Abstract
Vitamin D deficiency, prevalent in 30–50% of adults in developed countries, is largely due to inadequate cutaneous production that results from decreased exposure to sunlight, and to a lesser degree from low dietary intake of vitamin D. Serum levels of 25-hydroxyvitamin D (25-OH D) <20 ng/mL indicate vitamin D deficiency and levels >30 ng/mL are considered optimal. While the endocrine functions of vitamin D related to bone metabolism and mineral ion homoeostasis have been extensively studied, robust epidemiological evidence also suggests a close association between vitamin D deficiency and cardiovascular morbidity and mortality. Experimental studies have demonstrated novel actions of vitamin D metabolites on cardiomyocytes, and endothelial and vascular smooth muscle cells. Low 25-OH D levels are associated with left ventricular hypertrophy, vascular dysfunction, and renin–angiotensin system activation. Despite a large body of experimental, cross-sectional, and prospective evidence implicating vitamin D deficiency in the pathogenesis of cardiovascular disease, a causal relationship remains to be established. Moreover, the cardiovascular benefits of normalizing 25-OH D levels in those without renal disease or hyperparathyroidism have not been established, and questions of an epiphenomenon where vitamin D status merely reflects a classic risk burden have been raised. Randomized trials of vitamin D replacement employing cardiovascular endpoints will provide much needed evidence for determining its role in cardiovascular protection.
Keywords: Vitamin D, Vascular risk factors, Cardiovascular disease
Historical perspective
Identification of essential dietary nutrients that humans cannot synthesize started in the eighteenth century and led to the discovery of common diseases such as scurvy, beriberi, and rickets that were subsequently successfully treated by altering dietary intake. The fourth identified essential factor—referred to as ‘‘vitamin D’’ – was derived from cod liver oil and shown to be an effective cure for rickets. While vitamin D is found in foods, its name is a misnomer given that human skin can synthesize sufficient amounts with adequate exposure to sunlight.
Biochemistry and photobiology
Vitamin D is a group of fat-soluble molecules similar to steroids but with a ‘broken’’ ring that are referred to as secosteroids (seco- from Greek: ‘‘to cut’’) (Figure 1). Several forms of vitamin D exist; cholecalciferol (or vitamin D3) is synthesized in response to ultraviolet (UV) irradiation of the skin resulting in the photochemical cleavage of 7-dehydrocholesterol, a precursor of cholesterol in the skin. A second form of vitamin D, ergocalciferol (or vitamin D2) is produced by irradiation of ergosterol, a membrane sterol found in the Ergot fungus. Dietary sources of vitamin D include fish oils (D3), egg yolks (D3), and mushrooms (D2) as well as artificially fortified cereals and dairy products (D2 or D3).
Figure 1.
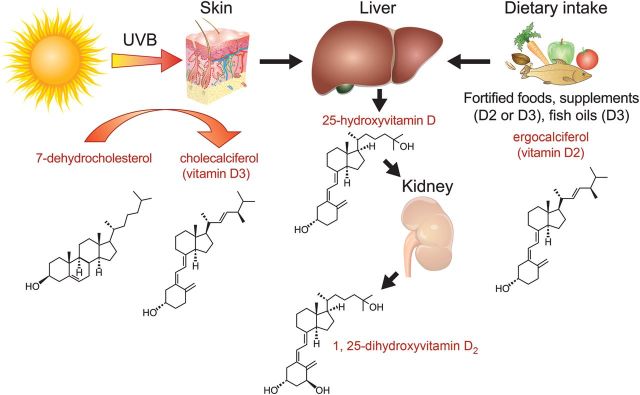
Vitamin D synthesis and metabolism. Vitamin D is photosynthesized in the skin and is also acquired by dietary intake. Two hydroxylation steps in the liver and the kidney are required for vitamin D activation, forming 1, 25-dihydroxyvitamin D. UVB, ultraviolet radiation in B-wavelength region (320–290 nm).
Energy received from the sun at UV wavelengths is most efficient in producing skin erythaema and hence cleavage of 7-dehydrocholesterol. However, the amount of energy that reaches the earth's surface is highly variable and is dependent on seasonal changes, distance from the equator and altitude, as well as the degree of ambient pollution and cloud cover. The ability to convert 7-dehydrocholesterol into vitamin D in the skin also decreases with age and with increasing skin pigmentation or sunscreen use.
Whether it is derived from the diet or synthesized cutaneously, vitamin D undergoes two successive hydroxylation steps. The first step occurs in the liver by mitochondrial and microsomal enzymes yielding 25-hydroxyvitamin D (25-OH D), the major circulating form of vitamin D. Less than 0.05% of 25-OH D is free in the circulation and the rest is bound to serum proteins with a half-life of 2 to 3 weeks. The second hydroxylation step occurs in the kidney by 1-α-hydroxylase, a tightly regulated enzyme found in the proximal convoluted tubule cells, that produces the hormonal form 1, 25-OH D2 or calcitriol. Although many cell types express 1-α-hydroxylase (e.g. cardiomyocytes, endothelial cells, and macrophages), renal 1-α-hydroxylation is the major contributor of circulating calcitriol under normal conditions.
The activity of renal 1-α-hydroxylase, unlike that produced by macrophages and other cell types, is under close modulation by a hormonal control loop that keeps calcitriol levels within a narrow range and maintains eucalcaemia. This dynamic process, in addition to calcitriol's short half-life and narrow physiological range, even with decreased cutaneous synthesis or nutritional intake of vitamin D, make it unsuitable for clinical testing to define vitamin D status. This is in contrast to 25-OH D which has a longer half-life (weeks vs. several hours for calcitriol). Nevertheless, calcitriol levels are measured in hypocalcaemic or hyperparathyroid patients and in those with a decreased renal mass (i.e. kidney disease) and reflect activity of renal 1-α hydroxylase.
Biologic effects of vitamin D result largely from its binding to the nuclear steroid hormone vitamin D receptor (VDR), which is found in virtually all tissues and is also closely related to the thyroid, retinoid, and peroxisome proliferator-activator receptors. Although all vitamin D metabolites bind the VDR, most biological effects are likely mediated by calcitriol because of its greater receptor affinity.
