Highlights
-
•
Alternation procedures in rodents are highly sensitive to manipulations of the hippocampus.
-
•
However as they require hand testing, they are low throughput and stressful for the animal.
-
•
An automated maze was developed for assessing alternation performance in mice.
-
•
Alternation performance was shown to be impaired in mice with lesions to the hippocampus.
Keywords: Automated, Hippocampus, Maze, Mouse
Abstract
Memory deficits associated with hippocampal dysfunction are a key feature of a number of neurodegenerative and psychiatric disorders. The discrete-trial rewarded alternation T-maze task is highly sensitive to hippocampal dysfunction. Normal mice have spontaneously high levels of alternation, whereas hippocampal-lesioned mice are dramatically impaired. However, this is a hand-run task and handling has been shown to impact crucially on behavioural responses, as well as being labour-intensive and therefore unsuitable for high-throughput studies. To overcome this, a fully automated maze was designed. The maze was attached to the mouse's home cage and the subject earned all of its food by running through the maze. In this study the hippocampal dependence of rewarded alternation in the automated maze was assessed. Bilateral hippocampal-lesioned mice were assessed in the standard, hand-run, discrete-trial rewarded alternation paradigm and in the automated paradigm, according to a cross-over design. A similarly robust lesion effect on alternation performance was found in both mazes, confirming the sensitivity of the automated maze to hippocampal lesions. Moreover, the performance of the animals in the automated maze was not affected by their handling history whereas performance in the hand-run maze was affected by prior testing history. By having more stable performance and by decreasing human contact the automated maze may offer opportunities to reduce extraneous experimental variation and therefore increase the reproducibility within and/or between laboratories. Furthermore, automation potentially allows for greater experimental throughput and hence suitability for use in assessment of cognitive function in drug discovery.
1. Introduction
The discrete trial rewarded alternation task has been widely used to study hippocampal function. The task is sensitive to lesions of the hippocampus [1], [2], [3] and to subtle manipulations of hippocampal synaptic plasticity [4]. Performance is relatively stable across repeated test sessions, hence the task is suitable for longitudinal designs such as those used to track progression of neurodegenerative pathology [5].
Alternation paradigms in rodents are based on win-shift behaviour that may reflect foraging in the wild [6], [7]. In the T-maze discrete trial rewarded alternation task, each trial consists of a sample run and a choice run. In the sample run, the mouse can only enter one of two goal arms to obtain a reward as the other arm is blocked. In the choice run, the mouse is allowed a free choice of either arm, but only the previously non-visited arm is baited (i.e. alternation is rewarded). One potential concern with maze protocols such as this is that they are manually run: the mouse is removed from the maze and returned to either the start box or a holding cage after each run. This intensive handling could alter the affective state of the mouse, producing effects on stress, anxiety and arousal that may alter cognitive performance [8], [9]. In addition, hand-run tasks are labour-intensive and therefore unsuitable for high-throughput studies such as those used in drug discovery.
Decreasing the level of human intervention would allow alternation performance to be assessed without the confound of stress and anxiety induced by handling. Previous attempts to develop alternation procedures that do not require human intervention have often resulted in a loss of hippocampal sensitivity. For example, in a continuous alternation procedure in a T-maze, in which the goal arms were connected to the start arm, complete bilateral hippocampal lesions had no effect on alternation performance. However, the requirement of the hippocampus was re-instated if the experimenter manually added a delay between trials [10]. Attempts have also been made to develop non-match or match to sample paradigms in operant boxes [11]. Unfortunately, sensitivity of these tests to hippocampal lesions in rats has proved difficult to confirm as animals may use alternative strategies to solve the tasks other than those requiring hippocampus-sensitive short-term memory [12], [13], [14], [15].
A mouse automated modular maze apparatus, with independently computer controlled doors connecting the different compartments of the maze, has been developed [16]. Mice can run through the maze without any human intervention, hence eliminating the stress induced by handling. As the maze is fully automated, it can be run during the night when mice are most active. The purpose of this study was to establish an automated rewarded alternation paradigm and test whether it was sensitive to hippocampal damage.
2. Materials and methods
2.1. Subjects
Eighteen adult (11 weeks old at the time of surgery) C57BL/6J male mice (Jax, ME, USA) were used in this study. They were housed in pairs in plastic cages (18.41 cm × 29.21 cm × 12.7 cm; Alternative Design, Siloam Springs, AR, USA) with aspen bedding material (Harlan Teklad, Madison, WI, USA) and 10 g of nesting material (Enviro-dri Eco-bedding, Fibercore, Cleveland, OH). The mice were kept on a 14:10 Light:Dark photoperiod (lights on at 06:00 am), at 20 °C ± 1 °C with 60 ± 10% relative humidity with food (Harlan Teklad, Madison, WI USA; Mouse diet 2019) and water given ad libitum. They were acclimatised to the laboratory for one week before surgery. Housing, care and experimental procedures were approved by the Institutional Animal Care and Use Committee of Purdue University. Experiments were carried out in accordance with the Guidelines laid down by the NIH in the US regarding the care and use of animals for experimental procedures.
