Abstract
This theoretical research is motivated by a recent model of microtubule (MT) transport put forward by Baas and Mozgova (Cytoskeleton 69:416–425, 2012). According to their model, in an axon all plus-end-distal mobile MTs move anterogradely while all minus-end-distal mobile MTs move retrogradely. Retrograde MT transport thus represents a mechanism by which minus-end-distal MTs are removed from the axon. We suggested equations that implement Baas and Mozgova’s model. We employed these equations to simulate transport of short mobile MTs from a region (such as the site of axonal branch formation) where MT severing activity results in generation of a large number of short MTs of both orientations. We obtained the exact and approximate transient solutions of these equations utilizing the Laplace transform technique. We applied the obtained solutions to calculate the average rates of anterograde and retrograde transport of short MTs.
Keywords: Microtubule transport, Neurons, Molecular motors, Exact solution
Introduction
Microtubules (MTs) are major tracks for transport of organelles and cytoskeletal elements in axons. However, short mobile MTs themselves are transported as part of a slow component “a”, moving with an average velocity of 0.002–0.1 μm/s in both anterograde and retrograde directions [2–4]. Mobile MTs are short; they do not exceed 7–10 μm in length [1]. It was established by Anthony Brown and colleagues that MTs move intermittently and asynchronously, alternating between short runs and long pauses. During the runs, mobile MTs move with velocities on the order of 1 μm/s, which is the velocity of known molecular motors, such as kinesin and dynein [2, 5]. However, it was not clear whether MTs are transported by the sliding filament mechanism or by the cargo mechanism. In the sliding filament mechanism, the motor is stationary and the mobile MT interacts with the motor domain. In the cargo mechanism, a mobile MT interacts with the cargo domain of the motor while the motor domain “walks” on a stationary track, such as a long stationary MT [1].
There has also been no agreement on what specific kinesin motors move MTs and what the role of dynein is. Gelfand and colleagues [6] recently presented results that suggest that conventional kinesin (kinesin-1) might be the motor that slides microtubules in axons. Their conclusion is based on an experimental investigation of MT transport in Drosophila neurons.
In contrast to Gelfand’s hypothesis [6], Baas and Mozgova [1] recently suggested a different model of MT transport. According to their model, short mobile MTs always move with their plus-ends leading. The role of dynein motors in axonal transport of MTs has been elucidated in many publications, including He et al. [7] and Ahmad et al. [8]. Baas and Mozgova [1] suggested that dynein may be the main motor responsible for MT transport. They assumed that the dynein motors that drive MTs are immobilized because the dynein cargo domains interact with either long stationary MTs or the actin cytoskeleton [9, 10]. The dynein motor domains then interact with short mobile MTs, causing their sliding, in accordance with the sliding filament mechanism. For this reason, all plus-end-distal mobile MTs move anterogradely while all minus-end-distal mobile MTs move retrogradely.
According to the hypothesis put forward in Baas and Mozgova [1], the above mechanism represents the error correction mechanism by which axons clear themselves of short minus-end-distal MTs before these MTs had a chance to elongate and become stationary. This enables axons to maintain the plus-end-distal MT polarity orientation. Baas and Mozgova’s hypothesis is consistent with experimental findings of Zheng et al. [11], who established that the number of minus-end-distal MTs increases in dynein mutant axons of Drosophila neurons, in which dynein function is suppressed.
Although long stationary MTs in axons are oriented plus-end-distally, short mobile MTs with both plus-end-distal and minus-end-distal orientations can be produced by first severing these long stationary MTs and then flipping some of the short MTs (see Fig. 5 in Baas and Mozgova [1]). Such severing events can occur, for example, at the site of axonal branch formation [12–14].
Fig. 5.
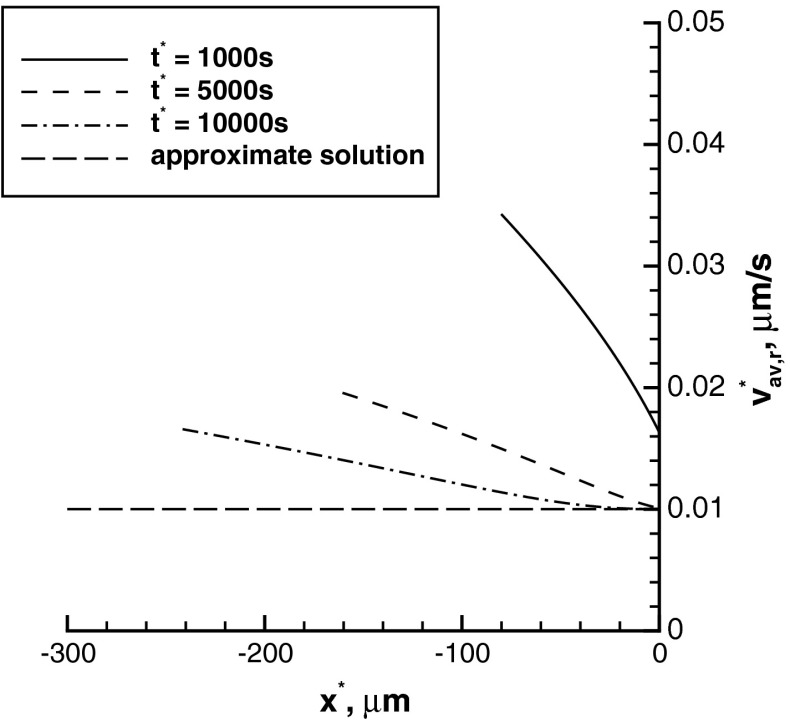
Average velocity of retrogradely moving short MTs
In this paper, we put the model of Baas and Mozgova [1] in the form of mathematical equations. We investigated transport of short mobile MTs generated by the above mechanism at the site of axonal branch formation. We then analytically solved these equations and investigated how concentration waves propagate from the site of branch formation. We also investigated the average velocities of anterograde and retrograde MT transport. The goal was to model the concept of the dynein-driven scenario proposed by Baas and Mozgova and to check whether Baas’s model stands up as a mechanistically feasible mechanism of MT transport in axons. By mechanistically feasible we mean that the model can be used to write self-consistent transport equations for short MTs and that the model predictions, in particular for the average velocity of short MTs, are reasonable. Demonstrating the feasibility of Baas’s model [1] is important in light of the alternate idea suggested by Gelfand [6].