Vitamin D receptor molecular activity
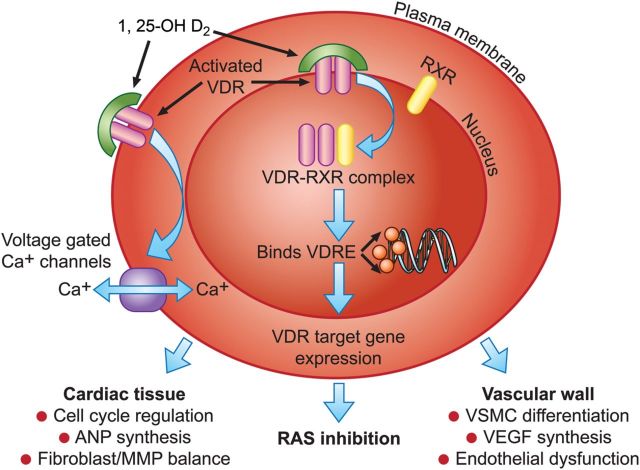
Elucidation of the molecular structure of VDR and recognition of its activity in non-classic tissues underscored the potential widespread physiological roles of vitamin D, and has prompted exploration of its extra-skeletal effects (Figure 2).1,2 Most well-known molecular effects of liganded VDR arise from its dimerization with the retinoid X receptor (RXR), forming a joint complex that serves as a transcription factor. This in turn binds to specific promoter regions referred to as vitamin D response elements (VDREs), modulating the expression of a multitude of genes.3 As with other hormonal nuclear receptors, a number of tissue- and target gene-specific co-activators and co-repressors dictate the milieu for liganded VDR activity.4 Non-nuclear, membrane-bound VDRs have been detected in several cells including cardiomyocytes, and may contribute to non-genomic functions. Nevertheless, these activities appear to be modulated by nuclear VDR activation and include controlling cation traffic across the cell membrane and regulating voltage-gated calcium channels.5,6
Figure 2.
Mechanisms by which vitamin D deficiency may confer cardiovascular risk. Potential effects of vitamin D metabolism on the cardiovascular system are divergent, but share common initial steps of nuclear and plasma membrane VDR activation. VDR, vitamin D receptor; 1, 25-OH D2, 1, 25-dihydroxyvitamin D; RXR, retinoid-X receptor; Ca+, calcium cation; ANP, atrial natriuretic peptide; MMP, matrix metalloproteinases; VDRE, vitamin D response elements (promoter region of target genes); RAS, renin–angiotensin system; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cells.
Vitamin D deficiency
Vitamin D metabolic pathways have been most extensively studied in areas related to its anti-rachitic effects and mineral ion homoeostasis, including the hormonal control loop involving serum calcium levels, parathyroid hormone, induction of 1-α hydroxylase, synthesis of 1, 25-OH D2, and the resulting alterations in intestinal and renal handling of mineral ions, as well as its effects on osteoblasts. Classically, clinical effects of vitamin D deficiency are considered to be the result of reduced intestinal absorption of calcium that in turn raises parathyroid hormone levels, and is accompanied by accelerated bone de-mineralization to maintain serum calcium concentration. Following chronic, severe vitamin D deficiency, frank hypocalcaemia ensues, but patients rarely present with acute symptoms (e.g. tingling or tetany), as this usually develops over an extended period of time. Rather, the most common presenting symptoms of vitamin D deficiency include vague, local, or diffuse musculoskeletal aches and pains.
Vitamin D and risk of morbid disorders
Increased prevalence of several metabolic, autoimmune, and malignant disorders has been long noted in geographic locales with increasing latitude from the equator.7 In addition, the incidence of many morbid events increases during periods of decreased sunlight exposure (i.e. winter season), suggesting that the lack of vitamin D photosynthesis is a potential underlying mechanism.8–10 Epidemiological studies then demonstrated increased risks of chronic morbid disorders with lower levels of serum 25-OH D.11 More recently, vitamin D deficiency was implicated as an independent risk factor for incident cardiovascular disease and overall mortality in the general population.12,13
Cardiovascular biology and vitamin D
Both VDR and 1-α-hydroxylase that convert vitamin D into the hormonal 1, 25-OH D2 (calcitriol) form are actively expressed in cardiovascular tissues, including cardiomyocytes, endothelial, and vascular smooth muscle cells.14,15
Cardiomyocytes
In ventricular myocytes isolated from neonatal rat hearts, calcitriol regulated the number of cells entering the synthesis phase of the cell cycle, therefore affecting subsequent maturation and differentiation.16 In addition, murine models lacking VDR exhibit increased ventricular mass, higher levels of atrial natriuretic peptide, in addition to cardiac metalloproteinases and fibroblast dyshomoeostasis that precipitate a fibrotic extra-cellular matrix. These changes eventually lead to ventricular dilation and impaired electromechanical coupling.17–19 Moreover, rats fed a vitamin D-deficient diet show higher systolic pressure and serum creatine phosphokinase that parallel decreases in calcium levels. All these effects are readily corrected by vitamin D analogues.20,21 Finally, vitamin D analogues attenuated the left ventricular hypertrophy associated with increased sodium load in salt sensitive rats via modulation of several protein kinase pathways.22,23
Vascular endothelial cells
Endothelial cells express VDR and its activation affects the development of immature cells, partly by modulating response elements in the vascular endothelial growth factor (VEGF) promoter.23 While VDR is up-regulated under stress in endothelial cells, active vitamin D analogues decrease cytokine induced expression of adhesion molecules and protect against advanced glycation products.24–27 Furthermore, vitamin D metabolites reduced endothelium-dependent vascular smooth muscle contractions and vascular tone in hypertensive models, an effect mediated by affecting calcium influx across endothelial cells.27
Renin–angiotensin–aldosterone system
Renin expression was shown to be highly deregulated in VDR knockout murine models, despite maintenance of a normal electrolyte balance. Tonic scission of angiotensinogen sharply increased angiotensin II levels and resulted in hypertensive heart disease. Similar abnormalities were observed with defective calcitriol synthesis in a wild-type model, independent of calcium metabolism, which were normalized following calcitriol administration.28 These findings suggest an active role for vitamin D in the pathogenesis of cardiovascular disorders and parallel results from clinical investigations.
Cardiovascular diseases and vitamin D
Although evidence confirms a robust association between vitamin D status and several cardiovascular disorders, a causal relationship remains to be fully elucidated. Mechanisms by which vitamin D deficiency may confer increased cardiovascular risk include the development of electrolyte imbalances, pancreatic β-cell dysfunction, and RAS activation.29 In addition, disrupted adaptive immune responses with severe vitamin D deficiency result in an inflammatory milieu that promotes vascular dysfunction and insulin resistance.30 Indeed, most epidemiological studies have reported an inverse relationship between vitamin D status and the prevalence of established cardiovascular risk factors such as age, hypertension, diabetes, and hypertriglyceridaemia (Table 1). Serum 25-OH D levels are also lower in women, in obesity, and in those with decreased physical activity.11,31
Table 1.
