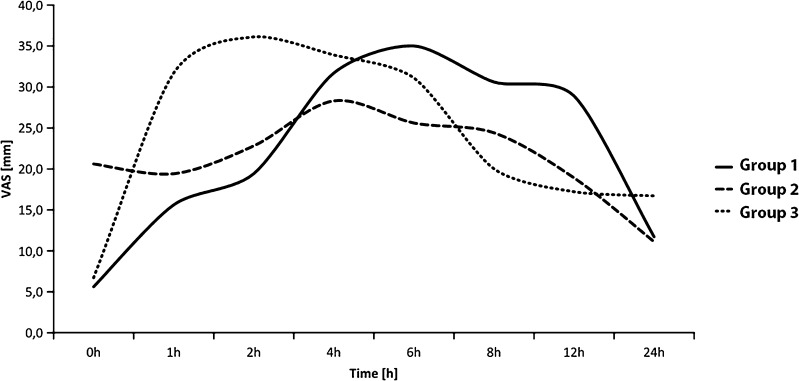
Differences between the groups according to the basic demographic parameters and duration of hospitalisation were not statistically significant (Table 1). In addition to the parameters listed in Table 1, groups were comparable regarding total blood loss (intra-operative plus post-operative), which was 1,378.9 ml (462.49) in group 1 (no), 1,548.3 ml (550.50) in group 2 (72 h) and 1,554.4 ml (498.65) in group 3 (14 days) (p = 0.501, ANOVA). Allogeneic blood transfusions were needed by six patients in group 1. In group 2, eight patients received an autologous blood transfusion and three patients both autologous and allogeneic blood transfusions. In group 3, one patient received only an allogeneic blood transfusion, eight patients only an autologous blood transfusion and two patients both autologous and allogeneic blood transfusions, so the groups were comparable according to this parameter (p = 0.211). Tables 2 and 3 show the most important parameters observed in this study (mean, standard deviation and results of statistical tests of significance). Statistically significant differences among the three groups were observed for pre-operative Hb, reticulocyte count and iron levels in the blood. Results of post hoc testing of these differences (not shown) indicate that the differences are due to significantly lower levels of Hb in group 1 compared with the two remaining groups and a significantly larger number of reticulocytes in group 3 compared with the remaining two groups. For iron levels, the statistical significance of differences between groups pre-operatively and on the second day after the operation are due to the differences between groups 1 and 3. Pain levels for the first 24 hours are shown in Fig. 2. Average pain level gradually increased from the immediate post-operative period (zero hours) and was at the highest peak after two hours in group 3, four hours in group 2 and six hours in group 1, after which the pain gradually decreased toward the end of the first post-operative day. The biggest difference, and the only one that showed statistical significance between the groups, was observed one or two hours after surgery. Figure 2 shows significantly higher levels of pain in group 3 when compared with the other two groups (p = 0.051 for one hour and p = 0.021 for two hours).
Table 1.
Demographic data
| Group 1 (no) | Group 2 (72 h) | Group 3 (14 days) | Difference groups 1 and 2 | Difference between all groups | |
|---|---|---|---|---|---|
| Gender, % women | 77.8 | 50.0 | 50.0 | 0.083a | 0.147a |
| Age, years (SD) | 64.9 (9.62) | 67.2 (7.20) | 66.3 (6.18) | 0.438b | 0.690c |
| BMI, kg/m2 (SD) | 29.9 (4.11) | 31.7 (5.39) | 28.8 (3.46) | 0.278b | 0.150c |
| Hospital stay, days (SD) | 9.9 (1.49) | 10.9 (2.00) | 10.0 (2.66) | 0.136b | 0.346c |
BMI body mass index
aChi-square
bStudent’s t test
cANOVA
Table 2.