2.2. Experimental design
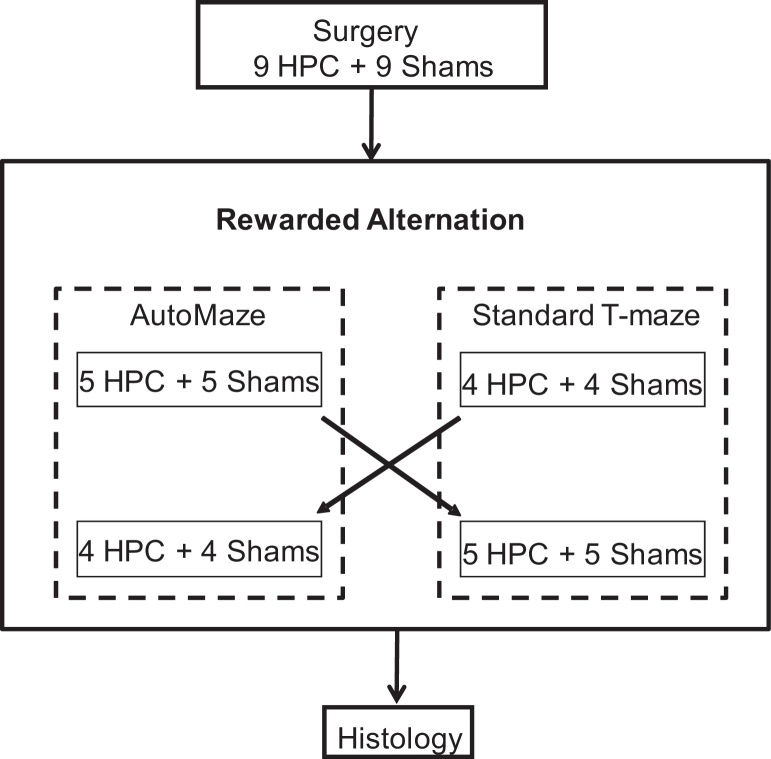
Many features of the mouse and its environment cluster at the cage level (e.g. rack position, date of surgery, or testing order), and can have a strong effect on behavioural measures. A matched-pair design, with one sham and one lesion animal per cage, was used to minimise the impact of such confounding factors [17], [18]. After habituation to the animal facility, mice from the same home cage were operated on the same day. They either received multiple bilateral injections of N-methyl-d-aspartic acid into the hippocampus (HPC) or multiple sham injections (SHAM). The mice were randomly assigned to each group. After recovery from surgery, mice were tested in each apparatus using a 2 × 2 factorial design (Fig. 1). One group started with the hand-run, discrete trial rewarded alternation task in the standard T-maze and then moved to the automated maze, whereas the other group did the opposite. To ensure that the order of left/right arm visits during the sample run of each task did not bias the comparison of performance, the same pseudorandom sequence (with no more than three consecutive visits in the same arm) was assigned to mice from the same cage. After completion of both tasks, mice were euthanized and their brains were removed and processed for histology.
Fig. 1.
Schema of the experimental design.
2.3. Surgery
Surgery was performed as described by Deacon et al. (2002) [19]. Before anaesthesia, mice were pre-treated with the non-steroidal anti-inflammatory drug Metacam (1 mg/kg, sc, Boehringer Ingheim, St Joseph, MO, USA), atropine (0.04 mg/kg, sc), and a benzodiazepine (10 mg/kg, sc, chlordiazepoxide hydrochloride, Sigma, UK). The mice were anaesthetised with isoflurane (1–3% in O2, with a rate of 2 L/min, Abbott, Chicago, IL, USA), their heads shaved and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) in a flat skull position. The scalp was incised and the skull exposed. Four microinjections of 10 mg/ml N-methyl-d-aspartic acid solution (Sigma, UK) were made per hemisphere to target the hippocampus. The stereotaxic coordinates used and the volumes of each injection are reported in Table 1. Each injection was made at a rate of 0.1 μl/min with a 5 μl Hamilton syringe mounted on the stereotaxic frame. Following injection, the needle was left in position for 3 min to allow absorption of the bolus and to minimise spread of the toxin along the needle track. Finally, the scalp was sutured and the mice put in a recovery chamber. SHAM-operated controls received an identical treatment and procedure with the exception that saline was injected instead of NMDA. To prevent seizures occurring during the recovery phase, the mice received an injection of chlordiazopexide (total of 10 mg/kg, sc in three bolus’: before, during and after surgery). There were 9 HPC- and 9 SHAM-operated mice in total.
Table 1.
Stereotaxic coordinates for hippocampal lesions.
| Site | AP (mm) | ML (mm) | DV (mm) | Volume (μl) |
|---|---|---|---|---|
| 1 | +2.1 | ±1.2 | −1.9 | 0.10 |
| 2 | +1.5 | ±1.7 | −1.9 | 0.15 |
| 3 | +1.0 | ±2.2 | −2.0 | 0.10 |
| 4 | +0.7 | ±2.8 | −4.0 | 0.20 |
Microinjections of NMDA into the hippocampus were performed at the stereotaxic coordinates above (Franklin & Paxinos). The antero-posterior values (AP) were measured from the interaural line; the medio-lateral values (ML) from the midline; and the dorso-ventral values (DV) from the surface of the skull at bregma.
2.4. Discrete trial, rewarded alternation
2.4.1. Standard T-maze
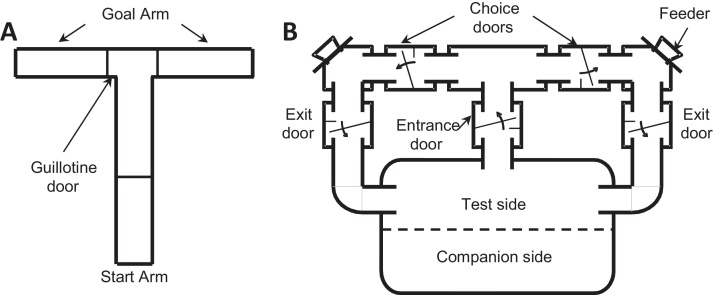
Apparatus. The T-maze was made of black opaque Perspex (constructed in-house at Eli Lilly & Co., IN, USA). It consisted of a start arm (45 cm × 10 cm × 10 cm) and two goal arms (30 cm × 10 cm × 10 cm, Fig. 2A). Each arm could be manually closed by a guillotine door.
Fig. 2.
Schematic representation of (A) the standard T-maze and (B) the automated maze. The automated maze was T-shaped and attached to a plastic home cage. Each door of the maze allowed access in one direction only (i.e. a mouse could not turn back through a door) and each could be independently controlled by a computer. This home cage was subdivided into a “test side” which was connected to a “companion side” via a perforated barrier (holes: 0.6 cm diameter). There was no access to the maze from the companion side. Each compartment measured 35 cm wide × 24 cm deep × 20 cm high. The partition allowed physical, auditory, and olfactory contact with the animal's cage mate (the companion mouse). This was to minimise disruption to the social organisation of the group, and to minimise distress. The companion mouse was provided with ad libitum food and water. The test mouse was provided with free access to water only. The test mouse earned all of its food by running through the maze during the night. The maze was closed during the day when mice are normally inactive and do not eat, thus imposing a naturalistic food deprivation. During the night the maze was opened according to different schedules. For example during the single trial training, the state of the maze alternated from being active for 15 min to being inactivate for 15 min; whereas during the rewarded alternation paradigm the maze was closed between the end of the test phase and the next sample phase for 10 min (ITI).
