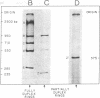
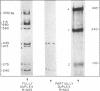
Abstract
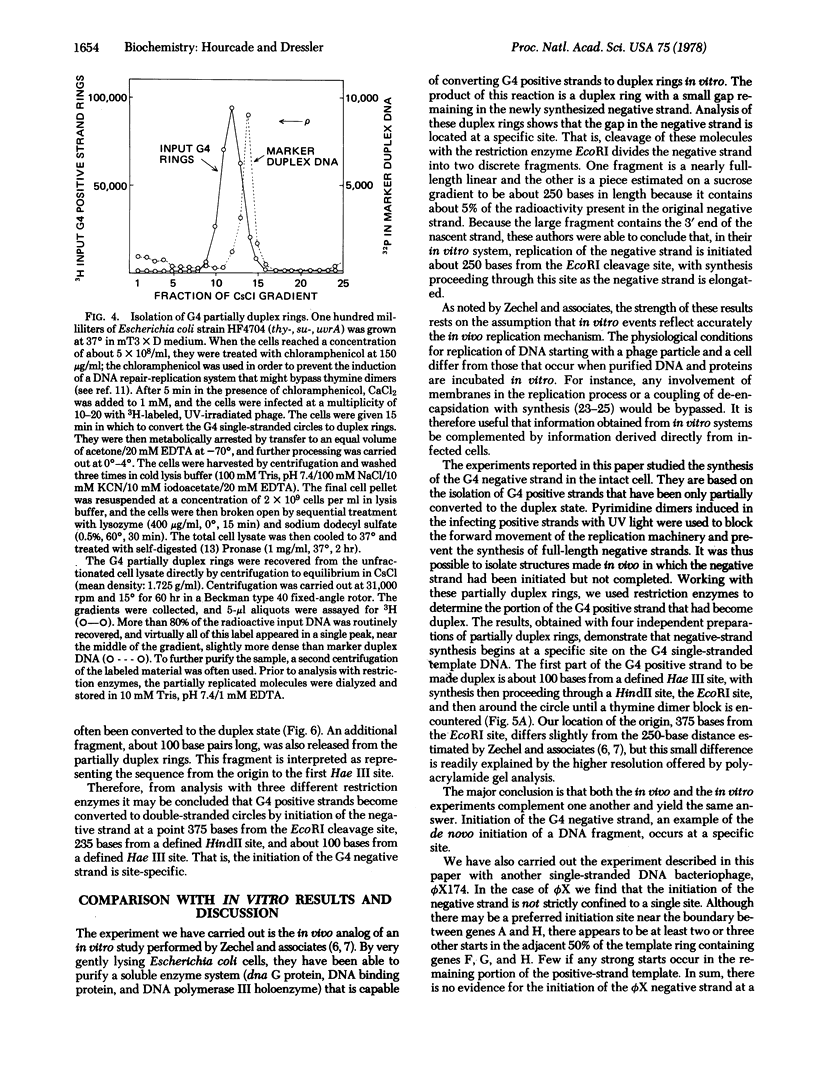
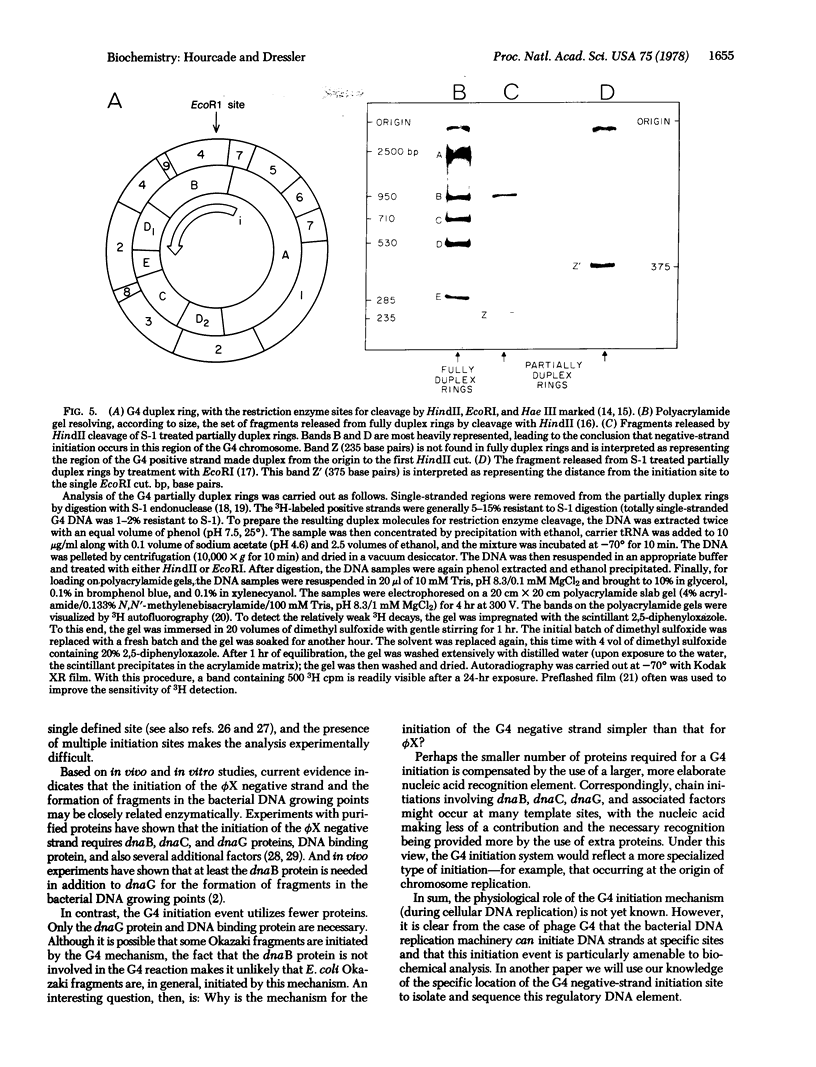
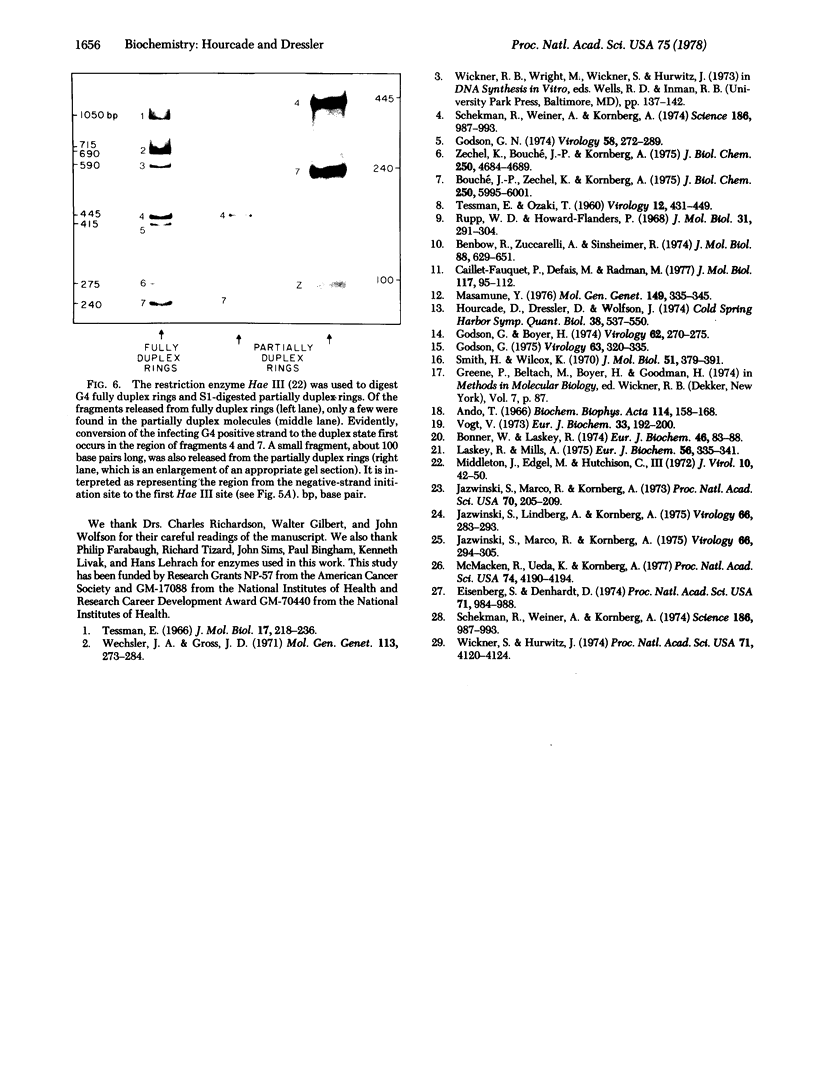
The first step in the replication cycle of the single-stranded DNA phages is the conversion of the infecting positive strand circle to a duplex ring. This event involves the de novo initiation of a negative strand, using the infecting positive strand cycle as a template. The synthesis of the negative strand is in many respects analogous to the formation of a fragment during cellular DNA replication. In this paper we describe the initiation of the negative strand of bacteriophage G4. The data establish that, in vivo, the synthesis of the G4 negative strand is initiated at a specific site, which we have mapped on the 5400-base viral chromosome.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Zuccarelli A. J., Sinsheimer R. L. A role for single-strand breaks in bacteriophage phi-X174 genetic recombination. J Mol Biol. 1974 Sep 25;88(3):629–651. doi: 10.1016/0022-2836(74)90414-8. [DOI] [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Denhardt D. T. Structure of nascent phiX174 replicative form: evidence for discontinuous DNA replication. Proc Natl Acad Sci U S A. 1974 Mar;71(3):984–988. doi: 10.1073/pnas.71.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Boyer H. Susceptibility of the phiX-like phages G4 and G14 to R-EcoRi endonuclease. Virology. 