Abstract
In brood-caring species, family members are faced with a conflict over resource distribution. While parents are selected to adapt the amount of care according to their offspring's needs, offspring might be selected to demand more care than optimal for parents. Recent studies on birds have shown that the social network structure of offspring affects the amount of care and thus the fitness of families. Such a network structure of repeated interactions is probably influenced by within-brood relatedness. We experimentally manipulated the group composition in a brood-caring spider to test how the presence of unrelated spiderlings affects the dynamics between female and brood as well as within broods. Broods consisting of siblings grew better and had a lower mortality compared with mixed broods, no matter whether the caring female was a genetic or foster mother. Interestingly, we found that foster mothers lost weight when caring for sibling broods, whereas females caring for mixed broods gained weight. This indicates that females may be willing to share more prey when the brood contains exclusively siblings even if the entire brood is unrelated to the female. Resource distribution may thus be negotiated by offspring dynamics that could have a signalling function to females.
Keywords: parent–offspring conflict, sociality, cooperation, social network structure
1. Introduction
In species with parental care, parents and offspring are faced with conflicts over resource distribution [1,2]. Offspring may be under selection to demand a higher amount of care than parents might be willing to provide, especially if parents need to retain resources for future broods. As parental care is limited, offspring compete with each other over sharing this resource [3,4]. Two main modes of resource allocation have been suggested to be relevant in this context: in scramble competition models, it is assumed that parents are rather passive and that competition between offspring determines how resources are distributed [5]. Honest signalling models assume that parents actively allocate resources depending on the need of the offspring [6–8]. Recent studies suggest that the resolution of parent–offspring conflicts involves repeated interactions among all group members [9,10]. Royle et al. [11] point out that determining control mechanisms is complex, and that offspring and female control represent the two ends of a ‘power continuum’. Hence, the resolution of the conflict strongly depends on the context, including a network of interactions [11].
While most studies dealing with conflicts over parental care show that offspring compete with each other over resources, siblings may also cooperate to increase the overall amount of parental care [4]. Cooperation will be favoured when inclusive fitness gains exceed the direct costs of cooperating [3,12]. Generally, interacting individuals in family conflicts are (at least partly) related so that any conflict will potentially entail indirect fitness costs. However, relatedness among broods may vary when females mate multiply (polyandry), mobile young immigrate into an existing family group [13] or due to brood parasitism [14]. Offspring should be less competitive towards closely related nest-mates [12], but should become less likely to share as relatedness decreases [15,16].
Offspring migration into a foreign group can be found in the subsocial crab spider Diaea ergandros. Spiderlings that lost their mother can migrate into foreign nests [13,17], and thus females may face a brood that contains a mixture of own and unrelated offspring. A molecular study (using allozyme markers) on the genetic structure of 28 sampled D. ergandros nests that contained a putative mother showed that in 75% of the nests offspring were probably produced by the present mother and a single father. In 21.4% of the nests, spiderlings could not be assigned to the mother's genotype, indicating that foreign spiderlings immigrate into nests. In one case (3.6%), paternity was shared between at least two fathers [13]. In these spiders, females hunt and share prey with the offspring [17,18], and some females are consumed by their offspring (matriphagy) [19], while others stay alive until the spiderlings mature (J.R. 2011–2012, personal observation). Evans [17] showed that females recognize own offspring. Cues that allow discrimination between kin and non-kin have not been identified in crab spiders, but in other subsocial spiders cuticular hydrocarbons are possible kin recognition cues [20]. As spiders digest externally [21], female D. ergandros cannot individually allocate food to specific spiderlings, but they may allow spiderlings to feed with them and leave more food for the brood. In a situation where broods consist of a mixture of own and foreign offspring, a female may selectively allow own offspring to join her feeding. Alternatively, females may share food independently of their relatedness to the brood. In this case, food distribution may depend on competition between offspring, while the female is mostly passive [11]. Accordingly, we predict that the dynamics between a female and the brood and also among the brood vary depending on the relatedness between them.