Key population study findings that relate vitamin D deficiency with cardiovascular disease and its risk factors
| Study | n | Findings |
|---|---|---|
| Third National Health and Nutrition Examination | +13 000 | ↑↑ Body mass index, blood pressure, lipids and hyperglycaemia in subjects with low 25-OH D levels |
| 1958 British Birth Cohort | 6810 | |
| German National Health Survey and Examination | 4093 | |
| Health Professional's Follow-up Study | 613 | 25-OH D <15 vs. ≥30 ng/mL with three-fold ↑↑ risk for future HTN |
| Nurse's Health Study | 1198 | |
| Framingham Offspring Study | 1739 | ↑↑ Incidence of CVD events with 25-OH D <15 ng/mL |
| Women's Health Initiative | 36 282 | No CVD benefit to low-dose vitamin D in postmenopausal women |
Vitamin D and hypertension
The association between vitamin D deficiency and elevated blood pressure perhaps offers the most convincing evidence for the involvement of vitamin D metabolism in the pathogenesis of cardiovascular disease.32 A cause–effect relationship is postulated based on experimental and translational evidence demonstrating vital modulatory effects of vitamin D on the RAS axis.
For example, studies in normotensive and hypertensive subjects reveal an inverse relationship between vitamin D metabolites and plasma renin activity, regardless of baseline renin levels or salt intake.33,34 In addition, dietary salt loading results in blood pressure increases that are worse with vitamin D deficiency, and are positively correlated with calcitriol synthesis.35,36 Importantly, high-dose cholecalciferol therapy (15 000 IU/day for 1 month) in obese, hypertensive patients increased renal plasma flow (RPF) and decreased mean arterial pressure. Moreover, infusion of angiotensin II following cholecalciferol therapy resulted in a greater RPF decline and higher aldosterone secretion when compared with pre-treatment infusions.37 This is similar to established findings of increased tissue sensitivity to angiotensin following RAS antagonist therapy.38
RAS activation and subsequent synthesis of angiotensin II are known to increase vascular tone and arterial stiffness, which precede and contribute to the development of hypertension and are also strong predictors of overall CVD risk.39 In this context, we observed a higher augmentation index (AIX) and a lower subendocardial viability ratio (SEVR), both considered as complex and composite markers of arterial wave reflections and systemic stiffening with lower 25-OH D levels, independent of concomitant vascular risk factors in healthy subjects (Figure 3). Furthermore, those with a normalized vitamin D status after 6 months exhibited significant improvements in vascular function measurements.40
Figure 3.
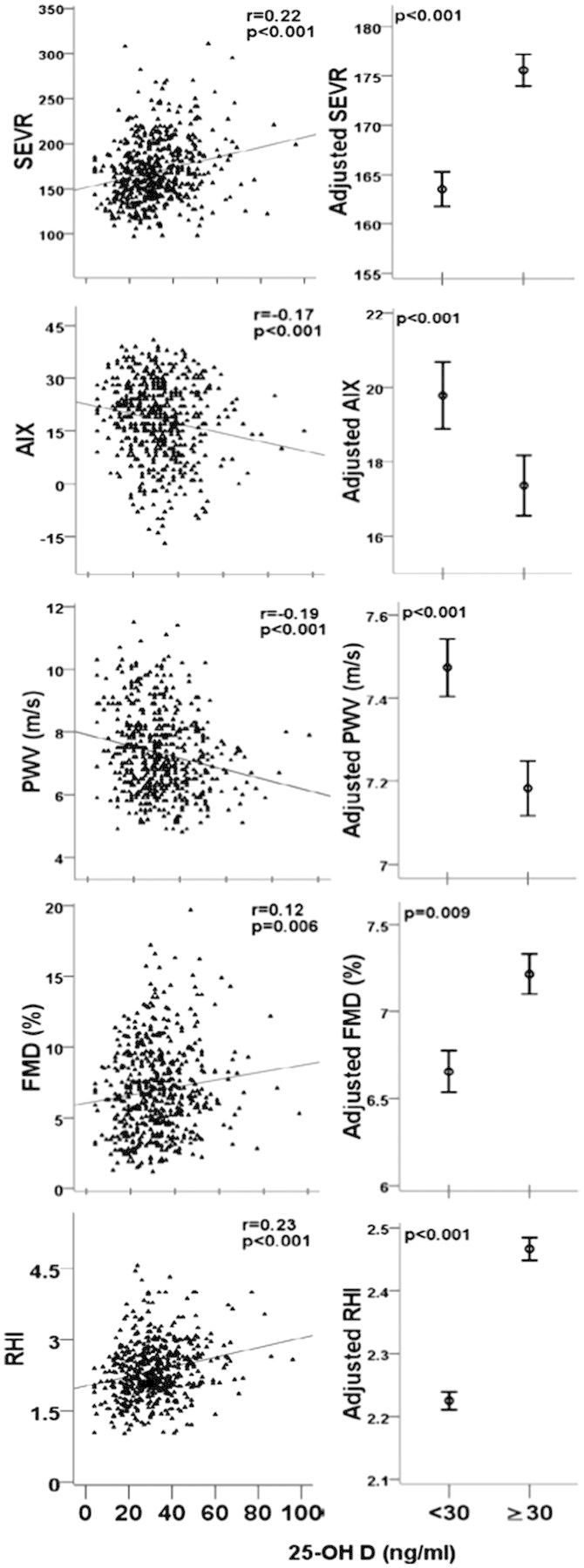
Relationship between 25-OH D levels, arterial stiffness, and vascular function. 25-OH D, 25-hydroxyvitamin D. RHI, reactive hyperaemia index; FMD, brachial artery flow-mediated dilation (percentage points); PWV, pulse wave velocity; AIX, augmentation index; SEVR, subendocardial viability ratio. Error bars represent mean and standard error of predicted values adjusted for age, gender, race, body mass index, total cholesterol, low-density lipoprotein, triglycerides, C-reactive protein, and medication use. r, Pearson's correlation. n = 554 (54% men).