Methods and models
Mathematical model of anterograde and retrograde transport of mobile MTs
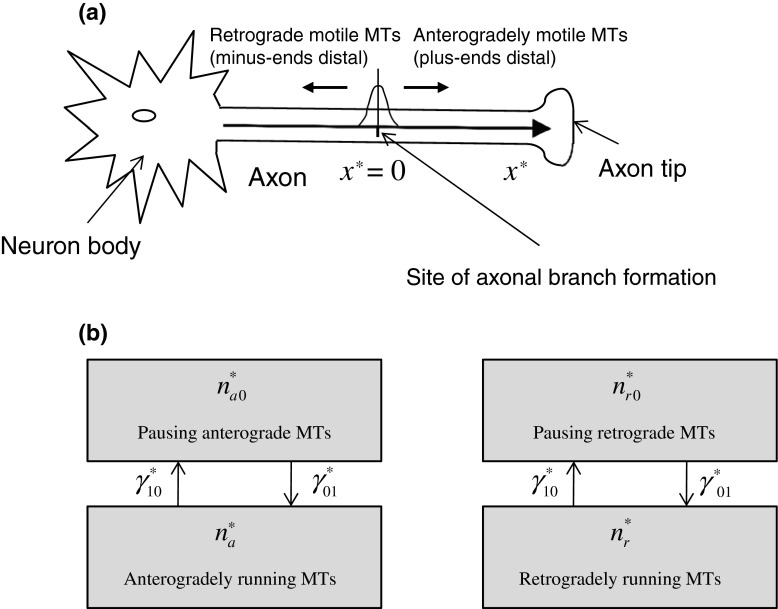
A sketch of a neuron and a coordinate system in the axon are shown in Fig. 1a. The origination point of the coordinate system is located at the site of axonal branch formation. We assumed that at x ∗ = 0 plus-end-distal and minus-end-distal mobile MTs are being produced. In developing transport equations for MTs, we relied on Jung and Brown’s stop-and-go model and their equations describing transport of neurofilaments (Eq. (7) in Jung and Brown [15], see also Li et al. [16]). Concentrations of mobile MTs in a particular kinetic state were characterized by the linear number density of MTs, which is the number of mobile MTs residing in that particular kinetic state per unit axon length (μm − 1). The governing equations for the plus-end-distal mobile MTs that move anterogradely are:
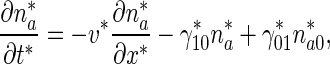 |
1 |
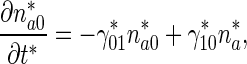 |
2 |
where 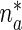 is the concentration of mobile MTs in the anterograde running state (μm − 1),
is the concentration of mobile MTs in the anterograde running state (μm − 1), 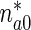 is the concentration of anterograde mobile MTs in the pausing state (μm − 1),
is the concentration of anterograde mobile MTs in the pausing state (μm − 1), 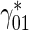 is a first-order rate constant describing the probability of transition of mobile MTs from the pausing to the running state (s − 1),
is a first-order rate constant describing the probability of transition of mobile MTs from the pausing to the running state (s − 1), 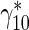 is a first-order rate constant describing the probability of transition of mobile MTs from the running to the pausing state (see Fig. 1b) (s − 1), v ∗ is the average velocity of dynein motors (μm/s), x ∗ is the linear coordinate along the axon (μm), and t ∗ is the time (s). Asterisks denote dimensional quantities.
is a first-order rate constant describing the probability of transition of mobile MTs from the running to the pausing state (see Fig. 1b) (s − 1), v ∗ is the average velocity of dynein motors (μm/s), x ∗ is the linear coordinate along the axon (μm), and t ∗ is the time (s). Asterisks denote dimensional quantities.