Values of Hb, platelets and reticulocytes (SD) during the study
| Hb (g/l) | Platelets (× 109/l) | Reticulocytes (× 109/l) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Gr. 1 | Gr. 2 | Gr. 3 | p | Gr. 1 | Gr. 2 | Gr. 3 | p | Gr. 1 | Gr. 2 | Gr. 3 | p |
| Preoperative | 137.67 (8.16) | 148.94 (9.94) | 145.39 (9.68) | 0.002 | 246.17 (53.11) | 228.56 (42.72) | 231.11 (58.9) | 0.551 | 11.89 (2.98) | 12.53 (3.06) | 17.16 (9.23) | 0.026 |
| 3 h after operation | 109.11 (12.52) | 111 (10.31) | 110.72 (10.65) | 0.861 | 188.39 (60.12) | 190.56 (45.67) | 211.61 (57.45) | 0.379 | 12.73 (5.4) | 12.84 (2.99) | 13.56 (2.98) | 0.796 |
| 30 min after transfusion | 111.89 (14.1) | 113.94 (11.38) | 115.12 (10.74) | 0.731 | 194.28 (56.77) | 174.12 (43.64) | 186.76 (59.7) | 0.541 | 12.98 (5.6) | 12.75 (3.02) | 13.42 (3.32) | 0.891 |
| +1 post-op day | 107.78 (11.42) | 109 (8.96) | 107.33 (7.58) | 0.862 | 185.06 (50.83) | 173.78 (43.29) | 200 (52.63) | 0.283 | 16.81 (6.71) | 15.95 (3.58) | 18.57 (10.95) | 0.616 |
| +2 post-op days | 91.72 (14.24) | 96.94 (7.57) | 95.89 (12.73) | 0.383 | 162.17 (46.79) | 160.5 (38.32) | 167.06 (42.6) | 0.891 | 16.86 (6.02) | 18.44 (6.32) | 17.26 (7.59) | 0.767 |
| +5 post-op days | 93 (11.25) | 92.89 (9.09) | 92.94 (10.9) | 0.999 | 212.67 (72.57) | 215.67 (54.43) | 202.72 (46.68) | 0.788 | – | – | – | – |
| Day before discharge | 96.5 (9.87) | 97.35 (8.09) | 98.47 (11.06) | 0.836 | 307.44 (106.82) | 352.76 (146.53) | 343.88 (83.31) | 0.469 | 31.58 (9.37) | 33.05 (13.37) | 33.3 (12.24) | 0.895 |
Table 3.
Values of PT, APTT and iron during study
| PT (s) | APTT (s) | Iron (μmol/l) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Gr. 1 | Gr. 2 | Gr. 3 | p | Gr. 1 | Gr. 2 | Gr. 3 | p | Gr. 1 | Gr. 2 | Gr. 3 | p |
| Preoperative | 0.98 (0.13) | 1.05 (0.13) | 1.04 (0.13) | 0.191 | 26.28 (3.02) | 26.97 (2.26) | 27.63 (2.86) | 0.341 | 14.05 (4.48) | 19.18 (5.85) | 15.79 (5.52) | 0.022 |
| +1 post-op day | 0.93 (0.07) | 0.91 (0.1) | 0.94 (0.1) | 0.683 | 28.32 (2.23) | 28.07 (3.69) | 28.81 (2.72) | 0.750 | – | – | – | |
| +2 post-op days | 0.89 (0.12) | 0.88 (0.14) | 0.94 (0.12) | 0.327 | 27.95 (3.31) | 28.52 (3.34) | 29 (3.42) | 0.645 | 5.47 (2.37) | 4 (1.66) | 4.13 (1.31) | 0.045 |
| +5 post-op days | 0.96 (0.2) | 1.12 (0.16) | 1 (0.25) | 0.090 | 27.71 (2.98) | 27.89 (2.49) | 27.99 (3.58) | 0.960 | – | – | – | |
| Day before discharge | 0.82 (0.21) | 0.78 (0.28) | 0.88 (0.2) | 0.426 | 27.32 (3.77) | 27.31 (3.68) | 29.18 (4.94) | 0.323 | 5.94 (2.21) | 6.71 (5.21) | 6.59 (2.87) | 0.800 |
Fig. 2.
