Abstract
We performed an in vitro cell culture experiment to ascertain whether rifampin exhibits bactericidal effects against Orientia tsutsugamushi, the causative agent of scrub typhus. ECV304 cells were infected with the Boryong or AFSC-4 strain of O. tsutsugamushi and then, the cultures were maintained in media with increasing concentrations of rifampin, azithromycin, doxycycline, or chloramphenicol for 4 days. On day 5, the media were replaced with fresh antibiotic-free medium and the cultures were maintained until day 28. On days 5, 13, and 28, immunofluorescence (IF) staining of O. tsutsugamushi was performed. IF staining on days 13 and 28 revealed increasing numbers of IF-positive foci in all cultures, even in cultures initially exposed to the highest concentration of rifampin (80 µg/mL), azithromycin (80 µg/mL), doxycycline (20 µg/mL), or chloramphenicol (100 µg/mL). The present study reveals that rifampin has no bactericidal effect against O. tsutsugamushi as observed for azithromycin, doxycycline, and chloramphenicol. A subpopulation of the bacteria that are not killed by high concentrations of the antibiotics may explain the persistence of O. tsutsugamushi in humans even after complete recovery from scrub typhus with antibiotic therapy.
Graphical Abstract
Keywords: Antibiotic Susceptibility Test; Azithromycin; Drug Resistance, Bacterial; Orientia tsutsugamushi; Rifampin; Scrub Typhus
INTRODUCTION
Scrub typhus is an easily treatable illness if antibiotics such as chloramphenicol, doxycycline, azithromycin, or rifampin are used properly in therapy. Relapse occurs several days after discontinuation of antibiotic therapy in certain patients, when the antibiotic treatment was initiated early in the course of the illness, and in those with short treatment duration (1-3). The reason of such a relapse is that Orientia tsutsugamushi, the causative agent of scrub typhus, persists in human body after recovery from scrub typhus (4), while chloramphenicol or tetracycline has only bacteriostatic effects, as documented in mice (3,5). In contrast to chloramphenicol and tetracycline, rifampin sometimes exhibits bactericidal effects on certain bacteria (6), and no relapse was observed in a clinical trial employing rifampin (7). Thus, rifampin may possess bactericidal effects against O. tsutsugamushi. In addition, in northern Thailand, where doxycycline-insensitive strains prevail, rifampin showed a more rapid response against scrub typhus (8).
There are several in vitro studies on antibiotic susceptibility against O. tsutsugamushi (9-12). However, these studies revealed only minimal inhibitory concentrations, and none of the studies evaluated the bactericidal effects of these antibiotics. Antibiotics with bactericidal properties against O. tsutsugamushi will prevent its persistence, and therefore, they may be used preferentially for the treatment of scrub typhus patients. Although macrolide antibiotics such as azithromycin exert bacteriostatic effects on conventional bacteria, there are no studies on the bacteriostatic effects of azithromycin against O. tsutsugamushi. In the present study, we performed an in vitro cell culture experiment to measure whether rifampin has bactericidal effects against O. tsutsugamushi. In addition, we assessed the bacteriostatic effects of azithromycin against O. tsutsugamushi.
MATERIALS AND METHODS
Infection of ECV304 cells with O. tsutsugamushi
In this study, we used the Boryong and AFSC-4 strains of O. tsutsugamushi. The former strain is the most prevalent variety in Korea and is susceptible to doxycycline. The latter, a strain isolated in Thailand and reported to be insensitive to doxycycline, was kindly provided by Dr. Daniel Strickman, Naval Medical Research Institute, Bethesda, MD, USA (13). Stock solutions of each strain were inoculated onto monolayers of ECV304 cells (14) seeded in culture flasks (75 cm2). When the infected ECV304 cells showed maximum cytopathic effects, the degree of infection of ECV304 cells was assessed by immunofluorescence (IF) staining to be approximately equal in each experiment. The cells were disrupted with glass beads, and centrifuged at 350 g for 5 min. The resultant supernatant was divided equally into 7 aliquots. Each aliquot was inoculated onto monolayers of ECV304 cells grown in a 6-well culture plate and incubated for 4 h. At the end of the initial incubation period, the inocula were replaced with fresh media containing rifampin (Sigma Co., St. Louis, MO, USA) at concentrations of 0, 0.1, 1, 10, 20, 40, and 80 µg/mL; azithromycin (Sigma) at concentrations of 0, 0.1, 1, 10, 20, 40, and 80 µg/mL; doxycycline (Sigma) at concentrations of 0, 0.1, 1, 10, 20, 40, and 80 µg/mL; or chloramphenicol (Sigma) at concentrations of 0, 2, 20, 40, 60, 80, and 100 µg/mL, and incubated for 4 days. Since doxycycline concentrations of 40 and 80 µg/mL were toxic and resulted in the death of ECV304 cells, further experiments were performed using doxycycline at 20 µg/mL or less. The cells were re-incubated in fresh antibiotic-free medium, which was changed every 3-4 days, for 23 days. Uninfected monolayers of ECV304 cells were used as controls.
