Abstract
Purpose
Bone stock reconstruction in TKR surgery is one of the biggest challenges for the surgeon. According to some, authors causes of bone stock loosening are multiple, including stress shielding, osteolysis from wear, septic or aseptic loosening, and bone loss caused by a poorly balanced implant. Moreover, bone loss may be iatrogenic at the time of implant removal, indicating that bone preservation during implant removal is critical.
Methods
Defect localization and extension affect the surgeon’s decisions about the choice of the surgical technique and the type of plant to be taken. Today there are several options available for bone deficiency treatment. The treatment choice is undoubtedly linked to the cause of revision, experience and personal philosophy, but it is necessary to consider also the patient's age, expectations of life, functional requirements and bone quality. Many authors prefer bone stock reconstruction techniques in patients with high bone quality and a better quality of life with more prospects. In patients with lower lease on life and lower bone quality the best bone replacement techniques are of modular systems, wedges, and augments. In cases with septic bone loss, more or less extended, different authors recommend reducing bone grafts in favor of modular prostheses to reduce the risk of graft contamination.
Results
All of these techniques have been shown to be durable in midterm outcomes, but concerns exist for a number of reasons, including disease transmission, resorption, fracture, immune reaction to allograft, the cost of custom prostheses, the inability to modify the construct intraoperatively and the overall technical challenge of applying these techniques.
Conclusions
The choice between different surgical options depends on bone defect dimension and characteristics but are also patient-related. Reestablishment of well-aligned and stable implants is necessary for successful reconstruction, but this can’t be accomplished without a sufficient restoration of an eventual bone loss.
Keywords: Bone loss, Total knee revision, Knee, Impaction grafting, Metaphyseal sleeves, Cones
Bone defects classification
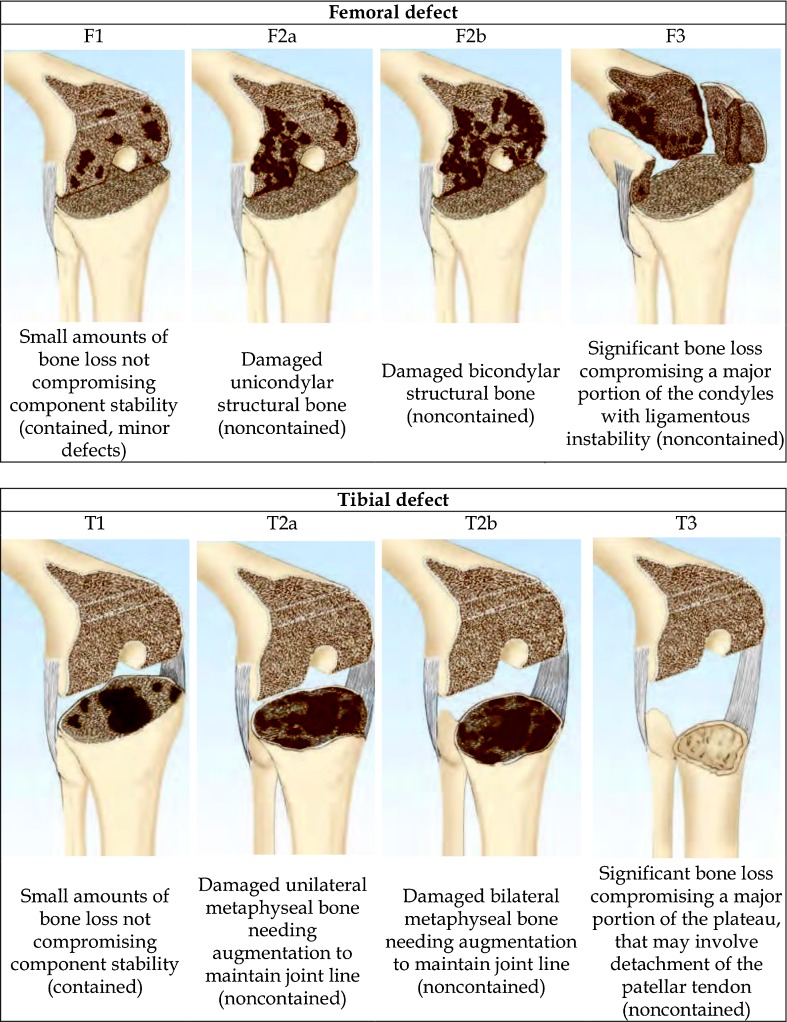
Bone defects are classified as minimal and severe. Their size and extension can only be assessed after the components have been removed, an act that may lead to a worsening compared to the pre-operative position. In the literature several classifications [1, 2] are reported for quantifying and defining the type of bone defect. Currently the most widely used is the classification of the Anderson Orthopaedic Research Institute (AORI) [3] that represents the evolution of the Engh classification [4]. In AORI classification development the authors wanted to create a classification that follows the criteria of simplicity and reproducibility and orientates the surgeon to the solutions to be taken in each type of defect. In this classification (Fig. 1) the femur (F) and the tibia (T) are considered separately, but with the same distinct deficit: type 1 (F/T), in which the bone is intact metaphyseally and there are minor defects that do not affect the stability of the implant, where it is preferable to use implants for the primary; and type 2 (F/T) where the metaphyseal region is damaged with loss of cancellous bone that can affect a condyle or tibial halfplate (F/T2A) or both (F/T2B); F/T2A and F/T2 require a reconstruction with the use of cement, bone graft or a substitution with augmentation to restore the joint line and the stability of the knee. Instead in type 3 defects (F/T) there is a resorption of the metaphyseal portion with both condyles or tibial halfplates impaired. In this severe defect allografts may be associated with detachment of the collateral ligaments or patellar tendon and therefore bound structural or modular implants are necessary.
