Abstract
Purpose
Biological reactions against wear particles are a common cause for revision in total knee arthroplasty. To date, wear has mainly been attributed to polyethylene. However, the implants have large metallic surfaces that also could potentially lead to metal wear products (metal ions and debris). The aim of this study was to determine the local release of cobalt, chromium, molybdenum and titanium in total knee arthroplasty during a standard knee wear test.
Methods
Four moderately conforming fixed-bearing implants were subjected to physiological loadings and motions for 5×106 walking cycles in a knee wear simulator. Polyethylene wear was determined gravimetrically and the release of metallic wear products was measured using high resolution-inductively coupled plasma-mass spectrometry.
Results
A polyethylene wear rate of 7.28 ± 0.27 mg/106 cycles was determined and the cumulative mass of released metals measured 1.63 ± 0.28 mg for cobalt, 0.47 ± 0.06 mg for chromium, 0.42 ± 0.06 mg for molybdenum and 1.28 ± 0.14 mg for titanium.
Conclusion
For other metallic implants such as metal-on-metal total hip arthroplasty, the metal wear products can interact with the immune system, potentially leading to immunotoxic effects. In this study about 12 % by weight of the wear products were metallic, and these particles and ions may become clinically relevant for patients sensitive to these materials in particular. Non-metallic materials (e.g. ceramics or suitable coatings) may be considered for an alternative treatment for those patients.
Keywords: Corrosion, Metal ion, Total knee arthroplasty, Wear
Introduction
Total knee arthroplasty (TKA) is a very successful treatment for degenerative joint disease, achieving good to excellent long-term results, and approximately one million knee replacements are performed worldwide each year [1]. Although patients generally benefit from joint replacement in terms of mobility and quality of life, the outcome of TKA is worse than for total hip arthroplasty (THA) [1]. TKA can fail over time and a mean revision rate of 6 % after five years has been reported in a current analysis of worldwide joint registers [2].
TKA failure is generally multifactorial even though the main reasons may be related to mechanical or biological causes [3]. Chronic inflammation following the generation of wear particles has been identified as the main biological mechanism leading to implant failure [4]. It is well known that metal wear products may cause systemic and local effects and that they are able to interact with the immune system. It has been shown that metal ions decrease the proliferation of osteoblasts, and therefore metal wear products might play a significant role in the aetiology of osteolysis [5]. In THA, metal-on-metal bearings have recently been shown to have high failure rates due to metallic wear debris [6]. Although systemic effects are rare [7], local effects including the formation of pseudotumours are frequently reported in MoM THA [8–10]. It is not yet clear whether these reactions are driven by immunotoxic or hypersensitive reactions [11, 12]. Although the incidence of allergic reactions to the implant is unclear, they seem to occur more frequently in TKA than THA [13]. In TKA, articulation typically occurs between the metallic femoral component and the tibial inlay made out of ultra-high-molecular-weight polyethylene (PE). Wear has been mainly attributed to the softer PE and in recent years much effort has been put into understanding the wear mechanism of PE and into optimising implants. However, evaluation of metal wear particles and ion release in TKA has been neglected so far and there are no studies that describe metallic wear kinetics of the local metal ion release for these implants. Total knee arthroplasty implants present large metal surface areas, which could lead to metal ion release due to corrosion processes. Furthermore, explanted femoral components show abrasive wear in the form of scratches on the metallic surfaces [14]. It must therefore be expected that TKA leads to an increasing release of metal wear products. In this study, we aimed to analyse the wear performance of a TKA using a knee wear simulator and to study the kinetics of metal ion and particle release in terms of cobalt (Co), chromium (Cr), molybdenum (Mo) and titanium (Ti).