Habituation. Mice were put on a restricted feeding schedule and maintained at 85% of their free-feeding weight during testing on the T-maze. The mice were weighed and handled by the experimenter on a daily basis. For the first 2 days, cage-mate paired mice were placed into the goal arm of the T-maze and left to explore freely and collect food rewards for 5 min. The pellets (25 mg, Test Diet TUM, Richmond, IN, USA) were scattered over the arms and were continuously supplied by the experimenter. The number of pellets supplied was progressively reduced until food was only available in the food well at the end of each arm. During the third day of habituation, subjects were individually placed into the goal arm of the T-maze and allowed to explore freely for 5 min. By this stage, all mice were eating from the food wells at the end of the arms. Experiments were performed from 2 to 6 pm.
Forced trial training. The mice were placed into the start arm and forced to visit a baited arm by blocking the access to the other arm. The order of left-right forced choices was determined by a pseudorandom sequence. Only one pellet was available at the end of the baited arm. A total of 20 forced trials were performed by each mouse.
Discrete trial rewarded alternation testing. The sample run began with the mouse being placed into the start arm. One goal arm of the T-maze was blocked with a guillotine door, and a single reward was available in the food cup at the end of the open arm. After the mouse had obtained the reward, the experimenter removed the mouse from the maze. The guillotine door was then removed and the experimenter returned the mouse to the start arm of the T-maze for the choice run of the trial. There was no additional delay hence the intra-trial interval was approximately 10 s. The mouse was rewarded with two pellets for choosing the previously unvisited arm (i.e., for alternating). The mouse was considered to have made a choice once its whole body had entered an arm. After an incorrect response, the mouse was immediately removed from the maze and returned to its holding cage. Each mouse ran one trial at a time with an inter trial interval (ITI) of 10–20 min, for a total of 10 trials per session (short sessions are essential in mice, which satiate quickly and lose motivation to solve the task). Each mouse was tested over 6 sessions on 6 days, resulting in a total of 60 trials. The apparatus was cleaned between mice with alcohol and rinsed with water and then dried. Typically, the completion of the hand task (from the start of habituation to the end of discrete trial rewarded alternation testing) required 13 experimental days per mouse.
2.4.2. Automated maze
Apparatus.Fig. 2B illustrates the automated T-shaped maze, connected to a home cage (constructed in-house at Purdue University, IN, USA). The maze was constructed of aluminium and plastic tubing [16]. The start arm (12.7 cm), goal arms (19.0 cm to feeding chamber and 19.8 cm return to test chamber) and different compartments were connected by one-way doors controlled by a computer. Both goal arms contained a computer-controlled feeder, which delivered food pellets. Infrared beams were positioned throughout the maze to locate the mouse and to record the times spent in different compartments of the maze. A computer equipped with custom-written software controlled the automated T-maze and recorded the data. All events (e.g. unlocking/locking a door, infrared beam breaks and pellet deliveries) were time-stamped to an accuracy of at least 0.01 s and collated in a database for subsequent analysis.
At the start of testing, the mouse was placed into the test compartment while the maze was switched off. The mouse could only obtain food whilst running into the maze. The maze was switched on between 6 pm and 6 am. During this time window, access to the maze was controlled automatically, to impose a session and/or trial timing structure (see below for details in the habituation, single trial or discrete trial rewarded alternation testing paragraph). When the maze was open, the mouse entered through the central tunnel and then ran to either the left hand or right hand reward chamber via one-way doors which prevented the mouse from returning to the previously visited compartment.
Habituation To reduce neophobia to food and the door system within the apparatus, a one-way door from the maze and some pellets were placed within the home cage for two days prior to training.
Single trial training. Initially 20 shaping trials were conducted in which a visit to either goal arm was rewarded. To maximise the motivation of the mouse to run through the maze, mice could access the maze in 15-min sessions, separated by 15 min inter-session intervals (in which the mouse was confined to the test side of the home cage) [16] (see Fig. 2B).
Discrete trial rewarded alternation testing. After completion of the pre-training, the maze automatically switched to the discrete trial rewarded alternation task. Each trial comprised a sample run and a choice run. The sample run began with the opening of the entrance door. When the maze was active this was signalled with an auditory cue and a light at the entrance to the maze was lit. The mouse was forced to go left or right: i.e. only one of the choice/one-way doors was unlocked. After the mouse had passed through the choice doors, it was able to consume a one-pellet reward. As soon as the mouse returned to the test compartment and hence completed the sample run, the test run started. For this, both choice one-way doors were unlocked but only the previously unvisited arm was baited. There was no additional delay imposed between the end of the sample run and the beginning of the test run; hence the automated task was performed at the pace of the mouse. On completion of a sample run and test run, the trial ended, and the maze locked the animal in the test side of the home cage for 10 min, before beginning the sample run of the next trial. Thus the mice could transition immediately from sample to trial runs, but experienced a forced 10 min ITI between two consecutive trials. The task ran continuously between 6pm and 6am. As with the standard T-maze, 60 trials were completed in total (which typically required 1–2 nights in the maze). The mice stayed in the automated maze throughout the training on this task. They were weighed before and after testing within the automated maze. If an animal only performed a few trials overnight, supplementary pellets were given in the test cage.
In addition to the percentage alternation measure, the automated maze could record time between events happening in the maze. As infrared beam break detectors were situated after each door, it was possible to record the time taken for the mouse to move around the maze. Three latency periods were defined: The entrance latency was the time taken for the mouse to enter the start arm. During the sample phase this was defined as the time from when the maze opened, signalled by a mechanical sound, to the point at which the mouse entered the maze through the entrance door. During the test phase, the entrance latency was defined as the time from passing through the exit door following the sample phase to re-entering the maze through the entrance door (Fig. 2B). The choice latency was defined during both phases as the time from when the animal passed through the entrance door to when it passed through the choice door. Finally, the run duration was the total time spent in the maze (i.e. from passing through the entrance door to passing out the exit door, see Fig. 2B).
The latencies were analysed to make sure that the lesions were not affecting performance indirectly – for example via motivation for reward or as a result of differences in the delay experienced between sample and test phases for the two groups. These confounds might be apparent in the time taken to enter the maze on sample and/or test runs. An additional analysis including the response (correct vs incorrect) was made to test how the HPC-lesion was impairing performance in the maze itself.