In the same way, measures of arterial stiffness inversely correlated with vitamin D status in the Baltimore Longitudinal Study of Aging and in a British multiethnic study, as well as in studies looking specifically at patients with diabetes, rheumatological conditions, peripheral arterial disease, and renal insufficiency.41–47 However, only a few studies examined effects of vitamin D therapy on vascular function, and so far results have been contradictory.48–51
Thus, current evidence indicates that vitamin D deficiency may promote vascular dysfunction and sustained RAS activation, while sufficient levels may afford ‘‘endogenous’’, proximal inhibition.29
Vitamin D deficiency and epidemiology of hypertension
The third National Health and Nutrition Examination (NHANES III) looked at serum 25-OH D in relation to CVD risk factors in over 13 000 US adults. After multivariable adjustment, those with 25-OH D levels in the lowest quartile had a significantly higher prevalence of hypertension compared with those in the highest quartile, and sufficient levels attenuated the expected age-related increases in blood pressure.31,52 Other population studies, including the 1958 British Birth Cohort and the German National Health Survey and Examination, confirm this inverse relationship.53–57
Moreover, in two prospective cohorts of healthcare professionals, the risk of incident hypertension was increased by three-fold in those with 25-OH D <15 ng/mL compared with those with levels >30 ng/mL. Similarly, in a study that estimated 25-OH D levels based on dietary surveys in over 110 000 healthcare professionals, those with low ‘‘predicted’’ 25-OH D levels had a higher incidence of hypertension during nearly 16 years of follow-up.58,59
Vitamin D therapy in hypertension
The cardiovascular benefits of vitamin D therapy in those with chronic kidney disease and hyperparathyroidism have been long recognized, including blood pressure reduction, improved electrolyte balance, and an overall reduced cardiovascular mortality in haemodialysis patients.60,61
It is less clear if vitamin D therapy in essential hypertension, without overt kidney disease or electrolyte disturbances, will provide similar benefits. Trials reporting these measurements have either shown no blood pressure changes or small reductions in BP; however, these were limited by small and heterogeneous study samples, widely variable dosing strategies, and a short duration of follow-up.62,63 Several meta-analyses and systematic reviews have also arrived at conflicting conclusions; while a net significant hypotensive effect of vitamin D replacement was reported by some, others found either no change or only reductions in systolic BP, which may be apparent in specific subgroups such as those with vitamin D deficiency at baseline.32,64–67
Another complication in determining effects of vitamin D on blood pressure is that exposure to UV light also causes reductions in blood pressure, independent of vitamin D photosynthesis. Significant, immediate hypotensive effects of erythaemal and pre-erythaemal doses of UV irradiation have been demonstrated in both normotensive and hypertensive subjects.68–70 These effects are likely to be the result of overall decreases in vascular resistance with diffuse skin vasodilatation, and this ‘‘photorelaxation’’ is thought to be partly mediated by increased nitric oxide release in cutaneous vascular beds.71,72
Vitamin D and diabetes mellitus
Vitamin D deficiency is associated with disorders of insulin synthesis, secretion, and sensitivity. Experimental evidence highlights mechanisms by which vitamin D may influence glycaemic control; these include modulation of pancreatic RAS activity and regulation of calcium ion traffic across β-cells that directly affect insulin synthesis and secretion. Furthermore, vitamin D deficiency results in aberrant immune responses that precipitate an inflammatory milieu and subsequent insulin resistance.2,73,74
However, discrepancies in experimental and clinical evidence underscore knowledge gaps in determining the relationship between vitamin D metabolism and glycaemic control.2 For example, while human adipocytes express membrane-bound VDR that modulates lipolysis and lipogenesis activity in vitro, VDR null murine models exhibit a lean phenotype and increased energy expenditure, associated with adipose tissue atrophy. Further, models heterozygous for VDR show a similar, albeit less severe phenotype.75 Alternatively, increased adiposity and body fat mass observed in most insulin-resistant subjects may partly account for the lower 25-OH D levels seen in this population, as lipid-soluble vitamin D may be sequestered in adipose tissue, thus decreasing 25-OH D bioavailability.76
Vitamin D deficiency and epidemiology of diabetes mellitus
Many retrospective, cross-sectional, case–control, and prospective studies demonstrate a higher incidence and prevalence of type I diabetes mellitus with depressed vitamin D status. Similarly, low serum 25-OH D correlates with insulin resistance, obesity, aberrant phasing of insulin responses to glucose loading, glucose intolerance, fasting hyperglycaemia, and frank type II diabetes mellitus.77–79
Vitamin D therapy in diabetes mellitus
Observational, case–control, and prospective evidence strongly suggests that supplementing infants with vitamin D may significantly reduce the future incidence of type I diabetes. Dosage and timing of therapy appear to modulate this protective effect.80 The evidence for type II diabetes is weaker. Recent results from the Women's Health Initiative in which 33 591 postmenopausal women were randomized to both daily calcium and cholecalciferol (1 g and 400 IU, respectively) or placebo demonstrated no primary prevention benefit of vitamin therapy in 2291 incident cases of diabetes mellitus after 7 years of follow-up.81 Limitations of this study include study subjects' enrolment in additional dietary and hormonal interventions, inclusion of subjects already taking vitamin D supplements and the exclusion of men.82
While several smaller and non-randomized clinical trials show promising improvements in glycaemic control with vitamin D therapy, a recent Endocrine Society statement emphasized the lack of solid evidence supporting benefits of vitamin D therapy in diabetes mellitus.2
The role of vitamin D in modulating adaptive immunity, vascular inflammation, and endothelial function
Other potential consequences of vitamin D metabolism on human vasculature derive from several lines of experimental investigation and include exacerbation of atherogenesis and acceleration of arterial calcification. For example, the established anti-lymphoproliferative effects of vitamin D extend to regulation of monocyte/macrophage differentiation and the concomitant response to, and secretion of, inflammatory cytokines.28,83–85 This in turn may determine monocyte infiltration and cholesterol retention in the vascular wall and may corroborate clinical evidence of increased plaque instability and incident myocardial infarctions in vitamin D-deficient patients, in addition to the observed improvements in inflammatory biomarker levels in heart failure patients following vitamin D therapy.86–89
Additionally, aberrant vitamin D signalling induced in murine models caused extensive calcification of medium and small sized arteries, resembling human age-related Mönckeberg's disease.90 In humans, vitamin D deficiency independently predicted prevalence, incidence, and progression of coronary calcification in 374 diabetic patients over 6 years of follow-up.91 Similar to experimental studies that show endothelial cell function modulation by vitamin D analogues;25,26 indices of endothelial function and microvascular reactivity assessed as brachial-artery flow-mediated dilation and reactive hyperaemia index, respectively, were independently correlated with 25-OH D levels in a study we conducted in asymptomatic individuals (Figure 3).