Material and methods
Wear and metal particle release were determined for four moderately conforming fixed-bearing TKAs (P.F.C.® SIGMA®, DePuy Orthopaedics Inc, Warsaw, USA) (Fig. 1). The posterior cruciate retaining femoral components (size 3) were made of cast CoCr28Mo6 alloy according to ASTM F75, and the tibial trays (size 3, 71 x 47 mm) were made of wrought Ti6Al4V alloy according to ASTM F136. The curved tibial inserts (size 3) made of PE (GUR 1020) were attached to the tibial trays by a snap-fit mechanism. The thickness of the γ-irradiated (dose ∼25 kGy) tibial inserts was ten millimetres.
Fig. 1.

The testing setup und implant fixation of one articular station are shown. To avoid contamination in terms of additional metal ion release from the setup, the simulation chambers were made from polyurethane (PU) and polycarbonate (PC) and the other metallic components were coated with per-fluoride ethylene-propylene (FEP)
In-vitro wear simulation, implant kinematics and PE wear
Force-controlled simulation according to ISO 14243–1:2009(E) was carried out on the AMTI knee simulator (Model KS2-6-1000, Advanced Mechanical Technology Inc., Watertown, MA, USA) [15] (Fig. 1). Compressive load was offset medially 5.0 mm from the varus-valgus axis to create higher forces on the medial compartment [16, 17]. All of the tibial components and articulating surfaces of the femoral components were immersed in diluted calf serum (PAA Laboratories GmbH, Pasching, Austria) throughout the wear test in sealed chambers maintained at 37 ± 1 °C.
A protein concentration of 20 g/l was chosen and 1.85 g/l sodium azide (NaN3) was added to retard bacterial growth. Prior to simulation, the tibial inserts were presoaked in serum and gravimetrically measured at weekly intervals. The presoak ceased once the incremental mass change of the inserts was less than 10 % of the cumulative mass change. The following test parameters were employed: a maximum load of 2600 N, a flexion angle of 0–58°, AP (anterior-posterior) force of −265 N to 110 N, and an IE (internal-external) rotational torque of −1 Nm to 6 Nm. As the laxity of ligaments influences the wear behaviour of TKA, asymmetric-nonlinear motion restraints were chosen for AP and IE to simulate the clinical situation of a sectioned anterior cruciate ligament and a retaining posterior cruciate ligament [15]. Simulation lasted a total of 5×106 cycles at a frequency of 1 Hz. One sample served as a soak control and was only loaded axially. Every 0.5×106 cycles the components were cleaned and gravimetrically measured according to ISO 14243–2:2009(E). To minimise inter-station variability tibial inserts were rotated between wear stations every 0.5×106 cycles. Serum was replaced in the same intervals. Kinematic implant data (AP translation and IE rotation) was recorded during simulation to evaluate the mobility of the implant systems.
Surface characterisation and metal release
To determine the wear mechanism, surface images of the worn PE inserts and metallic femoral components were taken using scanning electron microscopy (SEM) (Leo 440, Zeiss, Cambridge, United Kingdom) and by an optical camera of a co-ordinate measuring machine (MS 222, Mahr, Gottingen, Germany).
Surface alterations on the metallic femoral components were determined by measuring the surface roughness (arithmetical mean, Ra) at the beginning and end of wear testing using a roughness measurement instrument (Perthometer M2, Mahr, Gottingen, Germany).
The release of cobalt, chromium, molybdenum and titanium was determined by measuring the ion concentration in the serum every 0.5×106 cycles using high resolution-inductively coupled plasma-mass spectrometry (HR-ICP-MS) (Element2, Thermo Fisher Scientific, Bremen, Germany). The accuracy and validity of this method has previously been demonstrated [18]. To detect not just ions but also particles in the serum, the samples were first digested with high-purity nitric acid (HNO3) and hydrogen peroxide (H2O2) in Teflon containers under clean-room conditions in a microwave high-pressure autoclave (ultraCLAVE II Milestone, Bergamo, Italy). The sample solutions were diluted with ultra-pure water and examined with the HR-ICP-MS system. To use the full detection power of HR-ICP-MS, the potential risk of sample contamination had to be minimised. Thus, metallic components were avoided in the implant fixation and simulation chamber, as these tend to corrode and release ions. Therefore the implant fixation and simulation chambers were made from polyurethane (PU) and polycarbonate (PC) and metallic components were coated with per-fluoride ethylene-propylene (FEP) (Fig. 1). To distinguish between surface corrosion of the implant components and articulation-induced metal release (which can be seen as a combination of mechanical wear and corrosive wear), serum samples of the soak control were also analysed in the same intervals as the articulating implants. The validity, accuracy and precision of this analytical approach and the applicability of wear and corrosion determination have recently been shown [19, 20].