In addition, on day 5, a portion of the infected ECV304 cells grown in cultures containing the highest concentrations of the antibiotics was scraped with a glass scraper; half of the scraped cells were used for IF staining, and the remaining half was subcultured to fresh ECV304 cells.
Subculture
The portion of the above scraped cells was disrupted with glass beads and centrifuged at 350 g for 5 min. The resultant supernatants were passaged to fresh ECV304 cells, which were maintained in antibiotic-free medium until day 47, following which IF staining was performed as described below.
IF staining
On days 5, 13, 28, and 47, IF staining was performed. Scraped ECV304 cells infected with the Boryong or AFSC-4 strain were fixed with acetone. The infected cells were then stained with FS15 (15), a monoclonal antibody that reacts with the linear epitope on the 56 kDa major outer membrane protein of the bacterium, in phosphate-buffered saline (PBS) for 30 min at 37℃. Then they were rinsed briefly with PBS Tween-20 (PBST), treated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Jackson ImmunoResearch, West Grove, PA, USA) for 30 min in a moist chamber, and finally rinsed 3 times with PBST. To clearly define O. tsutsugamushi, the host cells were counter-stained with 0.003% Evans blue in PBS. After the slides were rinsed, they were placed in mounting medium (Vector Laboratories, Burlingame, CA, USA) and imaged after examination at ×200 magnification under a fluorescence microscope (Zeiss, Germany) with a confocal laser scanning system (Bio-Rad, Hercules, CA, USA).
RESULTS
Survival of O. tsutsugamushi in antibiotic-containing medium (day 5)
On day 5, IF-positive foci were observed in Boryong strain-infected ECV304 cells at the following concentrations: rifampin up to 80 µg/mL (Fig. 1), azithromycin up to 20 µg/mL (Fig. 2), doxycycline up to 10 µg/mL (Fig. 3), and chloramphenicol up to 40 µg/mL (Fig. 4). Only 1 or 2 foci were seen in 20 µg/mL azithromycin- or 10 µg/mL doxycycline-treated cells, whereas several IF-positive foci were evident in 80 µg/mL rifampin- or 40 µg/mL chloramphenicol-treated cells.
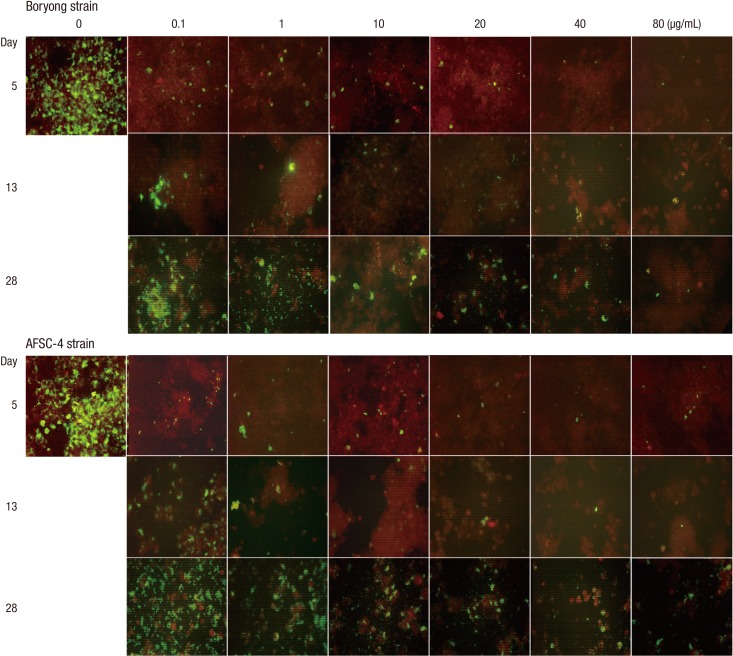
Fig. 1.