Fig. 1.
a Sketch of the problem showing a neuron body and an axon with a branch formation site, which is located at x ∗ = 0. MT severing at the axonal branch formation site results in a mixture of plus-end-distal and minus-end-distal mobile MTs. The plus-end-distal MTs move anterogradely and the minus-end-distal MTs move retrogradely. b Kinetic diagrams showing running and pausing MTs that are transported anterogradely (plus-end-distal MTs) and retrogradely (minus-end-distal MTs)
The governing equations for the minus-end-distal mobile MTs that move retrogradely are:
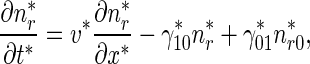 |
3 |
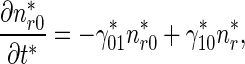 |
4 |
where 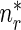 is the concentration of mobile MTs in the retrograde running state (μm − 1) and
is the concentration of mobile MTs in the retrograde running state (μm − 1) and 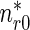 is the concentration of retrograde mobile MTs in the pausing state (μm − 1).
is the concentration of retrograde mobile MTs in the pausing state (μm − 1).
Note that the signs before 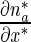 and
and 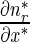 in (1) and (3) are different.
in (1) and (3) are different.
Equation (1) is a first-order partial differential equation (PDE) while (2) is an ordinary differential equation (ODE). Only one boundary condition can be imposed for the system (1), (2), and this is the boundary condition on 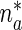 :
:
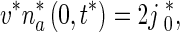 |
5 |
The value of 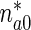 at x ∗ = 0 can then be determined by solving (1), (2), and (5).
at x ∗ = 0 can then be determined by solving (1), (2), and (5).
Similarly, the boundary condition for the system (3), (4) is
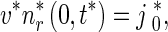 |
6 |
and the value of 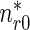 at x ∗ = 0 can then be determined by solving (3), (4), and (6).
at x ∗ = 0 can then be determined by solving (3), (4), and (6).
In (5) and (6), 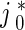 is the flux of retrogradely transported mobile MTs generated at the site where an axonal branch is being formed (see Fig. 5 of Baas and Mozgova [1]). Based on Wang and Brown [2], He et al. [7], Myers and Baas [17], we assumed that the flux of anterogradely transported mobile MTs is
is the flux of retrogradely transported mobile MTs generated at the site where an axonal branch is being formed (see Fig. 5 of Baas and Mozgova [1]). Based on Wang and Brown [2], He et al. [7], Myers and Baas [17], we assumed that the flux of anterogradely transported mobile MTs is 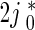 .
.
The initial conditions for (1)–(4) are
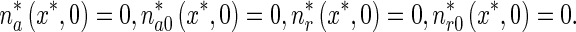 |
7 |
Non-dimensionalization
In order to reduce the number of parameters involved in the model, the model was recast into a dimensionless form. Dimensionless quantities were defined as follows:
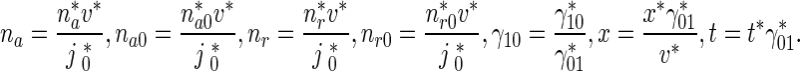 |
8 |
Dimensionless anterograde equations are
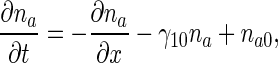 |
9 |
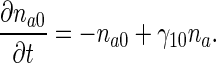 |
10 |
Dimensionless retrograde equations are
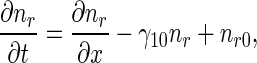 |
11 |
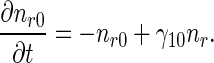 |
12 |
Dimensionless boundary conditions are
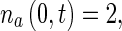 |
13 |
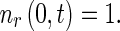 |
14 |
Results
Analytical solution by the Laplace transform method
Equations (9)–(12) for mobile MTs in the anterograde and retrograde pausing and running states were solved by the Laplace transform technique. Utilizing zero initial conditions, the subsidiary equations for the Laplace transforms of functions na , na0, nr, and nr0 are
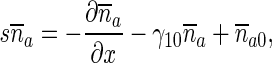 |
15 |
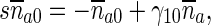 |
16 |
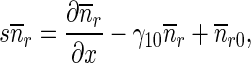 |
17 |
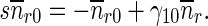 |
18 |
The Laplace transforms of boundary conditions (13) and (14) are
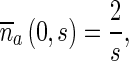 |
19 |
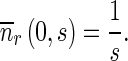 |
20 |
The solutions of the subsidiary equations are
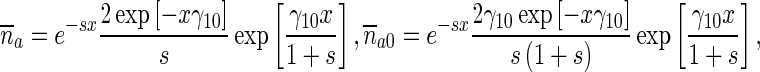 |
21 |
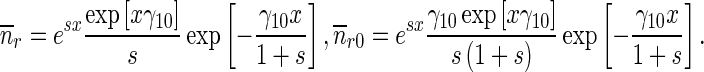 |
22 |
Calculating the inverse Laplace transform of the right-hand sides of (21), the solutions for the MT concentrations in the anterograde running and pausing states were obtained as, respectively:
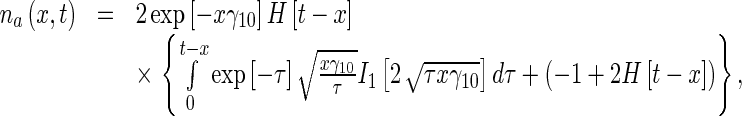 |
23 |
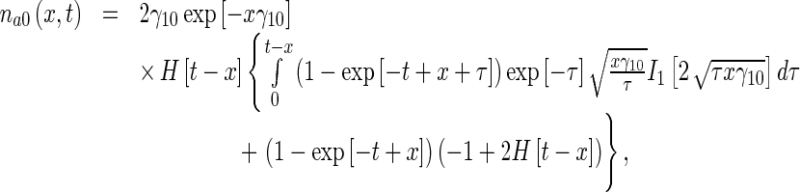 |
24 |
where I1(η) is the modified Bessel function of the first kind of order 1 and H(η) is the Heaviside step function.