Statistics
Student’s t-test for two independent parametric samples was used to compare surface roughness at the beginning and the end of the wear test. Regression analysis was performed on metal release and PE wear rate. All data are presented as mean ± standard deviation (SD) of the mean.
Results
PE wear and implant kinematics
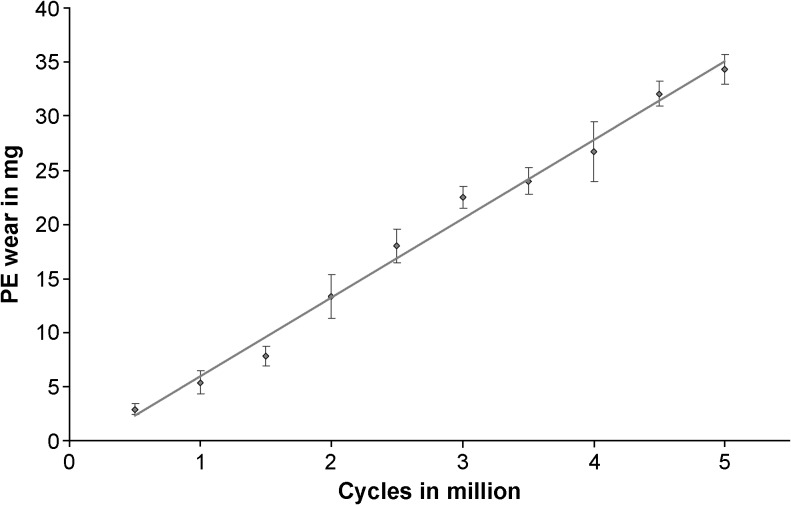
Taking weight increases of the soak-control inserts into account, an average PE wear rate of 7.28 ± 0.27 mg/106 cycles (R = 0.995; p ≤ 0.001) was determined (Fig. 2). The IE rotation averaged 8.74 ± 0.95° and the AP translation 8.57 ± 1.06 mm.
Fig. 2.
Polyethylene (PE) wear progression. The error bars show the standard deviations of the means for each measurement interval. To determine the wear rate a linear regression was calculated based on all means
Surface characterisation and metal release
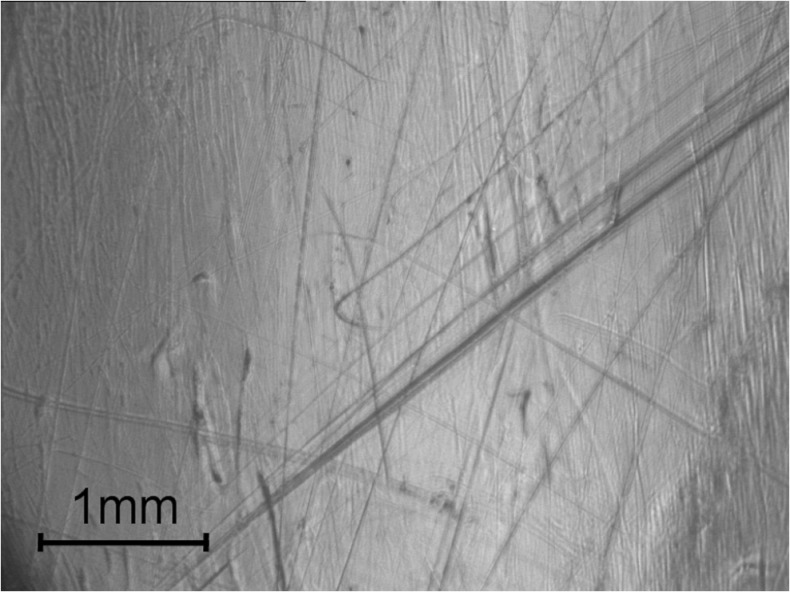
Polishing and burnishing were the dominant wear mechanisms on the PE inserts (Fig. 3).