Fig. 1.
Femoral and tibial defects classification
Pre-operative planning
The pre-operative planning is an essential time to plan a knee revision operation and to guide the choice for the most suitable technique and implant to use. The examinations include: lower limb standard radiographs in the anterior-posterior projection, knee X-ray in AP and LL, axial patella at 45° [5]. To assess the individual morphology of the healthy knee all the examinations have to be done under load and in comparison with the contralateral knee. The standard radiographic examinations often underestimate the bone loss [6]. CT examinations, with three-dimensional reconstructions, are necessary to perform, recognize and define the real bone loss.
In one study [7] performed on 31 patients with painful implants, CT examination showed all areas of osteolysis, in which only 17 % of cases had been identified in the standard X-ray examinations.
Examinations with implants sometimes aren’t enough to evaluate the real bone loss. In fact the implant presence underestimates the deficit. Only with careful intra-operative evaluation after implant and cement removal is it possible to define the real entities of the bone defect. Engh also argued: “the surgeon should anticipate the worst scenario, because often the defects turn out to be more severe than predicted from radiographs” [8].
In the defects, reconstruction is crucial to restore joint line [9], recreate symmetric spaces in flexion and extension resulting in an optimal ligament balance and proper joint kinematics [10]. For correct restoration of the joint line one must be aware of the anatomical references represented by the JL distance from medial epicondyle (about 20 mm) and lateral epicondyle (about 25 mm), tibial references (1 cm above the head of the fibula) and patellar reference (1 cm below the apex) [11].
In cases of revision for septic loosening, where the prosthetic components have been removed previously, the size of the bone defects extension is more accurately assessed by the information of the pre-operative implant examinations. However, the cement spacer removal, a meticulous and careful removal of the fibrous tissue adhering to the bone surface with the residue necrotic bone removal, can still reveal more extensive bone loss. Only after a complete bone exposure can one undertake its reconstruction on the basis of the deficit found.
Surgical options
AORI type I
In type 1 defects, femoral and tibial defects are characterized by lower metaphyseal bone intact with any instability of the prosthetic components.
Ritter and Dorr believe that in bone defects less than 5 mm in breadth and depth [12], the best surgical choice is a primary prosthetic implant with cement to fill the bone loss [13, 14]. This allows one to get a stability comparable to impaction bone grafting and the structural allografting when used in tibial bone defects. Use of cement is indicated only in content bone defects.
Fosco suggests using cement only for peripheral bone defects with an extension less than 50 % of the bone surface and less than 5 mm in depth [15].
In the case of mild contained or uncontained lesions more than 5 mm but less than 10 mm the use of cement with screws is indicated. Cement may be reinforced with embedded screws but their heads are slightly below the implant [16, 17]. The purpose of the screws is to distribute the load away from the joint line and cement bone interface.
The screws, of 5 or 6.5 mm in diameter, are placed 5–10 mm apart. According to some authors [14] the use of bone graft is recommended when the cement mantle below the tibial plateau is greater than 5 mm thick.
AORI type II
In type 2 defects, the metaphyseal bone is damaged, therefore, it must be reconstructed in order to ensure the stability of the prosthetic components. The defect may be limited to a femoral condyle/tibial half plate or both condyles/tibial half plate.
Bone grafts, already described, are also used in type 2 defects in patients with good prospects of life and good bone quality. Type 2 defect treatment involves the use of revision implant and the following surgical techniques.
Bone grafts
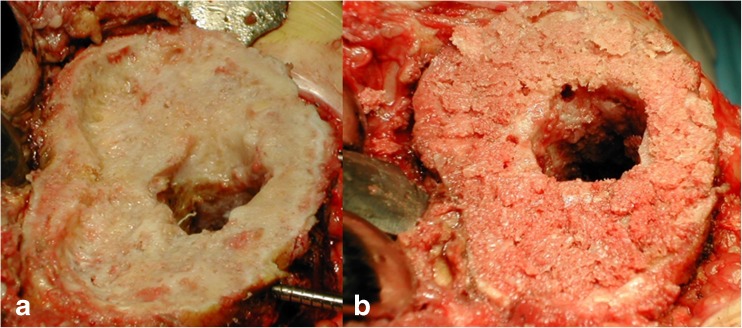
Impacted morsellized bone grafts (Fig. 2) enable restoration of living bone stock [18], especially in young patients in whom further revision operations are anticipated. Their use is also indicated when there is a contained bone loss larger than 10 mm or a mild uncontained defect less than 50 % of tibial plateau/condyles.
Fig. 2.
AORI TYPE II bone defect (a) treated with impacted morsellized bone grafts (b)
Impaction bone grafting is not recommended for repairing cortical or uncontained defects as the cortical rim is important to ensure stability of the tibial tray [19].
Host bone preparation should be performed as if it were for a nonunion [20].