The total concentration of anterograde MTs is then found as follows:
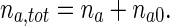 |
25 |
Next, calculating the inverse Laplace transform of the right-hand sides of (22), the solutions for the MT concentrations in the retrograde running and pausing states were obtained as, respectively:
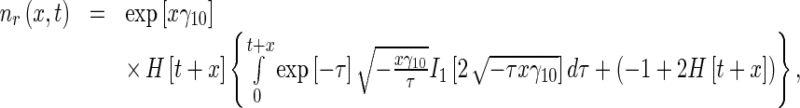 |
26 |
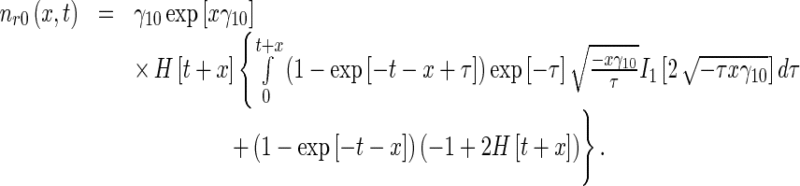 |
27 |
The total concentration of retrograde MTs is then found as follows:
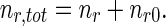 |
28 |
The average velocity of anterogradely moving MTs was calculated by dividing the total flux of anterograde MTs, 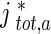 , at a certain location x ∗ , by the total concentration of anterograde MTs,
, at a certain location x ∗ , by the total concentration of anterograde MTs, 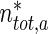 , in this location:
, in this location:
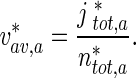 |
29 |
Here, 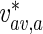 depends on the location in the axon.
depends on the location in the axon.
Similarly, the average velocity of retrogradely moving MTs was calculated as:
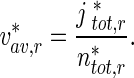 |
30 |
Since
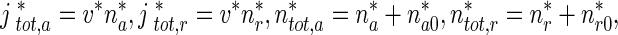 |
31 |
the average velocities of anterogradely and retrogradely moving MTs were obtained as, respectively:
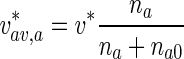 |
32 |
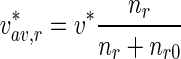 |
33 |
Approximate solution based on the equilibrium assumption
Much simpler approximate solutions of (9) and (10) can be obtained if one assumes that anterograde pausing and running MTs are in equilibrium. This assumption leads to
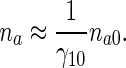 |
34 |
By adding (9) and (10) and using (34), one obtains:
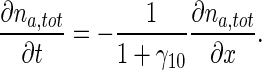 |
35 |
The boundary condition for (35) is obtained from (13) and (34), as:
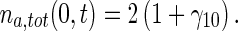 |
36 |
Solving (35) and (36) by the Laplace transform technique, one obtains
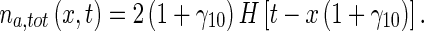 |
37 |
From the approximate solution given by (37), the average velocity of anterogradely moving MTs is
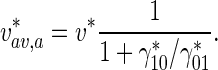 |
38 |
Equation (38) shows that the larger the ratio of the transition rate of short MTs to the pausing state to their transition rate to the running state,
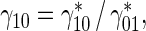 , the slower MT transport occurs.
, the slower MT transport occurs.
Similarly, if one assumes that retrograde pausing and running MTs are in equilibrium:
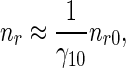 |
39 |
then an approximate analytical solution of (11) and (12), subject to boundary condition (14), can be obtained as:
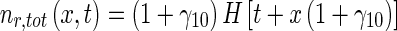 |
40 |
From (40), the average velocity of retrogradely moving MTs is again
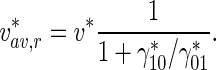 |
41 |
Estimation of parameter values
Estimated values of parameters involved in the problem are summarized in Table 1.
Table 1.