Vitamin D therapy and cardiovascular outcomes
Vitamin D deficiency has been implicated as an independent risk factor for incident cardiovascular events and all-cause mortality in several large prospective studies.12,13,92 To date, less than 60 randomized trials have reported cardiovascular outcomes, and less than half of these were designed with any a priori cardiovascular endpoints.67,93,94
The Women's Health Initiative, with pre-specified cardiovascular secondary efficacy endpoints, is the largest randomized trial of vitamin D therapy to date. One year following randomization of 36 282 postmenopausal women to hormonal replacement therapy and/or dietary modifications, participants were asked to participate in a double-blinded vitamin D plus calcium supplementation trial. After 7 years of follow-up, rates of incident myocardial infarction and coronary disease related death, revascularization, confirmed angina, strokes, and transient ischaemic attacks did not differ between the treatment and placebo groups.95 Interestingly, post hoc analysis in women not taking vitamin D or calcium at baseline revealed significant decreases in colorectal and breast cancer incidence, but not in fractures or overall mortality.82
In a British fracture prevention trial, thrice yearly administration of a large oral dose of cholecalciferol in 2686 elderly subjects (76% men) for 5 years resulted in a non-significant trend towards decreased all-cause mortality, which was the secondary outcome, compared with placebo.96 More recently, a smaller randomized vitamin D only trial also failed to elicit significant changes in conventional cardiovascular risk factors. Significant favourable changes in lipoprotein composition were noted in treated individuals, but were deemed to be clinically unimportant by study investigators.97
Despite widely variable study populations and dosing strategies, review of available randomized vitamin D trials report similar findings; possible small reductions (up to 7%) in relative mortality risk.67,93,98 Yet, different investigators arrived at disparate conclusions; some suggested positive effects in certain subpopulations (e.g. institutionalized, elderly or female patients), while others reported likely cardiovascular benefits only with cholecalciferol therapy in moderate-to-high doses.93,99 Alternatively, other investigators were unable to demonstrate significant cardiovascular benefits based on available vitamin D therapy trials data.67,95
A recent meta-analysis of prospective studies that assessed the relationship between vitamin D status and CVD risk from 1966 to 2012, revealed an inverse relationship between levels of 25-OH D and future risk of CVD endpoints, including coronary heart disease, stroke, and total CVD mortality. The investigators examined over 6123 incident events that occurred in over 65 000 subjects who participated in 19-independent studies.100
It should be emphasized that most randomized vitamin D therapy trials to date were designed to investigate its protective skeletal effects; therefore subjects' mean age exceeded 70 years, were mostly women (≈75%) and many had established cardiovascular disease or risk factors.93 Additionally, many studies were tertiary prevention trials directed at patients with established morbid conditions such as end-stage kidney failure, debilitating fractures, and pulmonary tuberculosis.101–103 This in turn may affect the applicability of any conclusion drawn from pooling of available randomized vitamin D therapy studies.
An ongoing trial will determine the effects of cholecalciferol (2000 IU/day), with or without omega-3 fatty acids supplementation, on the incidence of cardiovascular disease, stroke, and cancer in 20 000 healthy, middle-aged US adults. The mean treatment period in the VITamin D and Omega-3 triAL (VITAL) study is projected at 5 years, with a similar follow-up period. Baseline 25-OH D levels will be measured in the majority of subjects at baseline, allowing for subgroup analysis in deficient subjects, and repeat measurements will be performed in 6000 participants on follow-up.104 This large study and hundreds of other ongoing clinical trials (clinicaltrials.gov; search term: vitamin D AND cardiovascular disease), will soon provide much needed evidence for determining the relationship between vitamin D and CVD.
Vitamin D and cardiovascular disease: an epiphenomenon?
The independent association between vitamin D deficiency and incident cardiovascular disease, while implying a cause–effect relationship, is complicated by the fact that low 25-OH D levels may be a result of cardiovascular disorders rather than the cause of disease. Ambient sunlight exposure maintains physiological vitamin D levels and ambulatory subjects with normal outdoor exercise activities are likely to have higher 25-OH D levels and lower likelihood of cardiovascular disease, thus raising the concern that the link between CVD and vitamin D is an epiphenomenon. Indeed, we previously demonstrated an independent correlation between vitamin D status and cardiovascular fitness, measured by cardiopulmonary exercise testing in healthy adults.105
Additionally, though pharmacological preparations of cholecalciferol or ergocalciferol raise serum 25-OH D levels as effectively as sunlight exposure, other undefined physiological sequelae of either approach may vary. For example, cutaneous synthesis of cholecalciferol requires the cholesterol precursor 7-dehydrocholesterol and may produce other photoproducts which may affect lipid levels. This is supported by the observed seasonal variation in plasma lipid levels and lipoprotein composition, whereby higher total cholesterol and low-density lipoprotein are observed in the winter, and reach their nadir during the summer. These cyclical changes remain pronounced despite adjusting for dietary or physical activity changes.106 In contrast, short-term oral vitamin D therapy did not significantly alter lipid levels or lipoprotein composition in a recent randomized, placebo controlled clinical trial.107
Clinical considerations
While vitamin D deficiency is prevalent, non-institutionalized individuals that maintain moderate sun exposure will probably not benefit from additional supplementation. This is especially true in areas of lower latitude and in younger individuals who are physically active, with a normal body mass index and fairer skin complexions for which casual exposure of the face, arms and legs (as little as 10–15 min, thrice weekly) results in cutaneous production of sufficient amounts of vitamin D.11 Cutaneous synthesis raises serum 25-OH D to a plateau level above which sun exposure results in spontaneous 25-OH D degradation.108
In contrast, excessive intake of pharmacological preparations can cause severe 25-OH D elevation. Serum 25-OH D levels >240 ng/mL may displace protein-bound calcitriol and subsequently result in profound hypercalcaemia, with consequences ranging from hyperphosphataemia to nephro- and soft tissue-calcinosis. However, this condition is exceedingly rare and is usually the result of exposure to mega doses of pharmacological preparation of vitamin D.109
Although UV irradiation causes direct DNA damage and is now an established skin carcinogen, the incidence of non-melanoma skin cancer heavily depends on skin tone (blacks have 1 of 80 the lifetime risk compared with Caucasians).110,111 Moderate sun exposure should therefore not be discouraged in at-risk individuals with darker skin pigmentation, particularly in areas of higher geographic latitude. Even in sunny locations, expanding urbanization and concomitant air pollution, together with increasing concerns of skin malignancies and resultant sun avoidant behaviour all adversely contribute to the high prevalence of vitamin D deficiency.112
Supplementation of food materials vitamin D is now an established public health strategy in preventing deficiency worldwide. While food fortification with vitamin D, initially by direct UV irradiation and later by adding vitamin D2 (ergocalciferol) to various foods,113 was implemented in most of North America and European countries in the first decades of the twentieth century, an outbreak of presumed vitamin D toxicity in infants from consumption of over-fortified milk in Great Britain resulted in a ban on fortification across most of Europe by the end of 1950's.114 Nevertheless, supplementation of food material with vitamin D has been re-introduced in many parts of Europe, including milk supplementation, over the past decades.