Serial photographs of immunofluorescence staining of ECV304 cells infected with Orientia tsutsugamushi (×200). Infected ECV304 cells were incubated in medium containing rifampin of increasing concentrations for 4 days. Then, the inocula were replaced with antibiotic-free medium and the culture was continued until day 28. The highest concentration of rifampin at which IF-positive foci are observed is 80 µg/mL on days 5, 13, and 28 in cultures infected by the Boryong or AFSC-4 strain. Cultures in medium not containing rifampin showed cell death due to overgrowth of O. tsutsugamushi before day 13.
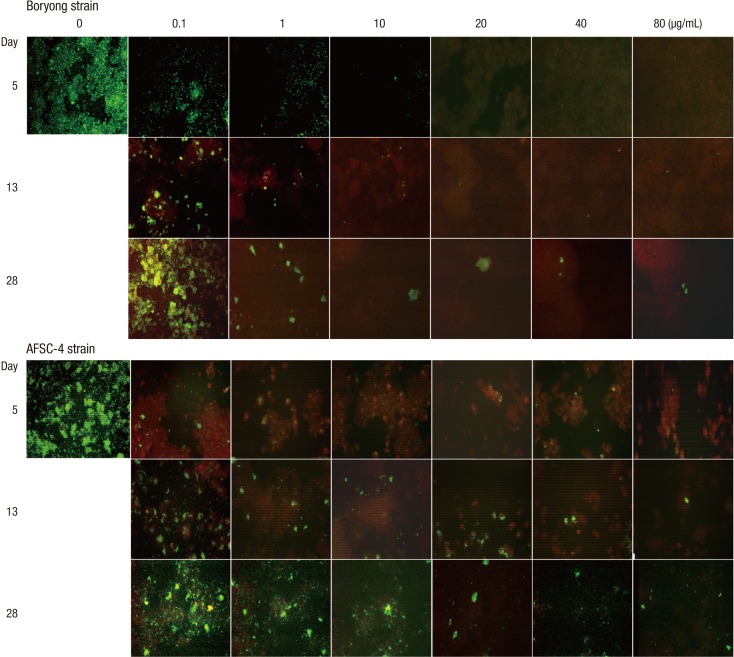
Fig. 2.
Serial photographs of infected ECV304 cells in medium containing azithromycin (see Fig. 1 for details) (×200). In the Boryong strain-infected cells, the highest concentrations of azithromycin at which IF-positive foci are observed are 10, 80, and 80 µg/mL on days 5, 13, and 28, respectively. In the AFSC-4 strain-infected cells, the highest concentration is 80 µg/mL on days 5, 13, and 28.
Fig. 3.
Serial photographs of infected ECV304 cells in medium containing doxycycline (see Fig. 1 for details) (×200). Doxycycline at 40 and 80 µg/mL was toxic, resulting in death of ECV304 cells. In the Boryong strain-infected cells, the highest doxycycline concentrations at which IF-positive foci are observed are 10, 20, and 20 µg/mL on days 5, 13, and 28, respectively. In the AFSC-4 strain-infected cells, the highest concentration is 20 µg/mL on days 5, 13, and 28. Cultures infected with the AFSC-4 strain in medium containing 0.1 µg/mL doxycycline showed cell death because of overgrowth of O. tsutsugamushi on day 20.
Fig. 4.
Serial photographs of infected ECV304 cells in medium containing chloramphenicol (see Fig. 1 for details) (×200). In the Boryong strain-infected cells, the highest concentrations of chloramphenicol at which IF-positive foci are observed are 40, 100, and 100 µg/mL on days 5, 13, and 28, respectively. In the AFSC-4 strain-infected cells, the highest concentration is 100 µg/mL on days 5, 13, and 28.