Some authors consider the grafts size should be 0.5–1 cm because it can integrate with the surrounding bone [20, 21]. In Whiteside’s opinion [20] chips less than 0.5 mm are reabsorbed by the inflammatory process while chips bigger than 10 mm have a slow and inappropriate integration [22]. Adequate impaction force makes morsellized bone grafts strong enough to carry the load, i.e. excessive impaction reduces host bone ingrowths [23]. The biological interaction between the graft and the host bone plays an important role in medium to long-term results [24]. Cancellous bone allografts enable bone remodelling through revascularization [25]. The incorporation of allografts involves two overlapping processes: the union of the graft-host bone interface and remodelling of the graft which is relatively slow [26, 27].
Mineralized cancellous grafts have greater osteoconductive but weaker capacity than demineralized cortical grafts [28]. Long-term stability and outcome are affected by the quality of bone graft, post harvesting, and vascularity of the host cavity. Frozen allografts provide solid grafts that are available and easy to work with but are associated with numerous risks: transmission of viral diseases, risk of infection which is reduced by 25 kGy radiation treatment that barely affects the solidity of the frozen allograft [29], and immunological reaction, for which the risk is reduced by ablation of the bone marrow by lavage.
Freeze-dried bone maintains its original mechanical properties, but is more fragile because of radiation and is more difficult to work with. The cement penetrates the cancellous bone, which helps fixation of the prosthesis after union. Morsellized allografts have no immediate resistance but they can be incorporated and are remodelled in reaction to surrounding loading pressures.
Modular metal augments
Metal augments in various shapes and sizes are available for both femoral and tibial defects and can be cemented or uncemented [30]. This technique is mainly used for AORI type 2 and 3 defects in elderly, low-demand patients [31]. Augments are especially used in uncontained bone defects with moderate and severe bone loss >50 % and > 5 mm of the femoral condyle and/or tibial plateau. The augments alone are advised if 40 % of the surface is not supported by host bone and if the defect exceeds 25 % of the peripheral rim [22]. According to some authors the best results came from augments using a graft. The failure rate for augments used alone stood at 48 % compared to only 19.2 % in the case of graft combination [32].
Augmented prostheses with a built-up metal wedge are mechanically superior to cement alone or cement with screws in terms of resisting movement when loaded [33]. The size of the metal augments usually corresponds to the size of the prostheses, with different thicknesses and angles to replace bone defects of various severities. The best-fit augment should be used to fill up the bone defect, while removing as little host bone as possible. Augments can be assembled to fit the femoral and tibial components with screws. Preference between wedges and blocks should be accorded to the augmentation that most closely fills the defect. On the tibial side, multiple sizes of metal wedges and blocks are available. Femoral defects can be reconstructed with metal blocks in increments of 5 mm; because bone loss in the femur is most often on the posterior and distal surfaces, augments fixed to the distal and posterior femoral condyles are used.
The surgical advantages of metal augments are the possibility to customize the implant intra-operatively (need not be incorporated into host bone and don’t carry a risk of nonunion or collapse). Despite the versatility and a wide geometry of augments, including hemi-wedges, full wedges, and symmetric spacers, these materials can manage only a limited defect, up to 20 mm deep. The success rates in the literature using these augments range from 84 % to 98 % good or excellent results [7, 34]. They allow rapid filling of bone defects that have been geometrically shaped with instruments. They provide stable support and transfer loading forces to the bone.
Posterior femoral augments make possible the use of a prosthesis that is large enough to obtain stability during flexion, and their bony support opposes rotational forces. Lateral posterior augments increase also external rotation of the femoral component and distal augments restore JL level. Augments are screwed or cemented to components. Bone cuts should be made after determining rotation of the component with the trial stem in place, as these variables change the position of the augment. The choice between a block and a wedge depends on the shape of the BL. It is not necessary to fill all the BL. The residual BL will be filled with cement or a bone graft. When the augment is resting on condensed bone, the cement can penetrate the drill holes.
The results of tibial wedges, first used for primary TKA, were satisfactory at mid-term follow-up, although there were frequent radiolucent lines [34]. Non progressive radiolucent lines have been observed between 27 % and 46 % of augmented tibial components [35, 36]. Metal augments confer the risk of fretting and corrosion [34, 37–39]. In the long run, the difference in elasticity between metal and bone may cause stress shielding and increase potential bone loss [40].
The current designs for modern metal augments maintain a high volumetric porosity (70 %–80 %) for bone ingrowth, with low modulus of elasticity and high frictional characteristics, making this metal conducive to biologic fixation [41].
AORI type III
The reconstruction appears much more complex in type III defects which have metaphyseal bone deficit with segmental impairment of both femoral condyles or tibial plateau occasionally associated with ligamentous detachment. The implant choice falls on constrained or resection prosthesis with stems. The surgical options for bone loss treatment are structural allografting, cones or metaphyseal sleeves.
Structural allograft
Structural allografts provide a stable and durable reconstruction of large or segmental bone deficiency. This technique is used in the treatment of segmental content defects not greater than 15 mm for the femur and greater than 20–45 mm for the tibia [42]. In patients with a life expectancy greater than ten years, structural bone grafts to restore bone stock is preferred [43]. Naturally, a prerequisite for its use is the host bone vitality. Femoral heads, distal femoral segments, and proximal tibial segments are most commonly used. Structural allografts may incorporate into the host bone and provide some stress protection to the implant [44]. Nonetheless, in cases of infection, the use of prosthetic augments and antibiotic bone cement is a safer option. The supply of allografts usually cannot satisfy the demand. In some cases, more bone is sacrificed to make the interface smooth, which may enlarge the defect. Sometimes it is necessary to use massive allografts due to the tibial or femoral condyle bone loss. In this case the host bone is modified to obtain a stable interface. The first step is to remove all the nonviable bone and soft tissue from in and around the defect; the presence of viable bone is absolutely necessary to maximize the likelihood of graft incorporation.