Treatment and prevention of vitamin D deficiency
In healthy individuals, prevention of vitamin D deficiency can be readily achieved by a combination of casual sunlight exposure, consumption of fatty fish or fish oils, in addition to fortified foods and/or supplements. While the current recommended dietary allowance of vitamin D in the USA ranges between 400 and 800 IU/day,115 as much as 2000 IU/day may be needed to maintain sufficient 25-OH D levels (≥30 ng/mL) in at-risk adults. As most diets generally provide less than the recommended daily allowance of vitamin D,116 pharmacological supplementation with vitamin D2 or D3 is, therefore, often required, particularly in locations where few foods are fortified with vitamin D or in individuals with increasing risk factors.117
Importantly, while an inverse linear dose–response relationship between 25-OH D and CVD risk exists at levels between 20 and 60 nmol/L (≈8–24 ng/mL), higher serum levels were not associated with a definitive increase or decrease in CVD risk. Thus, whereas vitamin D sufficiency confers a protective CVD effect compared with deficiency, further increases in 25-OH D by means of pharmacological supplementation may have no effects on CVD.
For treatment of documented vitamin D deficiency, a recent practice guideline statement by the The Endocrine Society recommends oral administration of 50 000 IU per week of either vitamin D2 or D3 for 8 weeks, followed by daily maintenance doses between 1500 and 2000 IU. Both loading and maintenance doses may be folds higher to in those with increasing risks for the development or recurrence of vitamin D deficiency. Concurrent calcium supplementation is a key component of effective therapy, and a preventative strategy should always address underlying causes, if possible.117,118
In addition to maintaining sufficient serum 25-OH D levels, patients with end-stage renal and/or hepatic disease impairing vitamin D activation and resulting in hypocalcaemia, in addition to those with secondary hyperparathyroidism or hypoparathyroidism require activated vitamin D therapy (e.g. 1, 25-OH D2; 0.25–0.5 µg/day).119,120 Patients with granulomatous disorders and dysregulated 1, 25-OH D2 activity may require vitamin D replacement, but 25-OH D levels >30 ng/mL can worsen the associated hypercalcaemia. Therefore, careful monitoring of vitamin D status, serum, and urinary calcium is necessary in these patients.117,121
Conclusion
Vitamin D deficiency is a highly prevalent condition and is independently associated with most CVD risk factors and to CVD morbidity and mortality. Despite a large body of experimental, cross-sectional, and prospective evidence that implicate vitamin D deficiency in the pathogenesis of CVD, the causality of this relationship remains to be established. Most importantly, randomized trials of vitamin D therapy with CVD endpoint are needed to support a role for vitamin D therapy in cardiovascular protection.
Conflict of interest: none declared.
References
- 1.McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O’Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235:1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- 2.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13:719–764. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- 4.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 5.Zhao G, Simpson RU. Membrane localization, caveolin-3 association and rapid actions of vitamin D receptor in cardiac myocytes. Steroids. 2010;75:555–559. doi: 10.1016/j.steroids.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farach-Carson MC, Ridall AL. Dual 1,25-dihydroxyvitamin D3 signal response pathways in osteoblasts: cross-talk between genomic and membrane-initiated pathways. Am J Kidney Dis. 1998;31:729–742. doi: 10.1053/ajkd.1998.v31.pm9531195. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 8.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 9.Cucu F, Schioiu-Costache L, Damsa T, Steinbach M. Effects of a multifactorial prevention trial of coronary heart disease on the seasonal variation of the incidence of major cardiovascular events. Rom J Int Med. 1992;30:175–185. [PubMed] [Google Scholar]
- 10.Intersalt: an International Study of electrolyte excretion and blood pressure. Results for 24 h urinary sodium and potassium excretion. Intersalt cooperative research group. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Luben R, Khaw KT, Bingham S, Wareham NJ, Forouhi NG. Dietary energy density predicts the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (epic)-norfolk Study. Diabetes Care. 2008;31:2120–2125. doi: 10.2337/dc08-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell TD, Berry JE, Jarvis AK, Somerman MJ, Simpson RU. 1,25-dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272:H1751–H1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 17.Simpson RU. Selective knockout of the vitamin D receptor in the heart results in cardiac hypertrophy: is the heart a drugable target for vitamin D receptor agonists? Circulation. 2011;124:1808–1810. doi: 10.1161/CIRCULATIONAHA.111.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258:E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 21.Mancuso P, Rahman A, Hershey SD, Dandu L, Nibbelink KA, Simpson RU. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008;51:559–564. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- 22.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in DAHL salt-sensitive animals. Proc Natl Acad Sci USA. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merke J, Milde P, Lewicka S, Hugel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond MA, Desormeaux A, Labelle A, Soulez M, Soulez G, Langelier Y, Pshezhetsky AV, Hebert MJ. Endothelial stress induces the release of vitamin D-binding protein, a novel growth factor. Biochem Biophys Res Commun. 2005;338:1374–1382. doi: 10.1016/j.bbrc.2005.10.105. [DOI] [PubMed] [Google Scholar]
- 25.Martinesi M, Bruni S, Stio M, Treves C. 1,25-dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int. 2006;30:365–375. doi: 10.1016/j.cellbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Talmor Y, Golan E, Benchetrit S, Bernheim J, Klein O, Green J, Rashid G. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am J Physiol Renal Physiol. 2008;294:F1059–F1064. doi: 10.1152/ajprenal.00051.2008. [DOI] [PubMed] [Google Scholar]
- 27.Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–H296. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- 28.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motiwala SR, Wang TJ. Vitamin D and cardiovascular risk. Curr Hypertens Rep. 2012;14:209–218. doi: 10.1007/s11906-012-0262-y. [DOI] [PubMed] [Google Scholar]