2.5. Histology
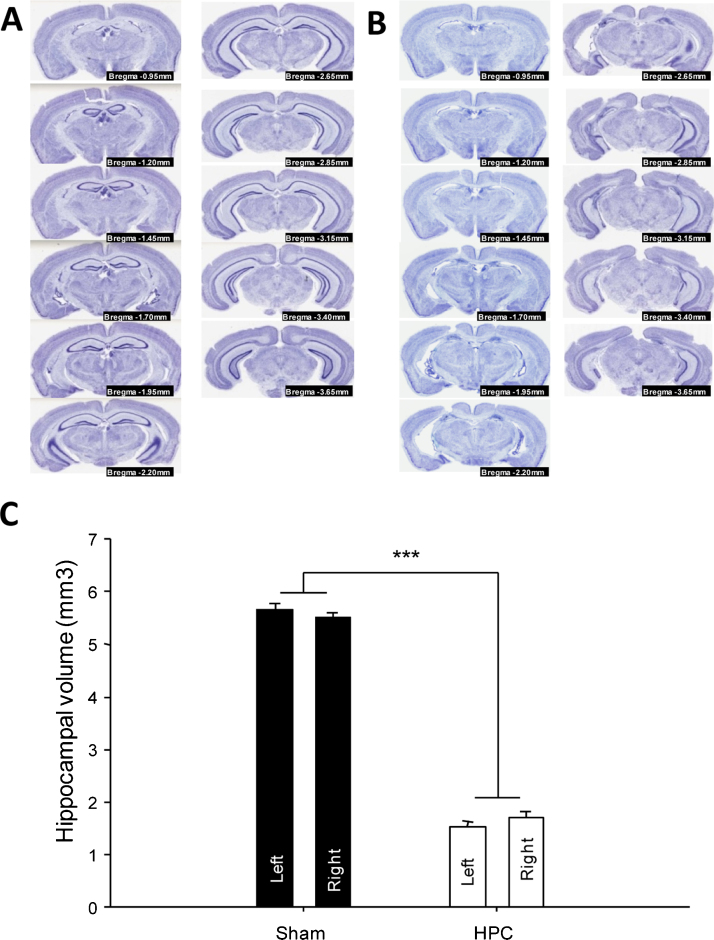
The mice were euthanized with a lethal dose of Pentobarbital (200 mg/kg, ip) and intracardially perfused with phosphate buffered saline (PBS) followed by 4% formalin in PBS. The brains were processed using MultiBrain Technology (NeuroScience Associates, Knoxville, TN, USA) and coronally sectioned at 35 μm thickness from bregma −0.10 mm to −5.34 mm according to the stereotaxic coordinates adapted from a mouse brain atlas [20]. A series of approximately 40 sections per mouse, equally spaced at 105 μm intervals across the entire hippocampus, were retained and mounted onto slides. The mounted tissue was stained with thionin for Nissl substance and coverslipped. Thionine Nissl stain is widely used in the histological assessment of neurotoxic lesions. Slides were scanned with a SanScopeXT (Aperio, Vista, CA) and analysed by the Cavalieri principle. An unbiased method based on serial section analysis and a uniform random systematic sample [21], [22] was used to assess the lesion. At alternate sections, the boundaries of the hippocampus were determined using the stereotaxic mouse atlas [20]. The volume was calculated by using the formula: V = td ∑S; where ∑S is the sum of the surface areas; t is the average section thickness; and d is the distance between two consecutive analysed sections. Examples of sections used to evaluate hippocampal volume are illustrated in Fig. 3A (SHAM) and B (HPC). At least 11 sections were assessed per mouse.
Fig. 3.
Photomicrographs of thionine Nissl stained section from representative (A) SHAM or (B) HPC lesion of the hippocampus. (C) Effect of intra-hippocampal NMDA micro-injections on the HPC volume. The two bars per group represent the two hemispheres. Statistical significance was determined by a repeated measure mixed model GLM approach followed by orthogonal contrasts. Data are expressed as least square mean ± standard error. ***p < 0.0001.
2.6. Statistics
Between-mouse variation was explicitly quantified and controlled using a repeated measures REML mixed model (JMP version 8: SAS Inc., Cary, NC). This method is more robust than a traditional least squares ANOVA. Like ANOVA or GLM, this approach statistically eliminates the influence of known and unknown environmental and intrinsic factors. For all mixed model analyses the assumptions of a mixed model, i.e. homogeneity of variance, normality of error, and linearity [23], were confirmed and suitable transformations applied as needed.
Different outcome variables used the same basic mixed model, which included mouse as a random effect, nested in group and order. Further relevant between and within subject factors were crossed with group, maze and order, and all suitable error terms for a mixed model included, following Newman et al. [24]. For the histology data this basic model also included side (hemisphere) as a within-subjects measure. For the discrete trial rewarded alternation paradigm, performance was expressed as the percentage alternation, both as a total across the whole of the session and per block of 10 trials. Latencies tend to have a strong skew, which typically become normal if log transformed. Thus, for the mixed model analysis of the latency data, each latency value was log transformed, and for each animal the mean for correct and incorrect choices calculated (the arithmetic mean of logged data = the log of the geometric mean of the same data). These data were then analysed, with response (correct vs. incorrect) added as a factor to the model which determines whether correct or incorrect trials differ on average in their preceding latencies. Therefore, to test whether individual latencies predict whether a trial will be correct or incorrect (i.e. whether latencies are reflecting processes causal to choice), a logistic regression implemented as a GLM was performed. This analysis was blocked for animal nested within group (to accommodate repeated measures) and included logged duration of the sample trial, and the trial number. The effect of treatment, logged entrance and choice latencies, and their interactions with treatment were also tested. Post hoc tests were performed with custom likelihood ratio tests, and Bonferroni corrected.
3. Results
3.1. Histology
Fig. 3A and B illustrate serial hippocampal sections from a representative SHAM-operated or HPC-lesioned mouse, respectively. Bilateral NMDA injections into the hippocampus induced a significant decrease in the volume of this structure (F(1,14) = 454.24, p < 0.0001, Fig. 3C). The lesions were bilaterally homogeneous with no difference in hippocampal volume found between the two hemispheres (F(1,14) = 0.02, p = 0.90, Fig. 3A, B and C). The dorsal hippocampus was completely lesioned. However, a small portion of the caudal part of the ventral hippocampus remained. There was minimal extra-hippocampal damage resulting from the injection.