In AFSC-4 strain-infected cells, IF-positive foci were seen at the following concentrations: rifampin up to 80 µg/mL, azithromycin up to 80 µg/mL, doxycycline up to 20 µg/mL, and chloramphenicol up to 100 µg/mL (Fig. 1-4). In cultures containing 80 µg/mL of rifampin, the number of IF-positive foci was larger in AFSC-4 strain-infected cells when compared with those in Boryong strain-infected cells. Uninfected ECV304 cells did not show any IF-positive foci.
Regrowth of O. tsutsugamushi in antibiotic-free medium (days 13 and 28)
The incubation of Boryong strain-infected cells in antibiotic-free medium until day 28 resulted in increasing numbers of IF-positive foci, even in cultures containing azithromycin (40, 80 µg/mL), doxycycline (20 µg/mL), and chloramphenicol (60, 80, 100 µg/mL) that had not initially shown any IF-positive foci. In azithromycin-treated cells, only 1-2 clonal growths were observed in cells treated with a concentration of 10 µg/mL or more, whereas several clonal growths in rifampin (40 µg/mL or more), doxycycline (10 µg/mL or more), or chloramphenicol (60 µg/mL or more). Similar results were observed in AFSC-4 strain-infected cells, although the degree of IF-positivity was greater than observed for Boryong strain-infected cells.
Growth of O. tsutsugamushi in subculture (day 47)
The subcultures of ECV304 cells infected with Boryong or AFSC-4 strain-infected cells, initially incubated at the highest concentrations of the antibiotics (80 µg/mL of rifampin, 80 µg/mL of azithromycin, 20 µg/mL of doxycycline, and 100 µg/mL of chloramphenicol), showed growth of O. tsutsugamushi, regardless of the absence of IF-positive focus in the initial cultures.
DISCUSSION
It has not been established how to measure bactericidal effects of antibiotics against O. tsutsugamushi as it requires viable host cells for its growth and partly due to the lack of a sensitive and quantitative method for growth measurement. In this study, we measured them by exposing O. tsutsugamushi to high concentrations of antibiotics that are not clinically tolerable and therefore cannot be achieved, and then continuing these cultures in medium not containing antibiotics, analogous to the experiments in mice performed previously (3,5). Compared with the mouse experiments, the present in vitro study revealed changes in the magnitude of O. tsutsugamushi growth as a function of time after antibiotic treatment. Our results show that azithromycin, doxycycline, and chloramphenicol showed IF negativity in the initial cultures containing the highest concentrations of each of the antibiotics and then IF positivity in the subsequent cultures and subcultures, suggesting that these antibiotics are bacteriostatic. In contrast, rifampin even at its highest concentration could not achieve IF negativity in the initial culture. The expected peak serum concentration of rifampin after administration of 600 mg is 7 µg/mL (16); however, several IF-positive foci were observed in ECV304 cells treated with rifampin at 10 µg/mL or more in our experiments. Therefore, this antibiotic cannot eradicate the bacterium in the endothelial cells, which is the main cellular target in O. tsutsugamushi infection. Although all 4 antibiotics tested failed to eliminate the bacterium, they successfully reduced the number of O. tsutsugamushi at the expected peak serum concentrations (0.09-0.44 µg/mL after treatment with 500 mg of azithromycin, 1.5-2.1 µg/mL after treatment with 100 mg of doxycycline, and 11-18 µg/mL after treatment with 1.0 g of chloramphenicol) (16) on day 5. These results indicate that these antibiotics are bacteriostatic and are effective in the treatment of scrub typhus. Together, these results support our previous clinical observation that viable O. tsutsugamushi persist in humans, despite complete recovery from scrub typhus with antibiotic treatment (4).
Interestingly, the AFSC-4 strain showed relatively low susceptibility to rifampin, azithromycin, doxycycline, and chloramphenicol. In cultures containing rifampin or azithromycin, the AFSC-4 strain showed a larger number of IF-positive foci than the Boryong strain, similar to the findings in cultures treated with doxycycline or chloramphenicol. A clinical trial comparing rifampin and doxycycline for the treatment of scrub typhus in Thailand showed that the fever clearance time was 52 hr in the doxycycline-treated group and 28 hr in the rifampin (600 mg/day)-treated group (7), which suggested that rifampin was more efficient against doxycycline-insensitive strains. However, considering our data, the superior efficacy of rifampin over doxycycline is its intrinsic property against O. tsutsugamushi, probably due to its low minimal inhibitory concentrations and high intra-endothelial concentrations (17), rather than its efficacy in the doxycycline-insensitive strains. However, the possibility of lower sensitivity of the AFSC-4 strain to doxycycline compared to rifampin could not be excluded with the present qualitative method.