Estimated values of dimensional parameters involved in the model
| Parameter | Notation | Dimension | Value | Reference |
|---|---|---|---|---|
| Average velocity of dynein motors | v ∗ | μm/s | 1 | King and Schroer [18], Toba et al. [19] |
| Rate constant describing the probability of transition of mobile MTs from the running to the pausing state |
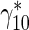
|
s − 1 | 0.093a | Jung and Brown [15] |
| Rate constant describing the probability of transition of mobile MTs from the pausing to the running state |
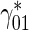
|
s − 1 | 0.00094b | see the explanation below Table 1 |
aThis estimate is based on the value reported in Jung and Brown [15] for slow neurofilament transport in the axon of a rat superior cervical ganglion neuron
bIf equilibrium between running and pausing kinetic states is assumed, the residence time of a mobile MT in the running state is proportional to 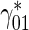 while the residence time of a mobile MT in the pausing state is proportional to
while the residence time of a mobile MT in the pausing state is proportional to 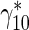 . Since MTs in the running state move with a velocity v
∗ and in the pausing state the MTs do not move, the average velocity of MT transport was estimated as
. Since MTs in the running state move with a velocity v
∗ and in the pausing state the MTs do not move, the average velocity of MT transport was estimated as 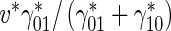 (this reasoning was used to estimate the average velocity of NF transport in Kuznetsov [20, 21]). According to Tytell et al. [22], tubulin is transported within the axon with an average velocity of 0.002...0.01 μm/s. Based on measurements in a squid giant axon, Galbraith et al. [23] reported a slightly larger average tubulin velocity of 0.025 μm/s. Using the value of 0.01 μm/s for the average tubulin velocity, the value of 1 μm/s for dynein velocity, and the value of 0.093 s − 1 for
(this reasoning was used to estimate the average velocity of NF transport in Kuznetsov [20, 21]). According to Tytell et al. [22], tubulin is transported within the axon with an average velocity of 0.002...0.01 μm/s. Based on measurements in a squid giant axon, Galbraith et al. [23] reported a slightly larger average tubulin velocity of 0.025 μm/s. Using the value of 0.01 μm/s for the average tubulin velocity, the value of 1 μm/s for dynein velocity, and the value of 0.093 s − 1 for 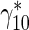 , we estimated
, we estimated 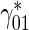 to be 0.00094 s − 1
to be 0.00094 s − 1
Data reported in Table 1 were used to estimate the value of the dimensionless parameter, γ102.
Table 2.
Estimated value of the dimensionless parameter involved in the model
| Dimensionless parameter | Notation | Definition | Value |
|---|---|---|---|
| Ratio of the rate of MT transitions from the running to pausing state to the rate of the backward transitions | γ 10 |
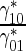
|
98.9 |
Numerical results
The effort required for coding the analytical solution is significantly less than the effort that would be required for numerical solution of the system of partial differential (1)–(4) by using a finite difference method. We implemented (23), (24) and (26), (27) using the software package Mathematica 9.0 (Wolfram Research, Champaign, IL). Numerical integration was implemented via the built-in function NIntegrate. Numerical results are then obtained within a few seconds on a standard laptop PC.
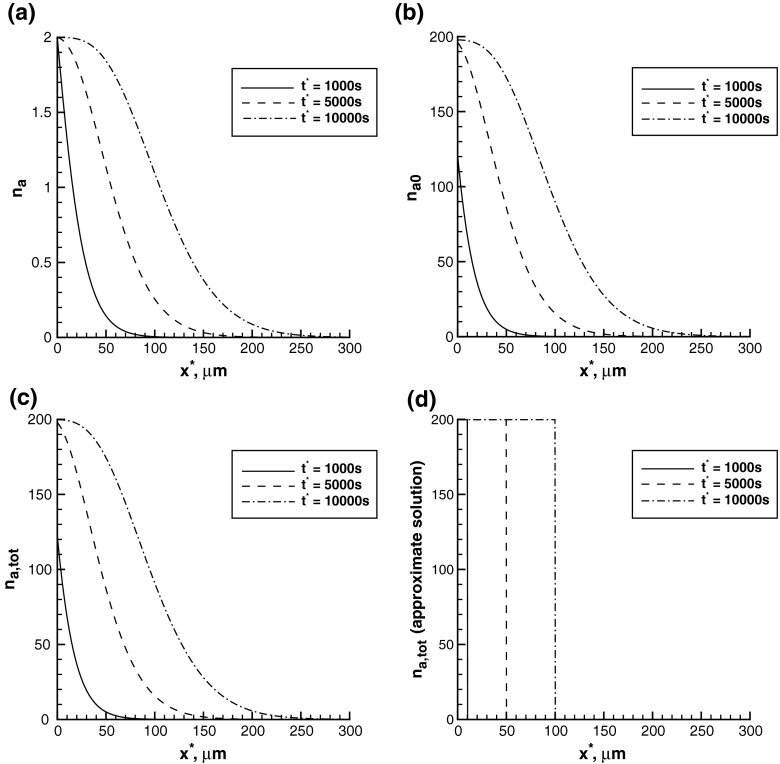
The concentration of running plus-end-distal mobile MTs, na, gradually advances from the site of axonal branch formation (Fig. 2a). It should be noted that the model expressed by (1) and (2) does not contain any diffusion; na decreases as x ∗ increases due to MT transport in the running kinetic state and MT transitions between the running and pausing kinetic states (see Fig. 1b). At x ∗ = 0, the dimensionless concentration of anterogradely running MTs is equal to 2, which is a consequence of (13). Physically, Eq. (13) posits a constant flux of short plus-end-distal MTs at the site of axonal branch formation. This flux is caused by severing of long MTs by MT-severing enzymes [24].