Pain levels for the first 24 h
Discussion
In this prospective randomised study, we have evaluated the efficacy of PABD in patients undergoing TKA according to the question of whether there is any need for autologous blood donations during TKA, and, if yes, for which group of patients. Despite improved surgical techniques, expected blood loss in TKA can be significant especially immediately after the surgery [1].
In our study there was no difference between the groups regarding the demographic data. The basic parameter for inclusion in the study was Hb > 120 g/l and all patients in groups 2 and 3 donated one dose of autologous blood. There was no difference between groups regarding total intra-operative and post-operative blood loss. Also, there was no significant difference between groups regarding the amounts of post-operatively collected and returned blood. According to Woolson and Wall [7] post-operative autotransfusion is equally effective as PABD in the prevention of risks associated with the use of allogeneic transfusion after TKA, while Cip et al. [18] in their prospective, randomised study showed that post-operative autotransfusion systems do not reduce the percentage of post-operative allogeneic blood transfusions after TKA. Statistically significant differences among the three groups were observed for preoperative Hb (p = 0.002), reticulocyte count (p = 0.026) and the level of iron in the blood (p = 0.022). The highest values of Hb were found in group 3 (148.94 g/l) and the lowest in group 1 (137.67 g/l). A similar distribution was found with blood iron levels on the second post-operative day (p = 0.022), with the highest values in group 2 (mean 19.18 μmol/l) and the lowest in group 1 (mean 14.05 μmol/l). This is probably related to the fact that both groups of patients that were included in PABD, immediately after blood collection, began with oral iron replacement, while the group that did not give blood did not receive pre-operative iron. According to Weisbach et al. [20] for patients without iron deficiency before PABD, if intravenous or oral iron therapy is started it increases the success rate of PABD but does not reduce the need for allogeneic blood transfusions. According to Biesma et al. [21] in PABD donors with normal levels of iron, iron compensation has no effect on erythropoiesis and is insufficient to maintain iron stores. Statistically significant differences between the groups were found for reticulocytes (p = 0.026), with the most significant increase in group 3 (17.16 × 109/l) and the lowest increase in group 1 (11.89 × 109/l). In both groups involved in PABD (groups 2 and 3) there was no statistically significant difference in the number of autologous blood transfusions (eight patients autologous and three patients allogeneic in group 2 and eight patients autologous and two patients allogeneic in group 3). Twelve patients in group 1 did not receive any blood transfusions. The cut-off value for the application of either autologous or allogeneic blood transfusion was Hb 80 g/l. Lower than 80 g/l values were seen on the second post-operative day in two patients in group 1, no patient in group 2 and three patients in group 3, which means that there were no statistically significant differences between the two groups. The majority of patients in this period had Hb levels between 80 and 90 g/l [14 patients (77.7 %) in group 1, 11 patients (61.1 %) in group 2 and ten patients (55.5 %) in group 3], which can be correlated with the fact that the majority of patients received a blood transfusion on the second post-operative day (according to the independent clinical evaluation of the anaesthesiologist). According to Couvret et al. [22], PABD which is based on a standard prediction of blood loss is prone to excessive and unnecessary pre-operative donations resulting in iatrogenic pre-operative anaemia and wasting large amounts of pre-operative autologous blood taken, which is often the case in late donated autologous blood. According to Martinez et al. [14], PABD for patients with high pre-operative Hb levels is unnecessary because it increases the likelihood of post-operative anaemia and perioperative need for transfusion. PABD is equally bad for anaemic patients because haematopoiesis is usually insufficient to regenerate red blood cells in sufficient amounts, which increases the likelihood for transfusion. In both PABD groups (groups 2 and 3) a large amount of autologous blood was discarded (seven patients in each group, 38.8 %), which unnecessarily increases the cost of treatment. Many, mostly retrospective studies showed that a large number of preoperatively taken autologous blood doses is discarded. According to Billote et al. 6 % of patients donated blood preoperatively and 45 % of the taken doses were discarded, and more than 10 % of patients received allogeneic blood transfusion despite PABD. According to Bierbaum et al. [1], in spite of intense autologous blood collection, there are still 6–9 % of such patients in whom allogeneic blood transfusions are needed. At the same time more than 45 % of pre-operatively donated blood doses are discarded. According to Forgie et al. [10], patients involved in PABD have lower preoperative haematocrit values in relation to those who have not donated blood pre-operatively, the number of used allogeneic blood doses decreased in the PABD group but the total number of transfusions increased and a large percentage of autologous doses was discarded. According to Keating et al. [23], in patients with preoperative values of Hb greater than 130 g/l, using universal protocols for autologous blood donation results in discarding 66 % of taken doses.