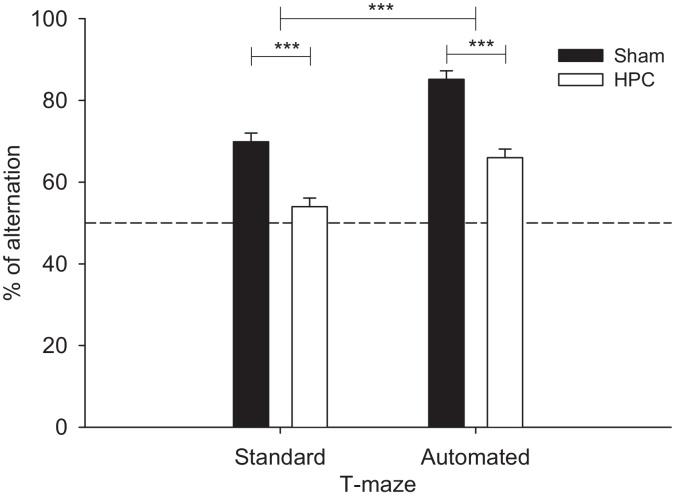
3.2. Percentage alternation in the discrete trial rewarded alternation task
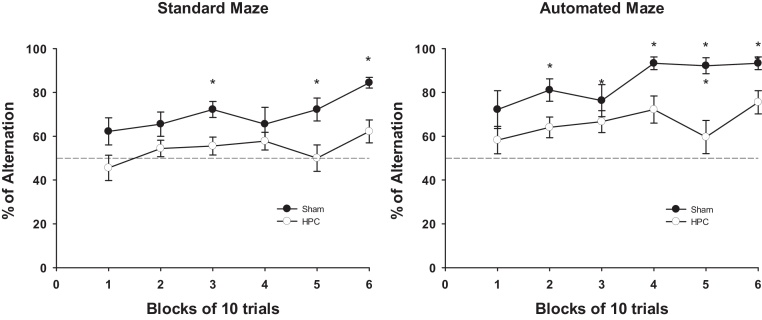
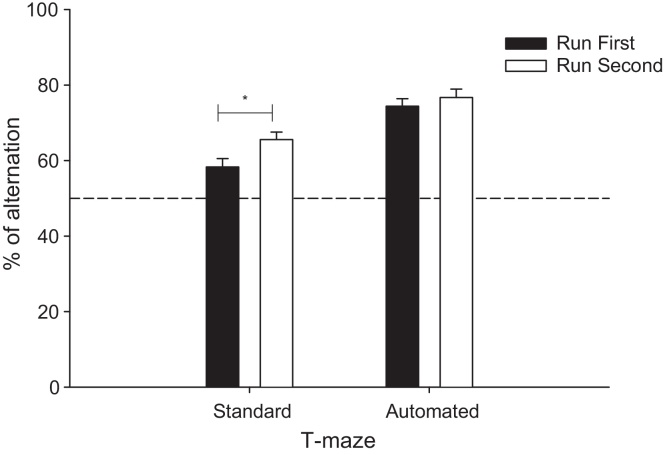
The percentage alternation of SHAM and HPC groups in both mazes is represented in Fig. 4. HPC mice showed lower performance than SHAM mice in both mazes (group effect: F(1,14) = 52.50, p < 0.0001). However, both groups showed significantly higher levels of performance in the automated maze than in the standard T-maze (maze: F(1,14) = 62.18, p < 0.0001). Group differences per block of 10 trials are illustrated in Fig. 5 for the automated and standard T-maze (block effect: F(5,70) = 4.28, p < 0.002). Notably, there was also a significant interaction between maze and order (F(1,14) = 7.42, p < 0.05): mice showed higher levels of alternation in the standard maze if they had experienced the automated maze first. In contrast, whether or not the mice had experienced the standard maze before the automated maze had no influence on performance in the latter (Fig. 6). Thus, the automated maze showed less variability as a function of previous experience than the hand run maze.
Fig. 4.
Effect of the HPC lesion on the percentage alternation on the total number of trials broken down by maze and group. Black filled bars depict the SHAM group, whereas white filled bars depict the HPC group. Statistical significance was determined by a repeated measures mixed model GLM followed by orthogonal contrasts. Data are expressed as least square mean ± standard error. ***p < 0.0001.
Fig. 5.
Effect of the HPC lesion on the percentage alternation broken down by block of 10 trials in the automated T-maze (left) and in the standard T-maze (right). Black filled circles depict the SHAM group, whereas empty circles depict the HPC group. Statistical significance was determined by a repeated measures mixed model GLM followed by orthogonal contrasts. Data are expressed as least square mean ± standard error. *p < 0.05.
Fig. 6.
Effect of the running order of the mazes on the percentage alternation. Black filled bars depict that the animals experience the maze first, whereas white filled bars depict that the maze was run second. Data are expressed as least square mean ± standard error. *p < 0.05.
3.3. Latency analysis in the automated maze
The first analysis tested whether correct or incorrect choices, and treatment group were associated with mean log latency to enter the maze. Neither group (REML mixed model: F1,14 = 0.05, p > 0.1), nor the group × response interaction (F1,14 = 2.20, p > 0.1) were significant. However, correct choices were on average associated with shorter mean log entry latencies than incorrect choices (response main effect: F1,14 = 9.50, p = 0.008).
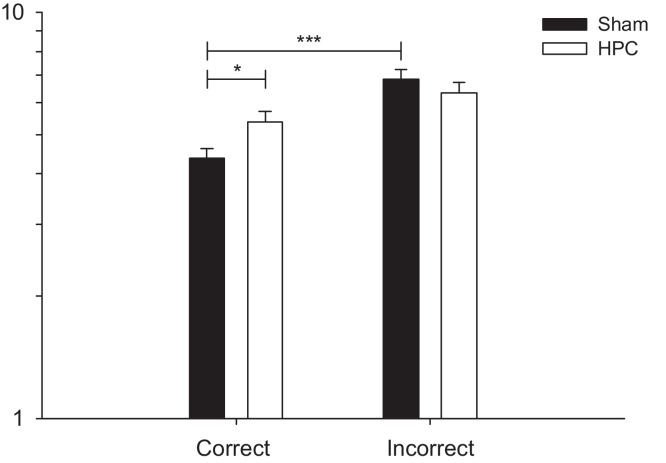
The next analysis was to determine whether correct or incorrect choices, and treatment group were associated with mean log latency to choose (choice latency). The group × response interaction (F1,14 = 6.16, p < 0.05) was significant (therefore no further testing of the marginal main effects was appropriate [21]). Planned orthogonal contrasts on the interaction showed that SHAM animals required a shorter time to choose a correct goal arm than an incorrect one (F1,14 = 30.65, p < 0.0001; Bonferroni critical alpha = 0.025). In contrast, the difference in choice latency between correct and incorrect responses was not significant for the HPC animals (F1,14 = 4.10, p = 0.06; Bonferroni critical alpha = 0.025, see Fig. 7).