Fig. 2.
Concentrations of plus-end-distal mobile MTs in the a running and b pausing kinetic states. c Total concentration of plus-end-distal mobile MTs obtained from the exact solution. d Total concentration of plus-end-distal mobile MTs obtained from the approximate solution. Plus-end-distal mobile MTs move anterogradely
The concentration of pausing plus-end-distal mobile MTs, na0, behaves similarly to na, but is about 100 times larger (Fig. 2b). This is because 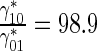 . This ratio of the kinetic constants reflects the fact that in order to move with an average velocity of 0.01 μm/s, which is 100 times smaller than the dynein velocity of 1 μm/s, mobile MTs have to spend approximately 100 times more time in the pausing state than in the running state (see Fig. 1b).
. This ratio of the kinetic constants reflects the fact that in order to move with an average velocity of 0.01 μm/s, which is 100 times smaller than the dynein velocity of 1 μm/s, mobile MTs have to spend approximately 100 times more time in the pausing state than in the running state (see Fig. 1b).
The total concentration of plus-end-distal mobile MTs (the sum of na and na0) displayed in Fig. 2c is only slightly larger than the concentration of plus-end-distal pausing MTs and has a similar shape. However, the approximate solution for the total concentration of anterogradely moving MTs (given by (37)) (Fig. 2d) is quite different from the total concentration based on the exact solution (Fig. 2c). This is because the approximate solution is based on the assumption that the running and pausing plus-end-distal MTs are in equilibrium at all times, which results in a constant average velocity of their transport and the total concentration exhibiting a sharp front (Fig. 2d).
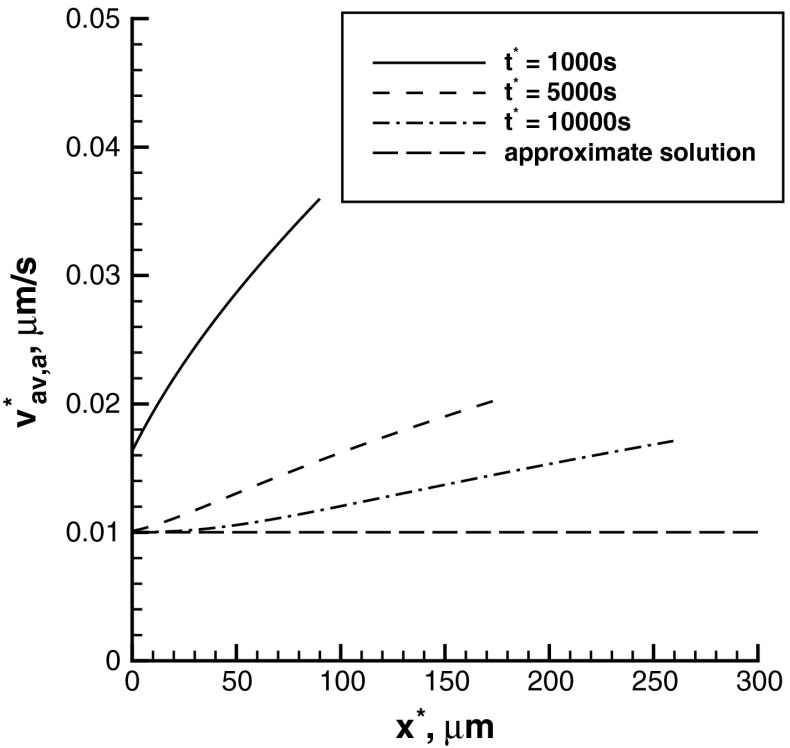
The average velocity of anterogradely moving MTs increases as the distance from the branch formation site increases (Fig. 3). This is because at the tip of the wave the portion of running MTs is larger (the ratio of running-to-pausing MTs is farther from equilibrium). Since MTs in the running kinetic state are the ones that are moving, at the wave’s tip their concentration must be larger. Apparently, it takes some time to reach equilibrium between the pausing and running states. For example, at x ∗ = 0 at times t ∗ = 5000 s and 10000 s the equilibrium is reached, but at t ∗ = 1000 s there are still less MTs in the pausing state than there are at equilibrium (Fig. 2b). For this reason, at x ∗ = 0 at time t ∗ = 1000 s the average velocity of MTs is approximately 1.6 times larger than the equilibrium value of 0.01 μm/s. Such a slow establishment of equilibrium is because of a constant supply of new mobile MTs at x ∗ = 0 due to severing of long MTs. In our model, we assumed that at the boundary there is a constant flux of newly produced mobile MTs in the running kinetic state (see (5)). The lines in Fig. 3 interrupt at the tip of the concentration wave, na (Fig. 2a). The approximate solution, which is based on the equilibrium assumption, predicts a constant average velocity of transport of plus-end-distal MTs (Fig. 3). This reconfirms our conjecture that the increase of the average velocity is caused by the deviation from equilibrium.