1974 Nov;62(1):270–275. doi: 10.1016/0042-6822(74)90321-3. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Evolution of phi chi 174. II. A cleavage map of the G4 phage genome and comparison with the cleavage map of phi chi 174. Virology. 1975 Feb;63(2):320–325. doi: 10.1016/0042-6822(75)90306-2. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Evolution of phi-chi 174. Isolation of four new phi-chi-like phages and comparison with phi-chi 174. Virology. 1974 Mar;58(1):272–289. doi: 10.1016/0042-6822(74)90161-5. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The nucleolus and the rolling circle. Cold Spring Harb Symp Quant Biol. 1974;38:537–550. doi: 10.1101/sqb.1974.038.01.058. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Lindberg A. A., Kornberg A. The gene H spike protein of bacteriophages phiX174 and S13. I. Functions in phage-receptor recognition and in transfection. Virology. 1975 Jul;66(1):283–293. doi: 10.1016/0042-6822(75)90198-1. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. A coat protein of the bacteriophage M13 virion participates in membrane-oriented synthesis of DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):205–209. doi: 10.1073/pnas.70.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. The gene H spike protein of bacteriophages phiX174 and S13. II. Relation to synthesis of the parenteral replicative form. Virology. 1975 Jul;66(1):294–305. doi: 10.1016/0042-6822(75)90199-3. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Masamune Y. Effect of ultraviolet irradiation of bacteriophage f1 DNA on its conversion to replicative form by extracts of Escherichia coli. Mol Gen Genet. 1976 Dec 22;149(3):335–345. doi: 10.1007/BF00268536. [DOI] [PubMed] [Google Scholar]
- McMacken R., Ueda K., Kornberg A. Migration of Escherichia coli dnaB protein on the template DNA strand as a mechanism in initiating DNA replication. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4190–4194. doi: 10.1073/pnas.74.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J. H., Edgell M. H., Hutchison C. A., 3rd Specific fragments of phi X174 deoxyribonucleic acid produced by a restriction enzyme from Haemophilus aegyptius, endonuclease Z. J Virol. 1972 Jul;10(1):42–50. doi: 10.1128/jvi.10.1.42-50.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- TESSMAN E. S., OZAKI T. The interaction of phage S13 with ultraviolet-irradiated host cells and properties of the ultraviolet-irradiated phage. Virology. 1960 Nov;12:431–449. doi: 10.1016/0042-6822(60)90165-3. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Bouché J. P., Kornberg A. Replication of phage G4. A novel and simple system for the initiation of deoxyribonucleic acid synthesis. J Biol Chem. 1975 Jun 25;250(12):4684–4689. [PubMed] [Google Scholar]