Fig. 3.
Wear scars on the polyethylene (PE) inserts. Polishing and burnishing were the dominant wear mechanisms
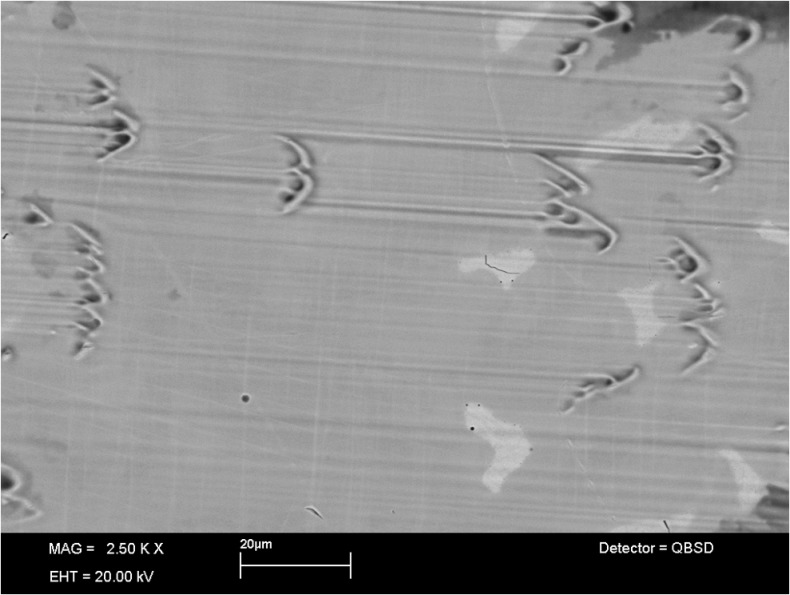
In Fig. 4 the microstructure of the CoCr28Mo6 alloy is shown. Besides the matrix, the material contains a network of eutectic carbides (bright area). The metallic femoral components mainly showed pitting as well as third body and abrasive wear mainly originating from the cobalt-rich matrix material.
Fig. 4.
The SEM image of the surface of the femoral components shows the microstructure of the CoCr28Mo6 alloy and wear scars. Mainly pitting and abrasive wear originate from the cobalt-rich matrix material
The surface roughness of the femoral components increased from 0.016 ± 0.004 μm (unworn) to 0.191 ± 0.270 μm (worn area) at the end of the test (p = 0.03).
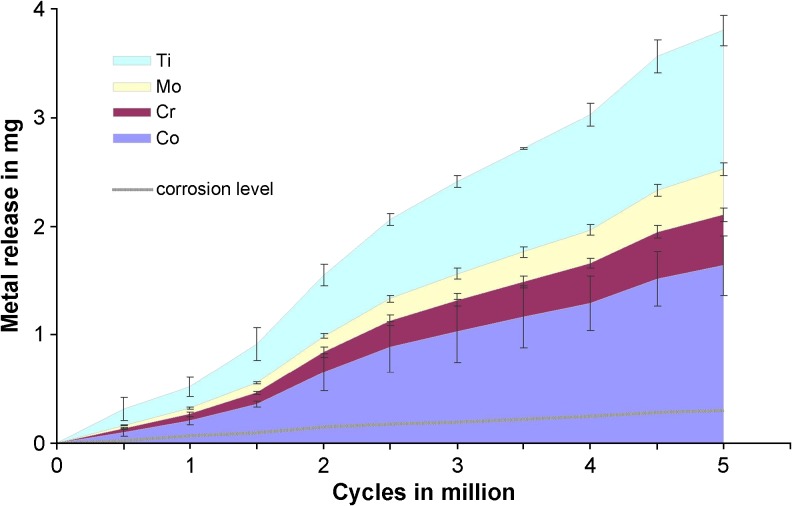
After 5×106 cycles, the cumulative metal release measured 1.63 ± 0.28 mg for cobalt, 0.47 ± 0.06 mg for chromium, 0.42 ± 0.06 mg for molybdenum and 1.28 ± 0.14 mg for titanium. The metal release progressed linearly and the rate of sole surface corrosion was 0.06 mg/106 cycles (R = 0.993; p ≤ 0.001), whereas the rate of articulation-induced metal release was found to be 0.80 mg/106 cycles (R = 0.996; p ≤ 0.001) (Fig. 5).