- 30.Kempker JA, Tangpricha V, Ziegler TR, Martin GS. Vitamin D in sepsis: from basic science to clinical impact. Crit Care. 2012;16:316. doi: 10.1186/cc11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third national health and nutrition examination survey. Am J Clin Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 32.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nature Rev. 2009;6:621–630. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2012;61:450–458. doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya A, Forman JP, Hopkins PN, Seely EW, Williams JS. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12:311–319. doi: 10.1177/1470320310391922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens. 1990;3:903–905. doi: 10.1093/ajh/3.12.903. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya A, Sun B, Larson C, Forman JP, Williams JS. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin ii akin to the action of a converting enzyme inhibitor in obese hypertensives: an interventional study. J Clin Endocrinol Metab. 2012;97:2456–2465. doi: 10.1210/jc.2012-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morganti A, Ambrosi B, Sala C, Cianci L, Bochicchio D, Turolo L, Zanchetti A. Effects of angiotensin ii blockade on the responses of the pituitary-adrenal axis to corticotropin-releasing factor in humans. J Cardiovasc Pharmacol. 1987;10(Suppl. 7):S167–S169. doi: 10.1097/00005344-198706107-00038. [DOI] [PubMed] [Google Scholar]
- 39.Quyyumi AA, Patel RS. Endothelial dysfunction and hypertension: cause or effect? Hypertension. 2010;55:1092–1094. doi: 10.1161/HYPERTENSIONAHA.109.148957. [DOI] [PubMed] [Google Scholar]
- 40.Patel RS, Al Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirro M, Manfredelli MR, Helou RS, Scarponi AM, Schillaci G, Bagaglia F, Melis F, Mannarino E. Association of parathyroid hormone and 25-OH-vitamin D levels with arterial stiffness in postmenopausal women with vitamin D insufficiency. J Atheroscler Thromb. 2012 doi: 10.5551/jat.13128. [DOI] [PubMed] [Google Scholar]
- 42.Lee JI, Oh SJ, Ha WC, Kwon HS, Sohn TS, Son HS, Cha BY. Serum 25-hydroxyvitamin D concentration and arterial stiffness among type 2 diabetes. Diabetes Res Clin Pract. 2012;95:42–47. doi: 10.1016/j.diabres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Reynolds JA, Haque S, Berry JL, Pemberton P, Teh LS, Ho P, Gorodkin R, Bruce IN. 25-hydroxyvitamin D deficiency is associated with increased aortic stiffness in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2012;51:544–551. doi: 10.1093/rheumatology/ker352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zagura M, Serg M, Kampus P, Zilmer M, Eha J, Unt E, Lieberg J, Cockcroft JR, Kals J. Aortic stiffness and vitamin D are independent markers of aortic calcification in patients with peripheral arterial disease and in healthy subjects. Eur J Vasc Endovasc Surg. 2011;42:689–695. doi: 10.1016/j.ejvs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 45.London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 46.Rezai MR, Wallace AM, Sattar N, Finn JD, Wu FC, Cruickshank JK. Ethnic differences in aortic pulse wave velocity occur in the descending aorta and may be related to vitamin D. Hypertension. 2011;58:247–253. doi: 10.1161/HYPERTENSIONAHA.111.174425. [DOI] [PubMed] [Google Scholar]
- 47.Giallauria F, Milaneschi Y, Tanaka T, Maggio M, Canepa M, Elango P, Vigorito C, Lakatta EG, Ferrucci L, Strait J. Arterial stiffness and vitamin D levels: the Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarcin O, Yavuz DG, Ozben B, Telli A, Ogunc AV, Yuksel M, Toprak A, Yazici D, Sancak S, Deyneli O, Akalin S. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 49.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 50.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, Pierce GL, White J, Holick MF, Zhu H. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95:4584–4591. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 51.Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS ONE. 2012;7:e36617. doi: 10.1371/journal.pone.0036617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the third national health and nutrition examination survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 53.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 54.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 55.Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone. 2009;44:1085–1091. doi: 10.1016/j.bone.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the medical research council Ely prospective study 1990–2000. Diabetes. 2008;57:2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gannage-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young middle-eastern population. Eur J Endocrinol. 2009;160:965–971. doi: 10.1530/EJE-08-0952. [DOI] [PubMed] [Google Scholar]
- 58.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 60.Ketteler M, Martin KJ, Wolf M, Amdahl M, Cozzolino M, Goldsmith D, Sharma A, Marx S, Khan S. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the impact SHPT study. Nephrol Dial Transplant. 2012;27:3270–3278. doi: 10.1093/ndt/gfs018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kovesdy CP, Kalantar-Zadeh K. Vitamin D receptor activation and survival in chronic kidney disease. Kidney Int. 2008;73:1355–1363. doi: 10.1038/ki.2008.35. [DOI] [PubMed] [Google Scholar]
- 62.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–646. [PubMed] [Google Scholar]
- 63.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 64.Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens. 2009;27:1948–1954. doi: 10.1097/HJH.0b013e32832f075b. [DOI] [PubMed] [Google Scholar]
- 65.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J. 2010;103:729–737. doi: 10.1097/SMJ.0b013e3181e6d389. [DOI] [PubMed] [Google Scholar]
- 67.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 68.Weber KT, Rosenberg EW, Sayre RM. Suberythemal ultraviolet exposure and reduction in blood pressure. Am J Med. 2004;117:281–282. doi: 10.1016/j.amjmed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 70.Forssander CA. Pre-erythema blood pressure changes following ultraviolet irradiation. Can Med Assoc J. 1956;74:730–733. [PMC free article] [PubMed] [Google Scholar]
- 71.Ergenekon E, Gucuyener K, Dursun H, Erbas D, Ozturk G, Koc E, Atalay Y. Nitric oxide production in newborns under phototherapy. Nitric Oxide. 2002;6:69–72. doi: 10.1006/niox.2001.0364. [DOI] [PubMed] [Google Scholar]
- 72.Buyukafsar K, Levent A, Un I, Ark M, Arikan O, Ozveren E. Mediation of nitric oxide from photosensitive stores in the photorelaxation of the rabbit corpus cavernosum. Eur J Pharmacol. 2003;459:263–267. doi: 10.1016/s0014-2999(02)02858-3. [DOI] [PubMed] [Google Scholar]
- 73.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 74.Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, Eizirik DL, Mathieu C. 1,25-dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 75.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150:651–661. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 77.Boucher BJ, John WG, Noonan K. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;80:1666. doi: 10.1093/ajcn/80.6.1666. author reply 1666–1667. [DOI] [PubMed] [Google Scholar]