In case of BL in both condyles a massive bone allograft is indispensable. The graft should not be carved too much, it will weaken it and favour resorption (perforations and exposed cancellous bone should be cemented). Ideally the allograft should be attached to the host bone by the stem alone but additional internal fixation may be necessary (screws, plate). To avoid using a plate, cortical allograft struts wrapped in cerclage wires may be used. The size of the implant will be chosen according to the size of the host epiphysis (be careful of large implants which can prevent closing). The implant and stem are cemented to the graft (without any cement between the graft and host bone interface). The stem is cemented into the shaft of the host or press-fit. Long stems which extend beyond the isthmus should be used; the interface is grafted and the remaining bone fragments and their attached ligaments are attached to the graft. Weight bearing is partial for six weeks and full after union (three to four months).
It is difficult to compare the results of published series [7, 19, 45, 46] because they differ in type of BL, graft fixation, length and type of stem fixation, constraint of the prosthesis and follow-up. The recent series by Bauman et al. clearly showed the limits of this technique [45]. Allografts also carry the risk of bacterial and viral disease transmission and biological issues such as immune response, graft disincorporation, resorption, load transfer, and fatigue fracture [40].
The goals of structural allograft reconstruction are to maximize the stability of the graft host bone contact and provide a stable platform for the implant fixation. Conversion of the oblique peripheral defects into rectangular space with vertical and horizontal surfaces has been demonstrated to improve stability for components fixation.
The angular patterns also have a biological advantage since it allows improving the contact area of the host-graft construct maximizing the probability of graft incorporation. Graft fixation is also an important step to be taken in consideration, i.e. mainly used are partially- or fully-threaded cancellous screws.
Several authors believe that they have a bulk allograft survival between 72 % and 86 % at eight to ten years of follow up [47]. In Engh’s opinion the survivorship after eight years is 92 % if bulk allograft is used with non vincolate prosthesis [48].
According to other authors Bulk allografts don’t undergo a process of revascularization, resorption or collapse.
Cones
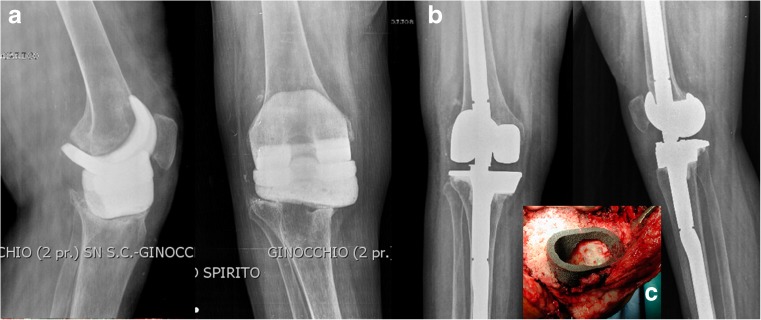
Filling metaphyseal defects with cones (Fig. 3) also extends [49] to elderly patients needing to replace the bone, while providing a base of mechanical support for the prosthesis implant.
Fig. 3.
AORI TYPE III bone defect in a case of total knee revision in treatment with antibiotic spacer (a). Radiographic results after bone loss treatment with cones and stemmed prosthesis (b). A particular type of cone used to fill metaphyseal bone loss (c)
The high variability of sizes and shapes allows a good adaptability of these modules to the metaphyseal bone deficiency, primarily for those types of cavities in which a reliable cortical shell in the face of a metaphyseal endosteal bone defect is present. A trial cone is chosen for suitable shape and size, and press-fit on bone guest. In case of a residual gap between the bone and the outline of the cone, it is useful to fill it with pressurized bone grafts to obtain a complete circumferential filling and contact, and ensure a further stability in the subsequent final cone application. The option of symmetrical or asymmetrical cones, allows us to face even the segmental defects, not only the extended cavity.
The trial cone may be useful to estimate the position of the defect, on both the medial-lateral and antroposterior plane, in relation to the centre of the medullary canal. If the defect is central it is appropriate to adopt a straight stem. However, if the defect is oriented asymmetrically with respect to the canal the use of stems with off-set reduces the risk of implant malposition.
After the choice of the most suitable cone and preparation of the defect is completed, the final cone with tight press-fit is applied, resulting in the reconstruction of the metaphyseal bone defect and constituting a stable scaffold for the remaining components. The tibial component is subsequently cemented definitive metaphyseal on the cone, while the intramedullary rod stabilizes the implant distally.