Fig. 5.
The articular-induced metal release for titanium (Ti), molybdenum (Mo), chromium (Cr) and cobalt (Co) is shown contrasting the corrosion level of the alloy. The error bars present the standard deviations of the means for each measurement interval
Discussion
To the authors’ knowledge, this is the first study that has experimentally measured the metal ion release and PE wear in an implant for TKA and distinguished between corrosive and abrasive wear. The investigated implant systematically released metallic wear products: cobalt, chromium, molybdenum and titanium. The metal release steadily increased during the course of the simulation and accounted for approximately 12 % of the PE wear by weight. In terms of wear volume, this corresponds to 1.7 % metallic wear as compared to the PE wear. Although PE is a soft material the release of metallic wear products seems to be mainly attributed to abrasive processes and corrosion of the metallic wear particles rather than corrosion of the bulk material.
Currently, there is a lack of data to compare the results of the determined metal ion kinetic with other metal-on-PE articulations. Compared to metal-on-metal (MoM) total hip arthroplasty (THA), the kinetics of the metal release of TKA seems to be different. Simulator studies have shown that the highest MoM THA wear occurs during the first 0.5––1.0∙106 cycles. This indicates running in wear followed by lower steady-state wear levels [21]. In MoM bearings, the running in process allows both counterparts to build up a characteristic wear patch that increases conformity between the components, obviously promoting fluid film lubrication and thus decreasing wear (steady state wear).
The metallic wear rate found in this study for TKA is low compared to MoM THA [22], and the wear mechanism of the metal-PE articulation in TKA seems to be different. Metallic wear products were released steadily without alterations in the wear progression. This may be due to the mode of lubrication; PE articulations are known to operate mainly in the boundary lubrication regime [23]. The viscoelasticity of PE may lead the material to creep and to adapt the worn metallic surface structure over time. Due to these aspects the promotion of full fluid film lubrication and therefore a wear reduction should not be expected in PE articulations. Clinically, metallic wear in metal-PE articulations has been attributed to third bodies such as bone cement or bone particles [14]. However, in this simulator study these types of third bodies were not present. Some studies reported the formation of calcium-phosphate layers on the articulating metallic surfaces [24], which may possibly chip off during articulation and cause third body wear.
On the metallic femoral components in this study (made of CoCr28Mo6 alloy) wear was dominant within the cobalt rich matrix (Fig. 4). Carbides are known to be very hard and rich in molybdenum and chromium [21]. Surprisingly, the proportion of molybdenum within the metallic wear was much higher compared to the composition of the alloy (Fig. 5). This possibly indicates that carbides (or fragments of these) were also released during the articulation. Consequently, the higher hardness of the carbides (or fragments) within the articulation may have caused the observed abrasive and third body wear on the femoral components.
Moreover, in comparison to the other elements, high titanium values were found. Titanium was released from the tibial plateau (made of Ti6Al4V alloy), although the tibial plateau is not intended for articulation. The titanium alloy has a very thin oxide layer which is sensitive to fretting [25]. It seems that even with a softer material like PE, micromotions (between the backside of the PE insert and the tibia plateau) may cause a relevant release of titanium wear products.