Fig. 7.
Effect of the HPC lesion on the time to choose the goal arm depending on the response type. A correct response refers to trials when the mouse had alternated; while an incorrect response refers to trials when the mouse had not alternated. Black filled bars depict the SHAM group, whereas white filled bars depict the HPC group. Statistical significance was determined by a repeated measures mixed model GLM followed by orthogonal contrasts. Data are expressed as least square mean ± standard error. *p < 0.05; ***p < 0.0001.
To test whether log latencies were predictive of individual correct or incorrect choices a repeated measures logistic regression was performed. Confirming other analyses, on any given trial, SHAM mice were more likely to choose correctly (Likelihood Ratio χ2 = 49.48; p < 0.0001), and for both groups the chance of choosing correctly increased with the number of trials completed (LR χ2 = 11.99; p < 0.001). For both groups, the longer the mice took to enter the maze following the sample phase, the less likely they were to choose correctly (LR χ2 = 6.50; p = 0.01). Finally, the choice latency (the time between entering the maze and making a choice) affected the two groups differently (LR χ2 = 4.033; p < 0.05) – for HPC animals this latency had no effect on the chance of choosing correctly (χ2 = 2.073; p > 0.1), while for SHAM animals, the longer the choice latency, the less likely they were to choose correctly (χ2 = 13.52; p < 0.001).
4. Discussion
The aim of this study was to evaluate a novel automated maze task and to contrast performance in this maze directly to performance using a traditional, discrete-trial rewarded alternation task in a hand-run T-maze. The protocol used in the automated version was designed to be as analogous as possible to the traditional T-maze for the purposes of validation. To this aim, mice received either complete lesions to the HPC or were SHAM-operated control animals. These animals then underwent testing in both versions of the maze in a counterbalanced order. SHAM animals showed significantly higher performance than HPC-lesioned animals in both versions of the task. Thus, the automated version of the non-matching to place paradigm was shown to be hippocampus-sensitive.
4.1. Latencies
It has been reported that HPC-lesioned animals are particularly impaired in spatial non-match to place procedures when the interval between sample run and test/choice run is increased [10]. As the automated maze was subject-paced it was important to run further analyses to ensure that the deficit in the HPC group was not attributable to differences in the running speeds of the two cohorts of animals. This might be apparent in all the latencies as the mice ran in the maze. However, this pattern was not seen, and HPC mice were only slower specifically when making a correct choice. The test phase entrance latency was not different between the two groups, which is important for eliminating other explanations. This latency is influenced by several processes, including general incentive motivation, arousal and on-task attention. Overall, the longer this latency, the less likely mice were to choose correctly. This could presumably reflect the memory trace decay between the sample run and the point at which the mouse chooses which arm to enter on the choice/test run, or the confidence or certainty of the animal when making its choice. However, as there was no difference between HPC and SHAM animals, these confounds can be ruled out.
The relationship between choice latency and the likelihood of choosing correctly was lost in HPC animals. As mentioned above, the latency data revealed an additional subtlety – HPC and SHAM animals differed only in their latency to make correct choices and did not differ in latencies on incorrect choices. SHAM animals made correct choices faster than incorrect ones. Their correct choice latency was also shorter than the correct choice latency of the HPC mice. These data are potentially consistent with earlier suggestions that distinct mechanisms exist for making correct choices, versus avoiding incorrect ones [25]; and that these two mechanisms learn independently and at different rates. HPC animals made correct choices at a similar speed to incorrect choices. One explanation for this is that the HPC lesioned mice are unable to rapidly engage a correct response. This potentially contrasts with other effects of hippocampal manipulations which have been described in terms of an inability to withhold or inhibit inappropriate responses (e.g. approach/avoidance behaviours on ethological, unconditioned tests of anxiety such as the elevated plus maze) [26], passive avoidance [27], differential reinforcement of low rates of responding (DRL) operant task [28], spatial discrimination in the watermaze [29]. This suggests that hippocampal lesions could impair performance on cognitive tasks for different reasons, although it is important to be cautious and not to over-interpret these latency data.
4.2. Comparison of the automated maze to other hippocampus-sensitive paradigms
The exponential increase in the use of transgenic mice in cognitive research into disorders such Alzheimer's disease or schizophrenia has increased the need for higher throughput hippocampus-sensitive tasks. While the Morris Water maze is widely employed it is not necessarily the most appropriated test for mice [30], [31]. In contrast, the non-matching to place paradigm in the standard T-maze is well established as a behavioural task sensitive to hippocampal lesions [4], and to subtle manipulations of hippocampal function. Indeed, the effect size for complete hippocampal lesions in rats is much higher for T-maze rewarded alternation (2.01) than for watermaze performance (1.51 for % time in training quadrant during probe tests; 0.24 for path lengths during acquisition training trials [3]). However, the low-throughput nature of T-maze-based procedures has proved a limiting factor for its widespread use in, for example, drug discovery. The automated maze combines the hippocampal lesion sensitivity afforded by a T-maze-based task with the potential for high throughput required for screening transgenic mice and novel therapeutics.