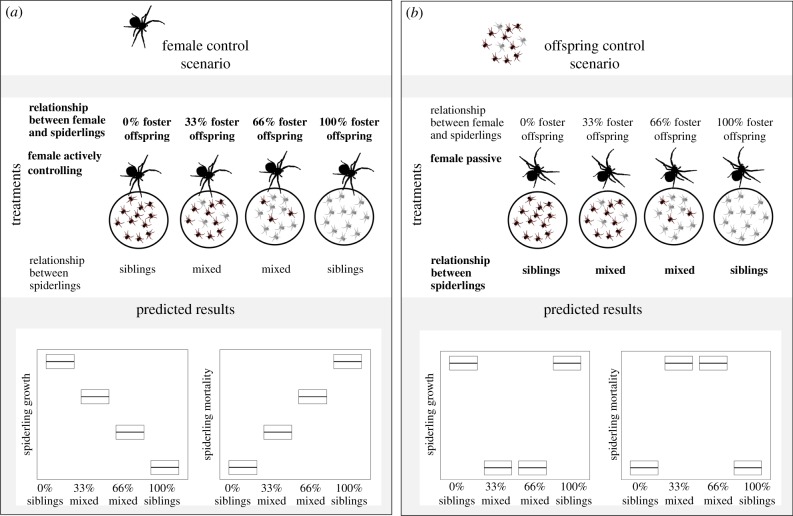
If females are largely in control of food allocation and base allocation on the presence of kin recognition cues, female care (food provisioning) should gradually decrease with increasing proportions of foster offspring in her brood. Reducing investment is beneficial for the mother if this increases the probability of producing a second brood. We observed that female D. ergandros are able to produce a second clutch in cases where the first one failed (J.R. 2011–2012, personal observation). In other semelparous subsocial spiders, females can also produce a second brood when the first brood is removed [22]. Reduced maternal investment probably results in higher spiderling mortality and lower spiderling growth when the proportion of foster offspring is high (figure 1a). Assuming that females mostly control the rate of food provisioning, we predict that females caring for own offspring leave more food to the brood and thus lose weight while those caring for foster offspring retain more food and gain weight. We further predict that only those females caring for own offspring tolerate matriphagy.
Figure 1.
Predicted outcomes for the two different scenarios for offspring growth and mortality. (a) Assuming that females are largely in control of resource allocation, we predict offspring growth to gradually decrease with increasing numbers of foster offspring and mortality to increase. (b) Assuming competition between offspring as major control over food distribution, we predict that the sibling broods have a higher growth and lower mortality than mixed broods.
If competition between offspring is the predominant influence on food distribution [4], spiderlings may adjust cooperation in sharing food to the average degree of relatedness within the brood [12]. If offspring mostly control food distribution and siblings cooperate more than non-siblings, we predict that broods consisting of siblings grow better and have a lower mortality compared with broods of mixed offspring (figure 1b).
To test these predictions about group dynamics between females and offspring, and the effects of immigrating spiderlings, we manipulated brood composition and decreased relatedness between females and their respective offspring groups. We then monitored female hunting behaviour, mass development and mortality, as well as offspring growth and mortality.
2. Material and methods
(a). Study species
Diaea ergandros Evans, 1995 (Thomisidae) is a semelparous, subsocial spider that inhabits the foliage of Eucalyptus trees in closed-canopy forests [23] as well as trees along roadsides in Southeastern Australia. Broods usually originate as the offspring of a single female that migrated from her natal colony after mating. Females construct nests from Eucalyptus leaves and produce a single egg sac [18]. After the spiderlings have hatched, the female continues to expand the nest and catches prey to feed her young. Offspring usually stay in their natal nest and communally continue to extend the nest with new leaves. Nests serve as foraging areas and spiders hunt without a capture web by ambushing prey [24].
(b). Experimental set-up
We collected D. ergandros nests from their natural habitat near the town of Yass in New South Wales (Australia, 34°55′20.50″ S, 149°6′15.53″ E) in February 2012. We cut whole nests off trees with either gardening cutters or expandable branch cutters in heights ranging from 50 cm to approximately 10 m. Nests were dissected in the laboratory, and spiderlings were counted and weighed to the nearest 0.1 mg by using an electronic balance (Mettler Toledo New Classic MS). Adult living females were weighed and their prosoma width was measured with digital callipers. We only collected nests with very young spiderlings, thereby decreasing the likelihood that foreign offspring have already migrated into the group. Asymmetries in relatedness in the broods due to multiply mating females cannot be excluded; however, the rate of polyandrous females was very low in a previous study [13].
For the experiment, we randomly assigned females into four treatments with varying degrees of relatedness to their allocated spiderlings: 0, 33, 66 or 100% of the brood were foster offspring, taken from a different female (= collected at least 5 km apart from each other to minimize maternal relatedness); the remaining percentage were the female's own offspring. As eggs mostly fail to hatch in the laboratory (J.R. 2011–2012, personal observation) we were unable to set up a cross-foster familiarity control, where offspring are assigned immediately after they hatch. This means that those females caring for own offspring may be more familiar with the brood compared with those females caring for foster offspring and that siblings are more familiar with each other compared with broods consisting of mixed offspring, which may affect group dynamics. The absence of a familiarity control does not allow conclusions about the mechanism of kin recognition, which however was not an aim of the study. When spiderlings migrate into foreign nests under natural conditions, it is assumed that they are neither familiar nor related to the group.