Fig. 3.
Average velocity of anterogradely moving short MTs
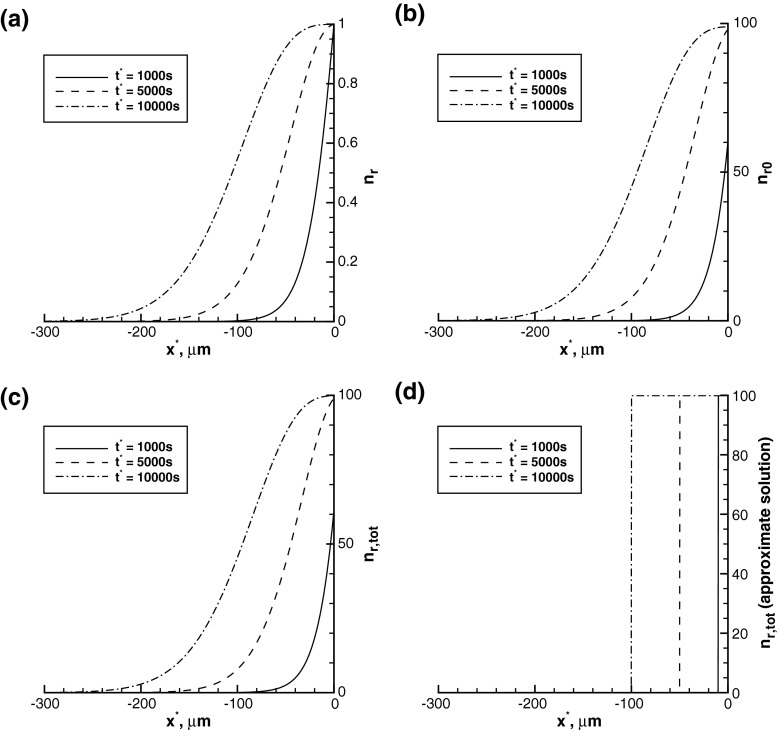
The concentrations of minus-end-distal mobile MTs in the running and pausing kinetic states as well as the total concentration of minus-end-distal mobile MTs (Fig. 4a, b, c) are similar to the concentrations in their plus-end-distal counterpart states (Fig. 2a, b, c), but the concentrations displayed in Fig. 4a, b, c are two times smaller. This is because we assumed that out of all newly generated mobile MTs at x ∗ = 0, two-thirds are plus-end-distal and only one-third is minus-end-distal (compare (13) and (14)). This is in agreement with reports of Wang and Brown [2], He et al. [7], Myers and Baas [17] that two-thirds of the short mobile MTs transit in the anterograde direction and only one-third transit in the retrograde direction. The total concentration of minus-end-distal MTs predicted by the approximate solution (40) is also two times smaller than the corresponding concentration of plus-end-distal MTs (compare Figs. 4d and 2d).
Fig. 4.
Concentrations of minus-end-distal mobile MTs in the a running and b pausing kinetic states. c Total concentration of minus-end-distal mobile MTs obtained from the exact solution. d Total concentration of minus-end-distal mobile MTs obtained from the approximate solution. Minus-end-distal mobile MTs move retrogradely
The absolute values of velocities of retrogradely moving MTs (Fig. 5) are similar to velocities of anterogradely moving MTs (Fig. 3). This is because, in accordance with [1], we assumed that all anterograde and retrograde motions of MTs are propelled by the same dynein motors.
Discussion and conclusions
We developed a continuum model for investigating transport of short mobile MTs. The model is based on a recent hypothesis put forward in Baas and Mozgova [1]. According to this hypothesis, plus-end-distal mobile MTs are always transported anterogradely while minus-end-distal mobile MTs are always transported retrogradely, and dynein motors propel both anterograde and retrograde MT transport by the sliding filament mechanism. Relying on the stop-and-go model of Jung and Brown [15], Li et al. [16], we developed governing equations for simulating MT transport from a site where short mobile MTs are produced by MT severing events, such as the site of axonal branch formation.
Although there is no MT diffusion in the model, the concentrations of anterogradely and retrogradely moving mobile MTs decrease with the increase of the distance from the branch formation site; the concentrations advance as time increases. This behavior is due to MT transitions between the running and pausing kinetic states and MT transport in the running states.
Our model predicts that the average velocity of anterogradely and retrogradely moving MTs increases as the distance from the branch formation site increases. This is because only MTs in the running kinetic state are moving, and since the tip of the wave should contain the fastest MTs, the concentration of running MTs at the wave’s tip must be largest. The equilibrium between running and pausing MTs is established first at the branch formation site, but it still takes more than a 1000 s to establish the equilibrium at this location. This is because new mobile MTs are constantly produced at the branch formation site (by severing of long MTs), and in the model we attributed these newly produced MTs to the running kinetic state.