Previous attempts to improve the specificity and throughput of rodent hippocampus-sensitive tasks have proved somewhat problematic. Operant or ‘Skinner’ boxes allow the development and running of automated paradigms where the animal can perform tasks without direct human intervention, typically via lever or touchscreen responses. However, automated spatial match/non match to position protocols in traditional operant chambers have been hampered by the fact that rodents often adopt mediating strategies to improve their performance [12], [13], [32], even when a supplementary task is included between the sample and test runs [33]. Indeed, the non-match to location paradigm has failed to show clear hippocampus-dependency in rats in operant chambers [14], [15], [33]. Maze-based continuous procedures have also been developed and deployed, such as continuous spontaneous alternation in a Y-maze and the continuous rewarded alternation task in a T-maze. Whilst the continuous Y-maze spontaneous alternation task has been shown to be hippocampus-sensitive [34], interpretation can be confounded by changes in locomotor activity and the total number of arm entries made by the animals, although it is possible to control for the number of arm entries in both the automated and standard mazes, and measure latencies in the former. Notably, however, animals can obtain a high alternation score in the continuous spontaneous alternation Y-maze task by adopting a consistent turn strategy (e.g. always turn left) which does not necessarily reflect their ability to remember which arms they have already visited. This is not possible in either the automated or standard T-maze. Furthermore, the continuous rewarded alternation T-maze paradigm is not hippocampus-sensitive unless delays are introduced to prevent mediating strategies (e.g. wall hugging) which could also mask the need for the mice to remember which arm they have just visited [10]. Therefore, the automated T-maze described in the present study represents a clear advance over these existing alternatives.
4.3. Stress and circadian rhythms
Performance levels in the automated maze were higher than in the standard maze for the SHAM and HPC lesioned groups. There are a number of possible explanations for this, including circadian differences or handling-induced stress effects on animals’ performance levels.
The standard maze is usually run during the daylight. Mice show an increase in global activity during the dark period of their circadian cycle presumably reflecting the fact that they are predominantly a nocturnal species [35]. Mice naturally sleep and fast during the day, and forage at night. Consequently, the time of the day at which cognitive and behavioural tasks are run, is likely to have an important impact on performance levels [35]. For example, it has been shown that mice perform extremely well on certain complex cognitive tasks when they are presented at dusk (when they are most hungry, and foraging is highly motivated) [37], although whether running during subjective night increases or decreases performance levels may depend on the nature of the task itself [35], [36]. However, this highlights one of the potential advantages of the automated maze: studies can easily be run at any time of the circadian cycle without the need for lengthy experimenter hours or for reverse light cycles to be employed. Furthermore, time of day can also have an important impact on the outcome of studies when comparing different experimental groups. For example, transgenic mice expressing the human amyloid precursor protein, a mouse model of amyloid pathology in Alzheimer's disease, showed a deficit in operant conditioning only when tested during the light phase but not during the dark phase [36].
Stress may also contribute to the lower performance levels on the hand-run, standard T-maze task. It is possible that the animals experienced a higher level of stress/arousal during the hand-tested standard alternation task as opposed to the automated maze. Handling can induce stress [8], and stress may impact an animals’ cognitive performance, including during T-maze rewarded alternation [9]. Therefore, another potential advantage of the automated maze is that the animals are not handled between trials. This may contribute to the overall higher level of accuracy and stability of performance across both groups of animals in the automated maze.
The control of stress is particularly important in the study of many cognitive disorders where the affective state of the animal models may be impacted, such as depression, Alzheimer's disease and schizophrenia. The introduction of a companion animal in the home cage compartment of the automated maze may also have reduced the possible impact of any social isolation on cognitive performance [38]. Stress may also account for the impact of prior testing history in the present study. Prior experience in the automated maze enhanced subsequent performance in the standard maze compared to experimentally naïve animals tested first in the standard maze. In contrast, performance in the automated maze was not affected by the presence or absence of prior maze experience. This stability of performance, regardless of prior experience, allows for reliable and reproducible data within or across laboratories with the use of automated maze procedures.
4.4. Conclusions
The automated maze confers many of the advantages of operant chambers, avoids the disadvantages of hand test maze-based procedures, or semi-automated mazes [39] while providing a robust and reliable test of hippocampus-sensitive short-term memory. A broader range of measures such as latencies can be accurately collected and analysed. Measurements are fully automated, and are not reliant on subjective measures such as judging the point at which the animal makes a choice. The maze is attached to a homecage hence the animals may be run remotely without the experimenter being present. This potentially allows animals to be run at any time during the 24 h of the day. Furthermore, a number of mazes can potentially be run simultaneously and over long periods. This provides a significant increase in throughput and a major advance over alternative HPC-sensitive maze-based tasks such as the standard T-maze, Morris watermaze or radial arm maze.
Acknowledgements
J.P.G. received a Purdue Research Trask Foundation Award to construct the automated T-maze. J.P.G. was employed at Purdue University for a portion of this study. Further details of an updated version of this apparatus can be acquired from Telos Discovery Systems (Lafayette, IN, USA; info@telosdiscoverysystems.com). D.M.B. was supported by the Wellcome Trust (Grant No. 087736).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Rawlins J.N., Olton D.S. The septo-hippocampal system and cognitive mapping. Behav Brain Res. 1982;5:331–358. doi: 10.1016/0166-4328(82)90039-0. [DOI] [PubMed] [Google Scholar]