From the offspring perspective, the group composition was the same in the 0 and 100% foster treatment (all siblings) as well as in the 33 and 66% foster treatment (mixed broods from two different females). All females and spiderlings experienced the same procedure of being separated and weighed before the groups were formed. As female body mass varied, the number of spiderlings per female was based on female mass (sum mass spiderlings = female mass × 2) and ranged between 16 and 35 spiderlings. Our rationale was that we wanted to standardize female body reserves in relation to offspring number. The body mass of the female was considered a resource in itself, because it has been found that females were consumed by their offspring [19]. There was a negative correlation between the number of spiderlings per female and the initial spiderling mass (meaning the mass spiderlings had prior to the experiment; Pearson: r = −0.59, p < 0.0001): the larger the spiderlings, the fewer were allocated to the female. The average number of allocated spiderlings per treatment as well as their mean initial body mass did not differ between treatments (ANOVAno spiderlings: r2 = 0.01, F3,36 = 0.16, p = 0.9; ANOVAbody mass: r2 = 0.05, F3,36 = 0.68, p = 0.6; table 1). In an additional control, females were kept without offspring to monitor their mass development and whether they would produce a second clutch. Spider groups were kept in 750 ml plastic containers that were covered with gauze to allow airflow. Groups were checked and sprayed with water every 2 days. We recorded all dead spiderlings and whether the respective female was alive. The experiment was terminated after nine weeks.
Table 1.
Initial female mass, number of given spiderlings and mean spiderling mass before the experiment was started.
| treatment | female mass (mg) | no. of added spiderlings | spiderling mass (average (mg)) | spiderling mass (median (mg)) | nreplicates |
|---|---|---|---|---|---|
| 0% foster | 28.6 ± 2.2 | 24.3 ± 1.6 | 2.38 ± 0.17 | 2.53 | 10 |
| 33% foster | 27.2 ± 2.1 | 24.2 ± 1.8 | 2.43 ± 0.19 | 2.60 | 10 |
| 66% foster | 24.7 ± 1.4 | 25.5 ± 1.4 | 2.05 ± 0.19 | 2.00 | 10 |
| 100% foster | 26.7 ± 1.9 | 25.0 ± 1.2 | 2.20 ± 0.20 | 1.98 | 10 |
| female | 27.6 ± 2.0 | n.a. | n.a. | n.a. | 12 |
(i). Female hunting behaviour
All groups were fed with one Calliphora sp. (Diptera, approx. 50 mg) once a week. These flies were too big to be caught by the spiderlings. Thus, females had to catch the flies and could either feed themselves or share the prey with the spiderlings. We recorded whether females had caught the fly 24 h after introduction to the containers.
(ii). Female mass development and matriphagy
Females were weighed every fortnight and we calculated their mass development by subtracting the initial mass from the final mass. To control for the overall effect of the presence of spiderlings on female mass development and mortality, we included a control of females without spiderlings. These were fed at the same frequency as the experimental females. In case a female died, we weighed the remains and calculated the loss of body mass from the previous weighing event.
(iii). Offspring mass development and mortality
Ten randomly chosen spiderlings of each group were individually weighed before the experiment and again after four weeks. We aimed to test whether female care affects offspring mass development and mortality depending on the relatedness to the female and between the offspring. Therefore, we analysed offspring mass development and mortality in the first four weeks when all females were still alive.
Spiders were anaesthetized with CO2 and transferred into 70% ethanol after the experiment; no ethics approval was necessary for working with invertebrates.
(c). Statistical analysis
Data analyses were performed using JMP v. 9.0.2 (SAS Institute Inc., Cary, NC) and R v. 2.15.3 [25]. Descriptive statistics are given as mean±s.e. Continuous data were tested for normality using the Shapiro–Wilk W-test. Data that were not normally distributed were analysed using non-parametric tests. The generalized linear model (GLM) and the generalized linear mixed model (GLMM) were performed in R using the lme4 package. We simplified maximal models by stepwise elimination of the least significant variable and comparing the models with ANOVAs. We used the minimal adequate model (indicated by the lowest AIC) to identify determinants of the response variable. Post hoc tests (Tukey contrasts) were performed with the multcomp package. The generalized estimating equation (GEE) was performed in R using the geepack package. We simplified the maximal model by stepwise elimination of the least significant variable and comparing the models with Wald statistics.