Lachiewicz et al. [50] found that tantalum cones were fully integrated after two years. Similarly at the Mayo Clinic tantalum cones were found to have osteintegrated in 100 % of tibial [51] and femoral cases [52]. The radiographic success of the tantalum cones is due to the highly porous nature of the material which allows early osteointegration [52]. The low modulus of elasticity of such components enables more load transfer and preservation of bone stock [53]. Tantalum does not react with or irritate bodily fluids. Trabecular metal cones help reconstruct large cavitary defects; these implants, along with offset stems when necessary, may eliminate the need for extensive bone grafting or allografting [34]. The distal femoral cones re-establish the metaphyseal–diaphyseal junction and create a stable base for the femoral component; these modular constructs absorb compressive loads, and provide structural and mechanical support. Tantalum has superior osteoconductive properties, but its high cost is a concern [54].
Metaphyseal sleeves
Femoral sleeves are indicated in elderly patients where there is a metaphyseal deficiency, if there is a limited bony support for the femoral component and if there is difficulty controlling the rotation of the femoral implant. Instead of preparing the metaphyseal bone free hand, metaphyseal sleeves are a solution which use a broach technique to prepare the bone for the press-fit implant. For tantalum cones the implant is bonded to the cone by cement but metaphyseal sleeves are bonded to the implant with a morse taper. Femoral sleeves may also be especially advantageous when there is significant posterior femoral condyle bone loss, as they can add rotational stability of the implant. The tibia is first prepared with the intramedullary technique. Care has to be taken to ensure that the reamer is going down to the centre of the tibial shaft while palpating the tibial crest as a guide. There are not offsets in this system for the stem. When the tibial canal reaming is complete the appropriately-sized stem is attached to the press-fit metaphyseal broach. The tibial sleeve can be impacted onto the tibial tray in as much as 20° of internal or external rotation. Once a secure fit is established the broach is left in place and the broach handle is removed. The top of the broach indicates the proximal tibial resection level. A flat saw blade is then used to resect the tibia to the top of the broach. This broach provides a guide for creating a level tibial surface. It is important to ensure the rotation of trial sleeve matches the rotation created during broaching. With the tibial trial in place the flexion and extension gaps are evaluated. After the tibia the preliminarily femoral component and the joint line have to be determined. The distal femoral cut establishes the joint line. If the stem is to be used then the same rigid straight reamers used for the tibia have to be used in he femur. The femoral canal is reamed keeping the reamer in relative varus and flexion to place the femoral component in the proper location. To properly achieve this the surgeon’s hand must be placed posterior and medial during the reaming process. A short femoral stem is preferred to a long femoral stem.
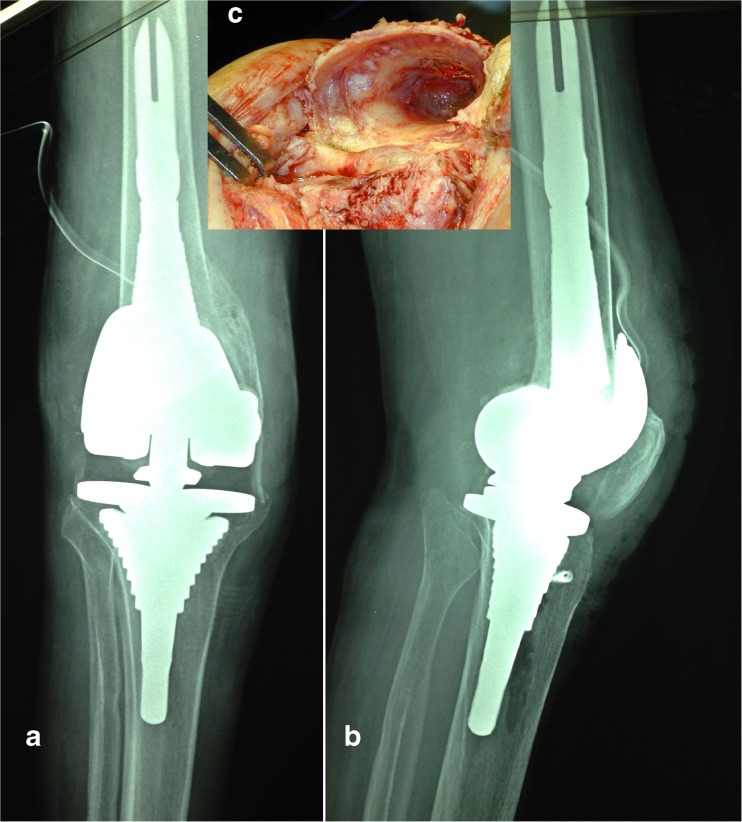
The sleeves (Fig. 4) can either be broached when starting the femoral component revision or at the end after the femoral component and augments have been established. The benefit of placing the femoral sleeve is that it makes it easier to hold the intramedullary rod and femoral cutting blocks. However, having the sleeve present before establishing the final implant makes it difficult to distally augment the prosthesis because the joint line has not been established. By contrast placing sleeves last means that the rotation will be established later. The sleeves should be inserted at the proper rotation but the exact rotation of the femur has yet to be determined.
Fig. 4.
AORI TYPE III bone defect treated with metaphyseal sleeves (a Preoperative X-ray. b Postoperative X-ray). An intraoperative particular of the defect (c)
After using an opening reamer the smallest broach is first used and the broaches are sequentially increased until a tight fit is achieved.
The most common complication from the use of metaphyseal sleeves is fracture when broaching the sleeves in the tibia or the femur. A fracture can also occur when impacting the final stem/sleeve. Treatment of a fracture may require cerclage wires to provide adequate fixation and postoperative weight bearing may be reduced to allow for fracture healing. The use of sleeves for revision TKA has demonstrated encouraging short-term results in several series [55, 56].