- 2.Hock B.J., Jr., Bunsey M.D. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannerman D.M., Yee B.K., Good M.A., Heupel M.J., Iversen S.D., Rawlins J.N. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- 4.McHugh S.B., Niewoehner B., Rawlins J.N., Bannerman D.M. Dorsal hippocampal N-methyl-d-aspartate receptors underlie spatial working memory performance during non-matching to place testing on the T-maze. Behav Brain Res. 2008;186:41–47. doi: 10.1016/j.bbr.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman P.F., White G.L., Jones M.W., Cooper-Blacketer D., Marshall V.J., Irizarry M., Younkin L., Good M.A., Bliss T.V., Hyman B.T., Younkin S.G., Hsiao K.K. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 6.Honig W.K. Studies of working memory in the pigeon. In: Hulse S.H., Fowler H., Honig W.K., editors. Cognitive processes in animal behavior. Erlbaum; Hillsdale, NJ: 1978. pp. 211–248. [Google Scholar]
- 7.Sanderson D.J., Bannerman D.M. The role of habituation in hippocampus-dependent spatial working memory tasks: evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus. 2012;22:981–994. doi: 10.1002/hipo.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst J.L., West R.S. Taming anxiety in laboratory mice. Nat Methods. 2010;7:825–826. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 9.Lyon L., Burnet P.W., Kew J.N., Corti C., Rawlins J.N., Lane T., De F.B., Harrison P.J., Bannerman D.M. Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and cognition. Neuropsychopharmacology. 2011;36:2616–2628. doi: 10.1038/npp.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ainge J.A., van der Meer M.A., Langston R.F., Wood E.R. Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus. 2007;17:988–1002. doi: 10.1002/hipo.20301. [DOI] [PubMed] [Google Scholar]
- 11.Dunnett S.B., Rogers D.C., Jones G.H. Effects of nucleus basalis magnocellularis lesions in rats on delayed matching and non-matching to position tasks. Eur J Neurosci. 1989;1:395–406. doi: 10.1111/j.1460-9568.1989.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 12.Herremans A.H., Hijzen T.H., Welborn P.F., Olivier B., Slangen J.L. Effects of infusion of cholinergic drugs into the prefrontal cortex area on delayed matching to position performance in the rat. Brain Res. 1996;711:102–111. doi: 10.1016/0006-8993(95)01404-7. [DOI] [PubMed] [Google Scholar]
- 13.Chudasama Y., Muir J.L. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- 14.Sloan H.L., Dobrossy M., Dunnett S.B. Hippocampal lesions impair performance on a conditional delayed matching and non-matching to position task in the rat. Behav Brain Res. 2006;171:240–250. doi: 10.1016/j.bbr.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Sloan H.L., Good M., Dunnett S.B. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Gaskill B.N., Lucas J.R., Pajor E.A., Garner J.P. Little and often? Maintaining continued performance in an automated T-maze for mice. Behav Processes. 2011;86:272–278. doi: 10.1016/j.beproc.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Richter S.H., Garner J.P., Auer C., Kunert J., Wurbel H. Systematic variation improves reproducibility of animal experiments. Nat Methods. 2010;7:167–168. doi: 10.1038/nmeth0310-167. [DOI] [PubMed] [Google Scholar]
- 18.Richter S.H., Garner J.P., Wurbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat Methods. 2009;6:257–261. doi: 10.1038/nmeth.1312. [DOI] [PubMed] [Google Scholar]
- 19.Deacon R.M., Bannerman D.M., Kirby B.P., Croucher A., Rawlins J.N. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G., Franklin K. Academic Press; San Diego: 2001. The mouse brain in stereotoxic coordinates. [Google Scholar]
- 21.West M.J. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 22.West M.J., Gundersen H.J. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 23.Grafen A., Hails R. Oxford University Press; Oxford/New York: 2002. Modern statistics for the life sciences. [Google Scholar]
- 24.Newman J.A., Bergelson J., Grafen A. Blocking factors and hypothesis tests in ecology: is your statistics text wrong? Ecology. 1997;78(5):1312–1320. Ecology, 75, 1312–20. [Google Scholar]
- 25.Olton D.S. Behavioral and neuroanatomical differentiation of response-suppression and response-shift mechanisms in the rat. J Comp Physiol Psychol. 1972;78:450–456. doi: 10.1037/h0032372. [DOI] [PubMed] [Google Scholar]
- 26.Gray J.A., McNaughton N. Oxford University Press; Oxford: 2010. The neuropsychology of anxiety: an enquiry in to the functions of the septo-hippocampal system. [Google Scholar]
- 27.Lovely R.H., Grossen N.E., Moot S.A., Bauer R.H., Peterson J.J. Hippocampal lesions and inhibition of avoidance behavior. J Comp Physiol Psychol. 1971;77:345–352. doi: 10.1037/h0031651. [DOI] [PubMed] [Google Scholar]
- 28.Sinden J.D., Rawlins J.N., Gray J.A., Jarrard L.E. Selective cytotoxic lesions of the hippocampal formation and DRL performance in rats. Behav Neurosci. 1986;100:320–329. doi: 10.1037//0735-7044.100.3.320. [DOI] [PubMed] [Google Scholar]
- 29.Bannerman D.M., Bus T., Taylor A., Sanderson D.J., Schwarz I., Jensen V., Hvalby O., Rawlins J.N., Seeburg P.H., Sprengel R. Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2012;15:1153–1159. doi: 10.1038/nn.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav Brain Res. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 31.Brown R.E., Wong A.A. The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem. 2007;14:134–144. doi: 10.1101/lm.473907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herremans A.H., Hijzen T.H. The delayed-conditional-discrimination task improves measurement of working memory in rats. Neurosci Biobehav Rev. 1997;21:371–379. doi: 10.1016/s0149-7634(96)00015-2. [DOI] [PubMed] [Google Scholar]
- 33.Young H.L., Stevens A.A., Converse E., Mair R.G. A comparison of temporal decay in place memory tasks in rats (Rattus norvegicus) with lesions affecting thalamus, frontal cortex, or the hippocampal system. Behav Neurosci. 1996;110:1244–1260. doi: 10.1037//0735-7044.110.6.1244. [DOI] [PubMed] [Google Scholar]
- 34.Dillon G.M., Qu X., Marcus J.N., Dodart J.C. Excitotoxic lesions restricted to the dorsal CA1 field of the hippocampus impair spatial memory and extinction learning in C57BL/6 mice. Neurobiol Learn Mem. 2008;90:426–433. doi: 10.1016/j.nlm.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Hossain S.M., Wong B.K., Simpson E.M. The dark phase improves genetic discrimination for some high throughput mouse behavioral phenotyping. Genes Brain Behav. 2004;3:167–177. doi: 10.1111/j.1601-183x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- 36.Kiryk A., Mochol G., Filipkowski R.K., Wawrzyniak M., Lioudyno V., Knapska E., Gorkiewicz T., Balcerzyk M., Leski S., Leuven F.V., Lipp H.P., Wojcik D.K., Kaczmarek L. Cognitive abilities of Alzheimer's disease transgenic mice are modulated by social context and circadian rhythm. Curr Alzheimer Res. 2011;8:883–892. doi: 10.2174/156720511798192745. [DOI] [PubMed] [Google Scholar]
- 37.Garner J.P., Thogerson C.M., Wurbel H., Murray J.D., Mench J.A. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 2006;173:53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Olsson I.A.S., Westlund K. More than numbers matter: the effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl Anim Behav Sci. 2007;103:229–254. [Google Scholar]
- 39.Schaefers A.T., Winter Y. Rapid task acquisition of spatial-delayed alternation in an automated T-maze by mice. Behav Brain Res. 2011;225:56–62. doi: 10.1016/j.bbr.2011.06.032. [DOI] [PubMed] [Google Scholar]