Box-and-whisker plots were plotted in R, and the upper and lower whiskers show the range, the box shows median and interquartiles. Individual dots indicate outliers.
The percentage of successful prey capture by females (n = 148 observations) was analysed using a GLMM with binomial error distribution. The maximal model to investigate whether a fly was caught (yes/no) included the following explanatory variables: treatment (percentage of foster offspring), female start mass and the week of the feeding observation. Female ID was included as a random factor to control for repeated measurements.
Female mass development was analysed with a non-parametric Kruskal–Wallis test. We analysed female mortality using a Cox proportional hazard model (n = 52).
Offspring mass development was calculated by subtracting the initial group mass from the final group mass. We fitted a GLM with normal error structure and included treatment (percentage of foster offspring) and the average spiderling initial mass as well as their interaction. Differences between the four treatments were analysed using Tukey contrast.
Offspring mortality was analysed using a GEE with binomial error structure and exchangeable association structure. Mortality (y/n) was the response variable, and treatment and spiderling start weight as well as their interaction were the explanatory variables (n = 989 spiderlings). Female ID was specified as grouping variable to control for measurements within the same group. Treatment was re-ordered to analyse differences between the treatments by comparing the coefficients of each treatment with the reference level.
3. Results
(a). Female hunting behaviour
We aimed to test whether the female's prey capture behaviour varies according to her relatedness with the brood. However, treatment (p = 0.3) did not significantly affect prey capture (average percentage of flies caught per treatment: 0% foster: 50.0±15.07; 33% foster: 41.7±18.0; 66% foster: 35.0±15.5; 100% foster: 40.9±21.2). Female initial mass was not significant either (p = 0.4), and both factors were eliminated from the final model, which showed that the percentage of successfully caught flies varied significantly between the feeding observations (FO), but without a discernable pattern of overall increase or decrease (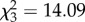 , p = 0.00017, nfemales = 40, nobservations = 148; average percentage of flies caught: FO 1: 45.7±4.1, FO 2: 88.2±6.4, FO 3: 8.9±6.4, FO 4: 24.9±1.7). We observed females of all treatments sharing prey with offspring. As the spiderlings were not individually marked, we could not differentiate whether females interacted differently with the offspring in the mixed broods.
, p = 0.00017, nfemales = 40, nobservations = 148; average percentage of flies caught: FO 1: 45.7±4.1, FO 2: 88.2±6.4, FO 3: 8.9±6.4, FO 4: 24.9±1.7). We observed females of all treatments sharing prey with offspring. As the spiderlings were not individually marked, we could not differentiate whether females interacted differently with the offspring in the mixed broods.
(b). Female mass development and matriphagy
Overall, female mass development was not significantly different between the five treatments over the duration of the experiment (Kruskal–Wallis test: 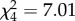 , p = 0.13). The median values initially increased with decreasing relatedness to the mother (figure 2), but then dropped in the 100% foster treatment, where we expected the greatest maternal weight gain. A Wilcoxon multiple comparison revealed that females of the 66% foster treatment gained significantly more mass than females of the 100% foster treatment (Z = 2.0, p = 0.045; figure 2). Females caring for own offspring (0% foster) lost mass, while females caring for 33% foster offspring and females without offspring neither gained nor lost mass; however, all treatment comparisons except for the abovementioned one were not significantly different (Wilcoxon each pair, all p > 0.05).
, p = 0.13). The median values initially increased with decreasing relatedness to the mother (figure 2), but then dropped in the 100% foster treatment, where we expected the greatest maternal weight gain. A Wilcoxon multiple comparison revealed that females of the 66% foster treatment gained significantly more mass than females of the 100% foster treatment (Z = 2.0, p = 0.045; figure 2). Females caring for own offspring (0% foster) lost mass, while females caring for 33% foster offspring and females without offspring neither gained nor lost mass; however, all treatment comparisons except for the abovementioned one were not significantly different (Wilcoxon each pair, all p > 0.05).
Figure 2.