Conclusion
The results showed that PABD in non-anaemic patients is not justified and is not economically feasible. With PABD there was no reduction in allogeneic blood transfusions and a large number of taken doses of autologous blood was discarded, which significantly increased the cost of treatment for these patients. We believe that in non-anaemic patients other factors can influence significant reduction of blood loss: good surgical techniques, regional anaesthesia, post-operative autotransfusion, oral iron therapy and maybe tranexamic acid [24] or platelet-rich plasma [25]. For patients undergoing TKA, PABD can provoke iatrogenic anaemia and thereby increase the likelihood of the need for allogeneic blood transfusion.
References
- 1.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Vielgut I, Kastner N, Pichler K, Holzer L, Glehr M, Gruber G, Leithner A, Labek G, Sadoghi P. Application and surgical technique of total knee arthroplasties: a systematic comparative analysis using worldwide registers. Int Orthop. 2013;37(8):1465–1469. doi: 10.1007/s00264-013-1933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735–739. doi: 10.1007/s00264-013-1801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bong MR, Patel V, Chang E, Issack PS, Hebert R, Di Cesare PE. Risks associated with blood transfusion after total knee arthroplasty. J Arthroplasty. 2004;19(3):281–287. doi: 10.1016/j.arth.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Madjdpour C, Heindl V, Spahn DR. Risks, benefits, alternatives and indications of allogenic blood transfusions. Minerva Anestesiol. 2006;72(5):283–298. [PubMed] [Google Scholar]
- 6.Cushner FD, Hawes T, Kessler D, Hill K, Scuderi GR. Orthopaedic-induced anemia: the fallacy of autologous donation programs. Clin Orthop Relat Res. 2005;431:145–149. doi: 10.1097/01.blo.0000150458.84389.f3. [DOI] [PubMed] [Google Scholar]
- 7.Woolson ST, Wall WW. Autologous blood transfusion after total knee arthroplasty: a randomized, prospective study comparing predonated and postoperative salvage blood. J Arthroplasty. 2003;18(3):243–249. doi: 10.1054/arth.2003.50058. [DOI] [PubMed] [Google Scholar]
- 8.Friederichs MG, Mariani EM, Bourne MH. Perioperative blood salvage as an alternative to predonating blood for primary total knee and hip arthroplasty. J Arthroplasty. 2002;17(3):298–303. doi: 10.1054/arth.2002.30409. [DOI] [PubMed] [Google Scholar]
- 9.Li T, Zhuang Q, Weng X, Zhou L, Bian Y. Non-continuous versus continuous wound drainage after total knee arthroplasty: a meta-analysis. Int Orthop. 2013 doi: 10.1007/s00264-013-2105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forgie MA, Wells PS, Laupacis A, Fergusson D. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a meta-analysis. International Study of Perioperative Transfusion (ISPOT) Investigators. Arch Intern Med. 1998;158(6):610–616. doi: 10.1001/archinte.158.6.610. [DOI] [PubMed] [Google Scholar]
- 11.Hatzidakis AM, Mendlick RM, McKillip T, Reddy RL, Garvin KL. Preoperative autologous donation for total joint arthroplasty. An analysis of risk factors for allogenic transfusion. J Bone Joint Surg Am. 2000;82(1):89–100. doi: 10.2106/00004623-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Billote DB, Glisson SN, Green D, Wixson RL. Efficacy of preoperative autologous blood donation: analysis of blood loss and transfusion practice in total hip replacement. J Clin Anesth. 2000;12(7):537–542. doi: 10.1016/S0952-8180(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 13.Etchason J, Petz L, Keeler E, Calhoun L, Kleinman S, Snider C, Fink A, Brook R. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995;332(11):719–724. doi: 10.1056/NEJM199503163321106. [DOI] [PubMed] [Google Scholar]