Conclusion
Revision surgery in total knee arthroplasty is very complex, especially when confronting potential failure to reconstruct the bone defect.
Re-establishment of well-aligned and stable implants is necessary for successful reconstruction, but this can’t be accomplished without a sufficient restoration of an eventual bone loss.
Defects classification is preoperatively difficult and their variability enable the adoption of different surgical techniques. Cement is cost effective but it cannot be used to address large bony defects. Custom-made implants can address severe bone loss but are very costly. Bone grafting carries the risk of nonunion and disease transmission which makes it a less desirable method of fixation in TKR. Using augments is helpful for filling defects but they still require cement for implant fixation. Another option for filling bony defects is using metaphyseal filling implants including metaphyseal sleeves or tantalum cones. The benefit of these implants is that they are press fit into bone which allows for bony ingrowth. An additional benefit to using metaphyseal sleeves is that bone loss can be addressed from both the tibia and femur. In contrast to structural allograft these implants avoid the risk of nonunion collapse and transmission of disease but lack potential for host bone restoration. Metaphyseal sleeves and cones appear to show promise for handling more difficult defects and may present an attractive alternative to structural allograft. Longer-term data are needed, however, to determine where these technologies fit in the armamentarium of the revision surgeon.
When there is significant bone loss it is often difficult to achieve stable rotational control of the tibial or femoral implant using just a diaphyseal stem with augments. Therefore to achieve stable fixation a press-fit metaphyseal femoral sleeve can be used to enhance the rotational stability of the femoral component.
References
- 1.Clatworthy MG, Ballance J, Brick GW, Chandler HP, Gross AE. The use of structural allograft for uncontained defects in revision total knee arthroplasty. A minimum five-year review. J Bone Joint Surg Am. 2001;83-A(3):404–411. doi: 10.2106/00004623-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Saleh KJ, Macaulay A, Raosevich DM, et al. The Knee Society Index of severity for failed total knee arthroplasty. Clin Orthop Relat Res. 2001;392:166–173. doi: 10.1097/00003086-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Engh GA, Ammen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1995;48:167–175. [PubMed] [Google Scholar]
- 4.Engh GA, Ammeen DJ. Classification and preoperative radiographic evaluation: knee. Orthop Clin N Am. 1998;29:205–217. doi: 10.1016/S0030-5898(05)70319-9. [DOI] [PubMed] [Google Scholar]
- 5.Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg Am. 1974;56(7):1391–1396. [PubMed] [Google Scholar]
- 6.Reish TG, Scott WN, Math KR. Osteolysis around total knee arthroplasty diagnosis by multi-detector computer tomography. Proceedings of American Academy Orthopaedic. San Francisco: Surgery; 2004. [Google Scholar]
- 7.Lucey SD, Scuderi GR, Kelly MA, et al. A practical approach to dealing with bone loss in revision total knee arthroplasty. Orthop. 2000;23:1036–1041. doi: 10.3928/0147-7447-20001001-14. [DOI] [PubMed] [Google Scholar]
- 8.Engh GA, Herzwurm PJ, Parks NL. Treatment of major defects of bone with bulk allografts and stemmed components during total knee arthroplasty. J Bone and Joint Surg Am. 1997;79(7):1030–1039. doi: 10.2106/00004623-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Innocenti M, Matassi F, Carulli C, Soderi S, Villano M, Civinini R. Joint line position in revision total knee arthroplasty: the role of posterior femoral off-set stems. Knee. 2013;20(6):447–450. doi: 10.1016/j.knee.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Booth RE, Jr, Engh GA, Laskin RS, Lotke PA, Jones RE, Vince KG. What would you do? Case challenges in knee surgery. J Arthroplast. 2006;21(4 Suppl 1):61–67. doi: 10.1016/j.arth.2006.02.153. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CL, Lonner JH, Rand JA, Lotke PA. Strategies of stem fixation and the role of supplemental bone graft in revision total knee arthroplasty. J Bone Joint Surg Am. 2003;85-A(Suppl 1):S52–S57. doi: 10.2106/00004623-200300001-00010. [DOI] [PubMed] [Google Scholar]
- 12.Toms AD, Barker RL, McCelland CL, Spencer-Jones R, Kuiper JH. Repair of defects and containment in revision total knee replacement: a comparative biomechanical analysis. J Bone Joint Surg Br. 2009;91:271–277. doi: 10.1302/0301-620X.91B2.21415. [DOI] [PubMed] [Google Scholar]