- 78.Hirani V. Relationship between vitamin D and hyperglycemia in older people from a nationally representative population survey. J Am Geriatr Soc. 2011;59:1786–1792. doi: 10.1111/j.1532-5415.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 79.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 80.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 81.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL, Siscovick DS, Weiss NS. Calcium plus vitamin D supplementation and the risk of incident diabetes in the women’s health initiative. Diabetes Care. 2008;31:701–707. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the women’s health initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–1149. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin D3–1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 84.Danielsson C, Nayeri S, Wiesinger H, Thieroff-Ekerdt R, Carlberg C. Potent gene regulatory and antiproliferative activities of 20-methyl analogues of 1,25 dihydroxyvitamin D3. J Cell Biochem. 1996;63:199–206. doi: 10.1002/(sici)1097-4644(19961101)63:2<199::aid-jcb7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 85.Manolagas SC, Provvedini DM, Murray EJ, Tsoukas CD, Deftos LJ. The antiproliferative effect of calcitriol on human peripheral blood mononuclear cells. J Clin Endocrinol Metab. 1986;63:394–400. doi: 10.1210/jcem-63-2-394. [DOI] [PubMed] [Google Scholar]
- 86.Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, Vyas YM, Aujla SJ, Finelli P, Blanchard M, Zeigler SF, Logar A, Hartigan E, Kurs-Lasky M, Rockette H, Ray A, Kolls JK. Vitamin D3 attenuates th2 responses to Aspergillus fumigatus mounted by cd4+ t cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 2010;120:3242–3254. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Int Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 90.Ignat M, Teletin M, Tisserand J, Khetchoumian K, Dennefeld C, Chambon P, Losson R, Mark M. Arterial calcifications and increased expression of vitamin D receptor targets in mice lacking tif1alpha. Proc Natl Acad Sci USA. 2008;105:2598–2603. doi: 10.1073/pnas.0712030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA, Garg SK, Rewers M. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care. 2011;34:454–458. doi: 10.2337/dc10-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 93.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011:CD007470. doi: 10.1002/14651858.CD007470.pub2. [DOI] [PubMed] [Google Scholar]
- 94.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 95.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 96.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Br Med J. 2003;326:469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, Simpson WG, Fraser WD, Reid DM, Macdonald HM. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors. A parallel-group, double-blind, placebo-controlled rct. J Clin Endocrinol Metab. 2012;97:3557–3568. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 98.LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, Gass M, Johnson KC, Ko M, Larson J, Manson JE, Stefanick ML, Wactawski-Wende J. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the women's health initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 100.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berggren M, Stenvall M, Olofsson B, Gustafson Y. Evaluation of a fall-prevention program in older people after femoral neck fracture: a one-year follow-up. Osteoporos Int. 2008;19:801–809. doi: 10.1007/s00198-007-0507-9. [DOI] [PubMed] [Google Scholar]
- 102.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Critical Care Med. 2009;179:843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 103.Khajehdehi P. Effect of vitamins on the lipid profile of patients on regular hemodialysis. Scand J Urol Nephrol. 2000;34:62–66. doi: 10.1080/003655900750016913. [DOI] [PubMed] [Google Scholar]
- 104.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The vitamin D and omega-3 trial (vital): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Al Mheid I, Ramadan R, Kavtaradze N, Morris A, Ali S, Aznaouridis K, Quyyumi F, Alexander RW, Brigham KL, Quyyumi A. Abstract 1723: vitamin D levels are associated with exercise capacity and measures of endothelial function in healthy humans. Circulation. 2009;120:s551. [Google Scholar]
- 106.Gordon DJ, Hyde J, Trost DC, Whaley FS, Hannan PJ, Jacobs DR, Ekelund LG. Cyclic seasonal variation in plasma lipid and lipoprotein levels: the lipid research clinics coronary primary prevention trial placebo group. J Clin Epidemiol. 1988;41:679–689. doi: 10.1016/0895-4356(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 107.Ponda MP, Dowd K, Finkielstein D, Holt PR, Breslow JL. The short-term effects of vitamin D repletion on cholesterol: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2012;32:2510–2515. doi: 10.1161/ATVBAHA.112.254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thieden E, Jorgensen HL, Jorgensen NR, Philipsen PA, Wulf HC. Sunbed radiation provokes cutaneous vitamin D synthesis in humans—a randomized controlled trial. Photochem Photobiol. 2008;84:1487–1492. doi: 10.1111/j.1751-1097.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 109.Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22(Suppl. 2):V64–68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 110.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 111.Svobodova AR, Galandakova A, Sianska J, Dolezal D, Lichnovska R, Ulrichova J, Vostalova J. DNA damage after acute exposure of mice skin to physiological doses of UVB and UVA light. Arch Dermatol Res. 2012;304:407–412. doi: 10.1007/s00403-012-1212-x. [DOI] [PubMed] [Google Scholar]
- 112.Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29:361–368. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Centers for Disease C, Prevention. Safer and healthier foods. MMWR Morb Mortal Wkly Rep. 1999;48:905–913. [PubMed] [Google Scholar]
- 114.Welch TR, Bergstrom WH, Tsang RC. Vitamin D-deficient rickets: the reemergence of a once-conquered disease. J Pediatrics. 2000;137:143–145. doi: 10.1067/mpd.2000.109008. [DOI] [PubMed] [Google Scholar]
- 115.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tangpricha V. Vitamin D in food and supplements. Am J Clin Nutr. 2012;95:1299–1300. doi: 10.3945/ajcn.112.039818. [DOI] [PubMed] [Google Scholar]
- 117.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 118.Fauci AS. Harrison's Principles of Internal Medicine. Mcgraw Hill Professional; 2012. 1online resource (xxxvii 2754 p. [Google Scholar]
- 119.Del Valle E, Negri AL, Aguirre C, Fradinger E, Zanchetta JR. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial Int. 2007;11:315–321. doi: 10.1111/j.1542-4758.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 120.de Francisco AL. Medical therapy of secondary hyperparathyroidism in chronic kidney disease: old and new drugs. Expert Opin Pharmacother. 2006;7:2215–2224. doi: 10.1517/14656566.7.16.2215. [DOI] [PubMed] [Google Scholar]
- 121.Baker K, Pehr K. Granuloma annulare associated with hypercalcemia secondary to hyperparathyroidism. Int J Dermatol. 2006;45:1118–1120. doi: 10.1111/j.1365-4632.2006.02868.x. [DOI] [PubMed] [Google Scholar]