- 14.Martinez V, Monsaingeon-Lion A, Cherif K, Judet T, Chauvin M, Fletcher D. Transfusion strategy for primary knee and hip arthroplasty: impact of an algorithm to lower transfusion rates and hospital costs. Br J Anaesth. 2007;99(6):794–800. doi: 10.1093/bja/aem266. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Altneu E, Monsef JB, King EA, Sculco TP, Boettner F. Nonanemic patients do not benefit from autologous blood donation before total knee replacement. HSS J. 2011;7(2):141–144. doi: 10.1007/s11420-011-9200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller U, Röder C, Pisan M, Orler R, El-Kerdi A, Eggli S. Autologous blood donation in total knee arthroplasties is not necessary. Acta Orthop Scand. 2004;75(1):66–70. doi: 10.1080/00016470410001708130. [DOI] [PubMed] [Google Scholar]
- 17.Buljan M, Nemet D, Golubic-Cepulic B, Bicanic G, Tripkovic B, Delimar D. Two different dosing regimens of human recombinant erythropoietin beta during preoperative autologous blood donation in patients having hip arthroplasty. Int Orthop. 2012;36(4):703–709. doi: 10.1007/s00264-011-1367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cip J, Widemschek M, Benesch T, Waibel R, Martin A. Does single use of an autologous transfusion system in TKA reduce the need for allogenic blood?: a prospective randomized trial. Clin Orthop Relat Res. 2013;471(4):1319–1325. doi: 10.1007/s11999-012-2729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisbach V, Skoda P, Rippel R, Lauer G, Glaser A, Zingsem J, Zimmermann R, Eckstein R. Oral or intravenous iron as an adjuvant to autologous blood donation in elective surgery: a randomized, controlled study. Transfusion. 1999;39(5):465–472. doi: 10.1046/j.1537-2995.1999.39050465.x. [DOI] [PubMed] [Google Scholar]
- 21.Biesma DH, Kraaijenhagen RJ, Poortman J, Marx JJ, van de Wiel A. The effect of oral iron supplementation on erythropoiesis in autologous blood donors. Transfusion. 1992;32(2):162–165. doi: 10.1046/j.1537-2995.1992.32292180147.x. [DOI] [PubMed] [Google Scholar]
- 22.Couvret C, Tricoche S, Baud A, Dabo B, Buchet S, Palud M, Fusciardi J. The reduction of preoperative autologous blood donation for primary total hip or knee arthroplasty: the effect on subsequent transfusion rates. Anesth Analg. 2002;94(4):815–823. doi: 10.1097/00000539-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Keating EM, Meding JB, Faris PM, Ritter MA. Predictors of transfusion risk in elective knee surgery. Clin Orthop Relat Res. 1998;357:50–59. doi: 10.1097/00003086-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Iwai T, Tsuji S, Tomita T, Sugamoto K, Hideki Y, Hamada M. Repeat-dose intravenous tranexamic acid further decreases blood loss in total knee arthroplasty. Int Orthop. 2013;37(3):441–445. doi: 10.1007/s00264-013-1787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal AK, Shashikanth VS, Marwaha N. Platelet-rich plasma prevents blood loss and pain and enhances early functional outcome after total knee arthroplasty: a prospective randomised controlled study. Int Orthop. 2013 doi: 10.1007/s00264-013-2136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]