- 13.Ritter MA, Keating EM, Faris PM. Screw and cement fixation of large defects in total knee arthroplasty. A sequel. J Arthroplast. 1993;8(1):63–65. doi: 10.1016/S0883-5403(06)80109-9. [DOI] [PubMed] [Google Scholar]
- 14.Dorr LD. Bone grafts for bone loss with total knee replacement. Orthop Clin N Am. 1989;20:179–187. [PubMed] [Google Scholar]
- 15.Fosco M, Ben Ayad R, Luca A et al. (2012) Management of bone loss in primary and revision knee replacement. In Fokter SK (ed) Recent advances in hip and knee arthroplasty. InTech, Italy, pp 203–222
- 16.Scott RD. Bone loss: prosthetic and augmentation method. Orthopedics. 1995;18:923–926. doi: 10.3928/0147-7447-19950901-42. [DOI] [PubMed] [Google Scholar]
- 17.Ritter MA. Screws and cement fixation of large defects in total knee arthroplasty. J Arthroplast. 1986;1(2):125–129. doi: 10.1016/S0883-5403(86)80050-X. [DOI] [PubMed] [Google Scholar]
- 18.Suarez MA, Murcia A, Maestro A. Filling of segmental bone defects in revision knee arthroplasty using morsellized bone grafts contained within a metal mesh. Acta Orthop Belg. 2002;68:163–167. [PubMed] [Google Scholar]
- 19.Toms AD, Barker RL, Jones RS, Kuiper JH. Impaction bone-grafting in revision joint replacement surgery. J Bone Joint Surg Am. 2004;86:2050–2060. doi: 10.2106/00004623-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Whiteside LA. Morselized allografting in revision total knee arthroplasty. Orthop. 1998;21(9):1041–1043. doi: 10.3928/0147-7447-19980901-40. [DOI] [PubMed] [Google Scholar]
- 21.Heyligers IC, Van Haaren EH, Wuisman PI. Revision knee arthroplasty using impaction grafting and primary implants. J Arthroplast. 2001;16(4):533–537. doi: 10.1054/arth.2001.22391. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker JP, Dharmarajan R, Toms AD. The management of bone loss in revision total knee replacement. J Bone Joint Surg Br. 2008;90(8):981–987. doi: 10.1302/0301-620X.90B8.19948. [DOI] [PubMed] [Google Scholar]
- 23.Tagil M, Aspenberg P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop Relat Res. 1998;352:231–238. [PubMed] [Google Scholar]
- 24.Linder L. Cancellous impaction grafting in the human femur: histological and radiographic observations in 6 autopsy femurs and 8 biopsies. Acta Orthop Scand. 2000;71:543–552. doi: 10.1080/000164700317362154. [DOI] [PubMed] [Google Scholar]
- 25.Tsiridis E, Narvani AA, Haddad FS, Timperley JA, Gie GA. Impaction femoral allografting and cemented revision for periprosthetic femoral fractures. J Bone Joint Surg Br. 2004;86:1124–1132. doi: 10.1302/0301-620X.86B8.14854. [DOI] [PubMed] [Google Scholar]
- 26.Tang T, Dai K, Zhu N, Chen Y. A histomorphometric and molecular study on stress adaptability of freeze-dried bone allograft. Chin Med J (Engl) 2001;114:1189–1192. [PubMed] [Google Scholar]
- 27.Yan CH, Chiu KY, Ng TP, Ng FY. Revision total hip arthroplasty with femoral impaction bone grafting. J Orthop Surg (Hong Kong) 2010;18:303–308. doi: 10.1177/230949901001800309. [DOI] [PubMed] [Google Scholar]
- 28.Judas F, Figueiredo M, Cabrita A, Proena A. Incorporation of impacted morselized bone allografts in rabbits. Transplant Proc. 2005;37(6):2802–2804. doi: 10.1016/j.transproceed.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Pour AE, Parvizi J, Sienker N, Purtill JJ, Sharkey PF. Rotating hinge total knee replacement: use with caution. J Bone Joint Surg Am. 2007;89a:1735–1741. doi: 10.2106/JBJS.F.00893. [DOI] [PubMed] [Google Scholar]
- 30.Radnay CS, Scuderi GR. Management of bone loss: augments, cones, offset stems. Clin Orthop Relat Res. 2006;446:83–92. doi: 10.1097/01.blo.0000214437.57151.41. [DOI] [PubMed] [Google Scholar]
- 31.Brand MG, Daley RJ, Ewald FC, Scott RD. Tibial tray augmentation with modular metal wedges for tibial bone stock deficiency. Clin Orthop Relat Res. 1989;248:71–79. [PubMed] [Google Scholar]
- 32.Hockman DE, Ammeen D, Engh GA. Augments and allografts in revision total knee arthroplasty. J Arthroplasty. 2005;20:35–41. doi: 10.1016/j.arth.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 33.Brooks PJ, Walker PS, Scott RD. Tibial component fixation in deficient tibial bone stock. Clin Orthop Related Res. 1984;184:302–308. [PubMed] [Google Scholar]
- 34.Patel JV, Masonis JL, Guerin J, Bourne RB, Rorabeck CH. The fate of augments to treat type-2 bone defects in revision arthroplasty. J Bone and Joint Surg Br. 2004;86:195–199. doi: 10.1302/0301-620X.86B2.13564. [DOI] [PubMed] [Google Scholar]
- 35.Brand MG, Daley RJ, Ewald FC, et al. Tibial tray augmentation with modular metal wedges for tibial bone stock deficiency. Clin Orthop Relat Res. 1989;248:71–79. [PubMed] [Google Scholar]