So far, metal wear has been mainly associated with MoM THA rather then TKA. However, a few clinical studies have also reported increased systemic metal ion levels for TKA patients, which were primarily reported after loosening or malfunctioning of the implant [26, 27]. A single study reported a significant increase in serum ion concentration (between one and four μg/l) for patients treated with TKA [28], but another study was not able to show any significant increase in metal ions [29]. However, the results of these clinical studies are not directly comparable to our study as they determined the systematic metal ion levels in patients’ blood or serum, which is different from the local ion release and absolute amount of wear products at the implant. Furthermore, these studies attributed the systemic metal ions mainly to corrosion of the implants. In contrast, our study showed the absolute release of wear products and their origin. Beside PE wear, approximately 12 % by weight of metallic wear products were also released for the implant investigated. Other wear mechanisms were observed in addition to corrosion.
There are some limitations to this study. This in-vitro study was performed in a relatively short time frame and so the effect of corrosion (of the bulk material or corrosion of released wear particles) may have been underestimated. The results are not necessarily applicable to other types of implants and may differ with other types of alloy or implant design.
Nevertheless, the method of analysing both, PE and metallic wear products is a powerful tool to study the overall wear performance of TKA and to investigate the efficacy of surface treatments (coatings, etc.) aimed at optimising the wear performance.
Conclusion
Although the systematic and long-term effects of metal ions and particles on the human organism and their effect on the clinical outcome of TKA are still unknown, this study shows that relevant levels of cobalt, chromium, molybdenum and titanium are continuously released from the implant to the local environment. If patients who are sensitive to metallic implant materials are eligible for TKA treatment, non-metallic materials (e.g. ceramics or suitable coatings) may be considered as an alternative.
Acknowledgments
The current study was funded through an internal research grant of the Department of Orthopaedics & Trauma Surgery, Heidelberg University Hospital, Germany. There are no conflicts of interest directly related to this study.
References
- 1.Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Labek G, Thaler M, Janda W, Agreiter M, Stockl B. Revision rates after total joint replacement: cumulative results from worldwide joint register datasets. J Bone Joint Surg Br. 2011;93:293–297. doi: 10.1302/0301-620X.93B3.25467. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald DW, Higgs G, Parvizi J, Klein G, Hartzband M, Levine H, Kraay M, Rimnac CM, Kurtz SM. Oxidative properties and surface damage mechanisms of remelted highly crosslinked polyethylenes in total knee arthroplasty. Int Orthop. 2013;37:611–615. doi: 10.1007/s00264-013-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992;276:7–18. [PubMed] [Google Scholar]
- 5.Hallab NJ, Vermes C, Messina C, Roebuck KA, Glant TT, Jacobs JJ. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res. 2002;60:420–433. doi: 10.1002/jbm.10106. [DOI] [PubMed] [Google Scholar]
- 6.Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410. doi: 10.1136/bmj.e1410. [DOI] [PubMed] [Google Scholar]
- 7.Rizzetti MC, Liberini P, Zarattini G, Catalani S, Pazzaglia U, Apostoli P, Padovani A. Loss of sight and sound. Could it be the hip? Lancet. 2009;373:1052. doi: 10.1016/S0140-6736(09)60490-6. [DOI] [PubMed] [Google Scholar]