The pattern of growth on day 28 in Boryong strain-infected cells was somewhat different between azithromycin and the other antibiotics. Azithromycin showed very less clonal growth at a relatively lower concentration (10 µg/mL), whereas such effects were observed at rather higher concentrations of rifampin (80 µg/mL) or chloramphenicol (80 µg/mL or more). If this concentration of azithromycin is clinically achievable, azithromycin may exhibit a prolonged post-antibiotic effect against O. tsutsugamushi, resulting in fewer early relapses than rifampin or chloramphenicol. It is speculated that early relapse occurs before the development of protective immunity, when the bacterium begins to grow after cessation of antibiotic therapy and then reaches a hypothetic threshold value at which the fever develops (18). Therefore, it is important to consider the factors related to immunity to avoid misinterpretation of relapse rates reported in several clinical studies. For example, as shown in our previous study (4), a relapse of scrub typhus was observed in a patient (case 1), who had been treated with a steroid that may impair the immunity, even after 1 week of azithromycin treatment. Because there are no established methods to measure immunity against O. tsutsugamushi, a plausible option may be to quantify bacteria in blood at the end of the antibiotic therapy.
The present study revealed that a small fraction of the bacterial population was not killed by even higher concentrations of the antibiotics. Although the mechanism of antibiotic resistance is unknown, the resistance observed in our study is different from that reported by Watt et al. (8). This resistance by persistent population was also observed in the Boryong strain, which is known to be susceptible to antibiotics and is not associated with delayed responses to antibiotics (18). Reports about the gyrA gene-associated resistance to fluoroquinolones (20, 21) are also not relevant to our observation. Since this subpopulation showed resistance to all 4 antibiotics of different classes, the resistance may be mediated by the mechanism common to a wide range of antibiotics, such as persistence, indifference, or heterotypic resistance (22, 23). These clones may be the main cause of persistence of O. tsutsugamushi in patients with scrub typhus who were treated with antibiotics; therefore, further studies are required to elucidate the mechanism of the resistance observed in the present study.
There are difficulties in presenting the results as figures. Since O. tsutsugamushi grows in aggregates, there are some variations between microscopic fields. Further, detachment of cells from the culture plates and plating onto IF slides make the cells distorted or overlapped yielding somewhat unclear findings. Faint immunofluorescent spots were observed in a few fields and even such weak positive signals were considered positive for analyses. Despite these limitations, our results clarify that these antibiotics are not bactericidal against O. tsutsugamushi.
Footnotes
This work was supported by an Inha University Research Grant.
Authors have no conflicts of interest to disclose.
References
- 1.Smadel JE, Bailey CA, Diercks FH. Chloramphenicol in the chemoprophylaxis of scrub typhus: IV. relapses of scrub typhus in treated volunteers and their prevention. Am J Epidemiol. 1950;51:229–241. [Google Scholar]
- 2.Sheehy TW, Hazlett D, Turk RE. Scrub typhus: a comparison of chloramphenicol and tetracycline in its treatment. Arch Intern Med. 1973;132:77–80. doi: 10.1001/archinte.132.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Smadel JE, Jackson EB, Ley HL., Jr Terramycin as a rickettsiostatic agent and its usefulness in patients with scrub typhus. Ann N Y Acad Sci. 1950;53:375–384. doi: 10.1111/j.1749-6632.1950.tb42172.x. [DOI] [PubMed] [Google Scholar]
- 4.Chung MH, Lee JS, Baek JH, Kim M, Kang JS. Persistence of Orientia tsutsugamushi in humans. J Korean Med Sci. 2012;27:231–235. doi: 10.3346/jkms.2012.27.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smadel JE, Jackson EB, Cruise AB. Chloromycetin in experimental rickettsial infections. J Immunol. 1949;62:49–65. [PubMed] [Google Scholar]