- 36.Pagnano MW, Trousdle RT, Rand JA. Tibial wedge augmentation for bone deficiency in total knee arthroplasty. A follow-up study Clin Orthop Related Res. 1995;321:151–155. [PubMed] [Google Scholar]
- 37.Takagi H, Iwata H, Ishiguro N, Kojima T, Oguchi T. Tibial wedge augmentation in total knee arthroplasty. Clin Rheumatol. 2001;13:289–292. [Google Scholar]
- 38.Werle JR, Goodman SB, Imrie SN. Revision total knee arthroplasty using large distal femoral augments for severe metaphyseal bone deficiency: a preliminary study. Orthopedics. 2002;25:325–327. doi: 10.3928/0147-7447-20020301-17. [DOI] [PubMed] [Google Scholar]
- 39.Gofton WT, Tsigaras H, Butler RA, Patterson JJ, Barrack RL, Rorabeck CH. Revision total knee arthroplasty: fixation with modular stems. Clin Orthop Relat Res. 2002;404:158–168. doi: 10.1097/00003086-200211000-00028. [DOI] [PubMed] [Google Scholar]
- 40.Stuchin SA. Allografting in total knee replacement arthroplasty. Semin Arthroplast. 1993;4:117–122. [PubMed] [Google Scholar]
- 41.Tigani D, Sabbioni G, Raimondi A. Early aseptic loosening of a porous tantalum knee prosthesis. Chirurgia Organi Movimento. 2009;93:187–191. doi: 10.1007/s12306-009-0047-x. [DOI] [PubMed] [Google Scholar]
- 42.Backstein D, Safir O, Gross A. Management of bone loss: structural grafts in revision total knee arthroplasty. Clin Orthop Related Res. 2006;446:104–112. doi: 10.1097/01.blo.0000214426.52206.2c. [DOI] [PubMed] [Google Scholar]
- 43.Mc Allister DR, Joyce MJ, Mann BJ, Vangsness CT. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35:2148–2158. doi: 10.1177/0363546507308936. [DOI] [PubMed] [Google Scholar]
- 44.Dennis DA, Little LR. The structural allograft composite in revision total knee arthroplasty. Orthopedics. 2005;28:1005–1007. doi: 10.3928/0147-7447-20050901-45. [DOI] [PubMed] [Google Scholar]
- 45.Eldridge J, Hubble M, Nelson K, Smith E, Learmonth I. The effect of bone chip size on initial stability following femoral impaction grafting. J Bone and Joint Surg Br. 1997;79(Suppl 3):S364. [Google Scholar]
- 46.Bolder SB, Schreurs BW, Verdonschot N, van Unen JM, Gardeniers JW, Slooff TJ. Particle size of bone graft and method of impaction affect initial stability of cemented cups: human cadaveric and synthetic pelvic specimen studies. Acta Orthop Scand. 2003;74:652–657. doi: 10.1080/00016470310018144. [DOI] [PubMed] [Google Scholar]
- 47.Dennis DA. The structural allograft composite in revision total knee arthroplasty. J Arthroplast. 2002;17(4 Suppl 1):90–93. doi: 10.1054/arth.2002.32456. [DOI] [PubMed] [Google Scholar]
- 48.Engh GA, Ammeen DJ. Use of structural allograft in revision total knee arthroplasty in knees with severe tibial bone loss. J Bone and Joint Surg Am. 2007;89(12):2640–2647. doi: 10.2106/JBJS.F.00865. [DOI] [PubMed] [Google Scholar]
- 49.Meneghini RM, Lewallen DG, Hanssen AD. Use of porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement. Surgical technique. J Bone Joint Surg Am. 2009;91(Suppl 2 Pt 1):131–138. doi: 10.2106/JBJS.H.01061. [DOI] [PubMed] [Google Scholar]
- 50.Lachiewicz B, Handerson RA, Soileau E, Vail TP. Can tantalum cones provide fixation in complex revision knee arthroplasty? Clin Orthop Relat Res. 2012;470:199–204. doi: 10.1007/s11999-011-1888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meneghini RM, Lewallen DG, Hanssen AD. Use the porous tantalum metaphyseal cones for severe tibial bone loss during revision total knee replacement. J Bone Joint Surg. 2008;90:78–84. doi: 10.2106/JBJS.F.01495. [DOI] [PubMed] [Google Scholar]
- 52.Howard JL, Kudera LE, Wallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg. 2011;93:478–484. doi: 10.2106/JBJS.I.01322. [DOI] [PubMed] [Google Scholar]
- 53.Maccauro G, Iommetti PR, Muratori F, Raffaelli L, Manicone PF, Fabbriciani C. An overview about biomedical applications of micron and nano size tantalum. Recent Patents Biotechnol. 2009;3:157–165. doi: 10.2174/187220809789389153. [DOI] [PubMed] [Google Scholar]
- 54.Henricson A, Linder L, Nilsson KG. A trabecular metal tibial component in total knee replacement in patients younger than 60 years: a two-year radiostereophotogrammetric analysis. J Bone Joint Surg Br. 2008;90:1585–1593. doi: 10.1302/0301-620X.90B12.20797. [DOI] [PubMed] [Google Scholar]
- 55.Jafarim A, Coyle C, Huang R, Austin M, Orozco F, Ong A (2011) Revision total knee arthroplasty using metaphyseal sleeves and short term follow up. Lombardi AV (ed) Annual Meeting American Academy of Orthophaedic Surgeons. San Diego
- 56.Pagnottom, Fedorka J, McGough R, Crossett I, Klatt B, Keating M (2011) Revision total knee replacement with porous coated metaphyseal sleeves. Lombardi AV (ed) Annual meeting 2011 American Academy of Orthopaedic Surgeons San Diego.