- 8.Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, Murray DW, Gill HS. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28:444–450. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- 9.Matthies AK, Skinner JA, Osmani H, Henckel J, Hart AJ. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-2201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Z, Kwon YM, Mehmood S, Downing C, Jurkschat K, Murray DW. Characterization of metal-wear nanoparticles in pseudotumor following metal-on-metal hip resurfacing. Nanomedicine. 2011;7:674–681. doi: 10.1016/j.nano.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Glyn-Jones S, Pandit H, Kwon YM, Doll H, Gill HS, Murray DW. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg Br. 2009;91:1566–1574. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- 12.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 13.Rau C, Thomas P, Thomsen M. Metal sensitivity in patients with joint replacement arthroplasties before and after surgery. Orthopade. 2008;37:102–110. doi: 10.1007/s00132-007-1186-0. [DOI] [PubMed] [Google Scholar]
- 14.Que L, Topoleski LD, Parks NL. Surface roughness of retrieved CoCrMo alloy femoral components from PCA artificial total knee joints. J Biomed Mater Res. 2000;53:111–118. doi: 10.1002/(SICI)1097-4636(2000)53:1<111::AID-JBM15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Kretzer JP, Jakubowitz E, Sonntag R, Hofmann K, Heisel C, Thomsen M. Effect of joint laxity on polyethylene wear in total knee replacement. J Biomech. 2010;43:1092–1096. doi: 10.1016/j.jbiomech.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Andriacchi TP, Stanwyck TS, Galante JO. Knee biomechanics and total knee replacement. J Arthroplasty. 1986;1:211–219. doi: 10.1016/S0883-5403(86)80033-X. [DOI] [PubMed] [Google Scholar]
- 17.Harrington IJ. Static and dynamic loading patterns in knee joints with deformities. J Bone Joint Surg Am. 1983;65:247–259. doi: 10.2106/00004623-198365020-00016. [DOI] [PubMed] [Google Scholar]
- 18.Krachler M, Heisel C, Kretzer JP. Validation of ultratrace analysis of Co, Cr, Ni and Mo in whole blood, serum and urine using ICP-SMS. J Anal Atom Spectrom. 2009;24:605–610. doi: 10.1039/b821913c. [DOI] [Google Scholar]
- 19.Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33:1531–1536. doi: 10.1007/s00264-009-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kretzer J, Krachler M, Reinders J, Jakubowitz E, Thomsen M, Heisel C. Determination of low wear rates in metal-on-metal hip joint replacements based on ultra trace element analysis in simulator studies. Tribol Lett. 2010;37:23–29. doi: 10.1007/s11249-009-9486-7. [DOI] [Google Scholar]
- 21.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg Am. 2008;90(Suppl 3):125–133. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- 22.Kretzer JP, Kleinhans JA, Jakubowitz E, Thomsen M, Heisel C. A meta-analysis of design- and manufacturing-related parameters influencing the wear behavior of metal-on-metal hip joint replacements. J Orthop Res. 2009;27:1473–1480. doi: 10.1002/jor.20921. [DOI] [PubMed] [Google Scholar]
- 23.Scholes SC, Unsworth A. The effects of proteins on the friction and lubrication of artificial joints. Proc InstMechEng [H] 2006;220:687–693. doi: 10.1243/09544119JEIM21. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, McKellop H, Liao P, Benya P. Potential thermal artefacts in hip joint wear simulators. J Biomed Mater Res. 1999;48:458–464. doi: 10.1002/(SICI)1097-4636(1999)48:4<458::AID-JBM9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27:1533–1544. doi: 10.1002/jbm.820271210. [DOI] [PubMed] [Google Scholar]
- 26.Savarino L, Tigani D, Greco M, Baldini N, Giunti A. The potential role of metal ion release as a marker of loosening in patients with total knee replacement: a cohort study. J Bone Joint Surg Br. 2010;92:634–638. doi: 10.1302/0301-620X.92B5.23452. [DOI] [PubMed] [Google Scholar]
- 27.Willis-Owen CA, Keene GC, Oakeshott RD. Early metallosis-related failure after total knee replacement: a report of 15 cases. J Bone Joint Surg Br. 2011;93:205–209. doi: 10.1302/0301-620X.93B2.25150. [DOI] [PubMed] [Google Scholar]
- 28.Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb WC. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res. 2007;461:136–142. doi: 10.1097/BLO.0b013e31806450ef. [DOI] [PubMed] [Google Scholar]
- 29.Garrett S, Jacobs N, Yates P, Smith A, Wood D. Differences in metal ion release following cobalt-chromium and oxidized zirconium total knee arthroplasty. Acta Orthop Belgica. 2010;76:513–520. [PubMed] [Google Scholar]