- 6.Finberg RW, Moellering RC, Tally FP, Craig WA, Pankey GA, Dellinger EP, West MA, Joshi M, Linden PK, Rolston KV, et al. The importance of bactericidal drugs: future directions in infectious disease. Clin Infect Dis. 2004;39:1314–1320. doi: 10.1086/425009. [DOI] [PubMed] [Google Scholar]
- 7.Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet. 2000;356:1057–1061. doi: 10.1016/S0140-6736(00)02728-8. [DOI] [PubMed] [Google Scholar]
- 8.Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P, Bhodhidatta D, Corcoran KD, Dasch GA, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348:86–89. doi: 10.1016/s0140-6736(96)02501-9. [DOI] [PubMed] [Google Scholar]
- 9.Miyamura S, Sato N, Tamura A. In vitro susceptibility of recent clinical isolates of Rickettsia tsutsugamushi to chemotherapeutic agents. Kansenshogaku Zasshi. 1985;59:486–488. doi: 10.11150/kansenshogakuzasshi1970.59.486. [DOI] [PubMed] [Google Scholar]
- 10.Miyamura S, Ohta T, Tamura A. Comparison of in vitro susceptibilities of Rickettsia prowazekii, R. rickettsii, R. sibirica and R. tsutsugamushi to antimicrobial agents. Nihon Saikingaku Zasshi. 1989;44:717–721. doi: 10.3412/jsb.44.717. [DOI] [PubMed] [Google Scholar]
- 11.Urakami H, Tamura A, Miyamura S, Yamamoto S, Kawabata N. Susceptibilities of recent clinical isolates of Rickettsia tsutsugamushi to chemotherapeutic agents in vitro. Kansenshogaku Zasshi. 1988;62:931–937. doi: 10.11150/kansenshogakuzasshi1970.62.931. [DOI] [PubMed] [Google Scholar]
- 12.Kim ES, Kim MK, Lee HM, Chung MH, Lee JS, Park JE, Kang JS. In vitro antibiotic susceptibility of Orientia tsutsugamushi strain Boryong measured by flow cytometry. Infect Chemother. 2008;40:212–217. [Google Scholar]
- 13.Strickman D, Sheer T, Salata K, Hershey J, Dasch G, Kelly D, Kuschner R. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob Agents Chemother. 1995;39:2406–2410. doi: 10.1128/aac.39.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown J, Reading SJ, Jones S, Fitchett CJ, Howl J, Martin A, Longland CL, Michelangeli F, Dubrova YE, Brown CA. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab Invest. 2000;80:37–45. doi: 10.1038/labinvest.3780006. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Kim MK, Kang JS. Improved antibiotic susceptibility test of Orientia tsutsugamushi by flow cytometry using monoclonal antibody. J Korean Med Sci. 2007;22:1–6. doi: 10.3346/jkms.2007.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amsden GW. Tables of antimicrobial agent pharmacology. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 635–700. [Google Scholar]
- 17.Darouiche RO, Hamill RJ. Antibiotic penetration of and bactericidal activity within endothelial cells. Antimicrob Agents Chemother. 1994;38:1059–1064. doi: 10.1128/aac.38.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smadel JE. Influence of antibiotics on immunologic responses in scrub typhus. Am J Med. 1954;17:246–258. doi: 10.1016/0002-9343(54)90262-4. [DOI] [PubMed] [Google Scholar]
- 19.Kim ES, Kim MK, Lee HM, Kil SH, Chung MH, Lee JS, Kang JS. Doxycycline resistance in Orientia tsutsugamushi isolated from Korean patients. Infect Chemother. 2008;40:259–265. [Google Scholar]
- 20.Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, Phetsouvanh R, Raoult D, Rolain JM. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents. 2010;35:338–341. doi: 10.1016/j.ijantimicag.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang HC, Choi SM, Jang MO, Ahn JH, Kim UJ, Kang SJ, Shin JH, Choy HE, Jung SI, Park KH. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667–671. doi: 10.3346/jkms.2013.28.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin BR, Rozen DE. Non-inherited antibiotic resistance. Nat Rev Microbiol. 2006;4:556–562. doi: 10.1038/nrmicro1445. [DOI] [PubMed] [Google Scholar]
- 23.Suchland RJ, Geisler WM, Stamm WE. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob Agents Chemother. 2003;47:636–642. doi: 10.1128/AAC.47.2.636-642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]