The obtained results suggest that Baas’s model [1] is mechanistically feasible. More insight can be gained in the future by directly comparing the predictions of Baas’s [1] and Gelfand’s [6] models, but in order to do that, more details need to be provided for Gelfand’s model, so that it could also be written in terms of transport equations.
In this research, we assumed that all mobile MTs are transported as part of slow comportment “a”. However, a portion of tubulin, at least in some neurons, moves in slow component “b” (that is 5-10 times faster) [2, 25–27]. Future models should account for both populations of mobile MTs, those moving in slow component “a” and those moving in slow component “b”.
Acknowledgements
The authors are indebted to the anonymous reviewers for their constructive comments. AVK gratefully acknowledges support of the Alexander von Humboldt Foundation though the Humboldt Research Award.
Contributor Information
I. A. Kuznetsov, Email: ikuznet1@jhu.edu
A. V. Kuznetsov, Email: avkuznet@ncsu.edu
References
- 1.Baas PW, Mozgova OI. A novel role for retrograde transport of microtubules in the axon. Cytoskeleton. 2012;69:416–425. doi: 10.1002/cm.21013. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Brown A. Rapid movement of microtubules in axons. Curr. Biol. 2002;12:1496–1501. doi: 10.1016/S0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- 3.Baas P, Karabay A, Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–524. doi: 10.1016/j.tcb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Baas P, Nadar C, Myers K. Axonal transport of microtubules: the long and short of it. Traffic. 2006;7:490–498. doi: 10.1111/j.1600-0854.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LSB, Yang ZH. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Ann. Rev. Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr. Biol. 2013;23:1018–1023. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Francis F, Myers KA, Yu WQ, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J. Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad F, He Y, Myers K, Hasaka T, Francis F, Black M, Baas P. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. doi: 10.1111/j.1600-0854.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 9.Myers KA, Baas PW. Microtubule–actin interactions during neuronal development. In: Gallo G, Lanier LM, editors. Neurobiology of Actin. New York: Springer; 2011. pp. 73–96. [Google Scholar]
- 10.Hasaka T, Myers K, Baas P. Role of actin filaments in the axonal transport of microtubules. J. Neurosci. 2004;24:11291–11301. doi: 10.1523/JNEUROSCI.3443-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, Zimmerman S, Jan LY, Jan YN. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat. Cell Biol. 2008;10:1172–1180. doi: 10.1038/ncb1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent E, Callaway J, Szebenyi G, Baas P, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J. Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W, Liang Q, Solowska JM, Karabay A, Korulu S, Baas PW. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson DA, Ma L. Developmental regulation of axon branching in the vertebrate nervous system. Development. 2011;138:183–195. doi: 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung P, Brown A. Modeling the slowing of neurofilament transport along the mouse sciatic nerve. Phys. Biol. 2009;6:46002. doi: 10.1088/1478-3975/6/4/046002. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Jung P, Brown A. Axonal transport of neurofilaments: a single population of intermittently moving polymers. J Neurosci. 2012;32:746–758. doi: 10.1523/JNEUROSCI.4926-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J. Cell Biol. 2007;178:1081–1091. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 19.Toba S, Watanabe TM, Yamaguchi-Okimoto L, Toyoshima YY, Higuchi H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc. Nat. Acad. Sci. U. S. A. 2006;103:5741–5745. doi: 10.1073/pnas.0508511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuznetsov AV. An exact solution describing slow axonal transport of cytoskeletal elements: effect of a finite half-life. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012;468:3384–3397. doi: 10.1098/rspa.2012.0061. [DOI] [Google Scholar]
- 21.Kuznetsov, A.V.: An exact solution of transient equations describing slow axonal transport. Comput. Methods Biomech Biomed. Eng. 16, 1232–1239 (2013) [DOI] [PubMed]
- 22.Tytell M, Brady S, Lasek R. Axonal-transport of a subclass of tau-proteins—evidence for the regional differentiation of microtubules in neurons. Proc. Nat. Acad. Sci. U. S. A. 1984;81:1570–1574. doi: 10.1073/pnas.81.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galbraith JA, Reese TS, Schlief ML, Gallant PE. Slow transport of unpolymerized tubulin and polymerized neurofilament in the squid giant axon. Proc. Nat. Acad. Sci. U. S. A. 1999;96:11589–11594. doi: 10.1073/pnas.96.20.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roll-Mecak A, Mcnally FJ. Microtubule-severing enzymes. Curr. Opin. Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Black M, Lasek R. Slow components of axonal-transport: two cytoskeletal networks. J. Cell Biol. 1980;86:616–623. doi: 10.1083/jcb.86.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oblinger M, Brady S, McQuarrie I, Lasek R. Cytotypic differences in the protein-composition of the axonally transported cytoskeleton in mammalian neurons. J. Neurosci. 1987;7:453–462. doi: 10.1523/JNEUROSCI.07-02-00453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznetsov IA, Kuznetsov AV. Analytical comparison between Nixon-Logvinenko and Jung-Brown theories of slow neurofilament transport in axons. Math. Biosci. 2013;245:331–339. doi: 10.1016/j.mbs.2013.08.001. [DOI] [PubMed] [Google Scholar]