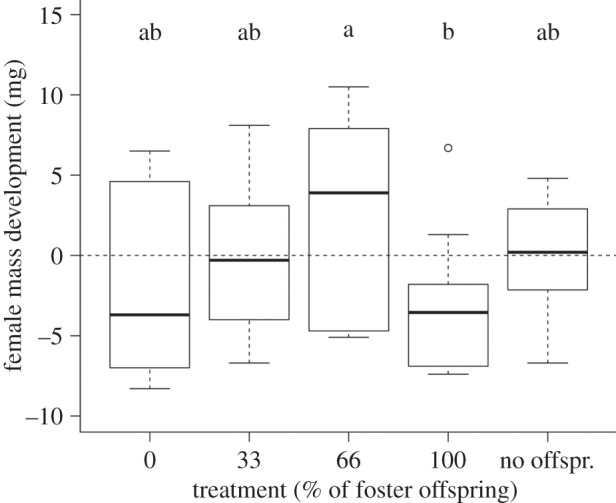
Female mass development depending on their relatedness to the offspring (0, 33, 66 and 100% foster treatment) and for females without brood. Females caring for sibling broods (0 and 100% foster) lost weight and there was a difference with females of the 100% foster treatment losing significantly more mass than females caring for 66% foster offspring (a and b express the statistical difference, Wilcoxon multiple comparisons p < 0.05).
Twenty-five females died over the course of the experiment but female mortality was not significantly different between the five treatments (Cox proportional hazard model: 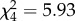 , p = 0.2). We never observed matriphagy, and the mass loss between the last weighing event before the female had died and the day she died was not significantly different between the treatments (ANOVA: r2 = 0.07, F4,21 = 0.41, p = 0.8). A single female (in the 0% foster treatment) lost more than 80% of body mass, but it is unclear whether the spiderlings may have fed on her. We regularly observed spiderlings sitting on the body of alive females (across all treatments), but we never observed them feeding on a female body. Across all treatments, the average mass loss of females was 28.6±4.3% and suggests that matriphagy was not relevant in this experiment. None of the females produced a second clutch.
, p = 0.2). We never observed matriphagy, and the mass loss between the last weighing event before the female had died and the day she died was not significantly different between the treatments (ANOVA: r2 = 0.07, F4,21 = 0.41, p = 0.8). A single female (in the 0% foster treatment) lost more than 80% of body mass, but it is unclear whether the spiderlings may have fed on her. We regularly observed spiderlings sitting on the body of alive females (across all treatments), but we never observed them feeding on a female body. Across all treatments, the average mass loss of females was 28.6±4.3% and suggests that matriphagy was not relevant in this experiment. None of the females produced a second clutch.
(c). Offspring mass development and mortality
Initial spiderling body mass had an influence on final mass (meaning that spiderlings with a higher initial body mass had a higher final body mass; Pearson: r = 0.86, p < 0.0001) and was therefore included as a covariate (there was no difference between treatments prior to the experiment; see table 1). Corrected for initial body mass (GLM: F1,35 = 4.49, p = 0.04), spiderling mass development was significantly different between the four treatments (GLM: F3,35 = 3.89, p = 0.017, figure 3), with spiderlings of the sibling treatments (0 and 100% foster) gaining more weight than spiderlings of mixed broods.
Figure 3.
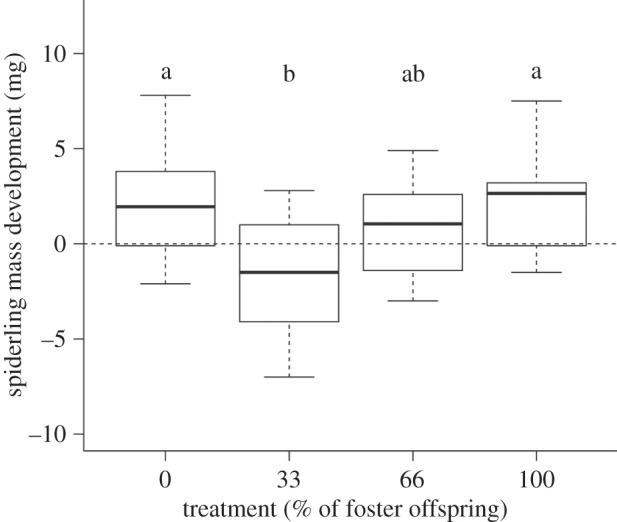
Offspring mass development depending on their relatedness to the caring female (0, 33, 66 and 100% foster treatment). Spiderlings of the sibling broods (0 and 100% foster) gained significantly more mass than those belonging to the 33% foster treatment, while the 66% foster treatment was not significantly different from the other treatments (a and b express the statistical difference, Tukey contrasts p < 0.05).
Offspring mortality was predicted by treatment (GEE: 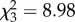 , p = 0.03) and negatively affected by spiderling start mass (
, p = 0.03) and negatively affected by spiderling start mass (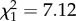 , p = 0.008). Spiderlings belonging to the 0% foster treatment had a significantly lower mortality than spiderlings belonging to the 66% foster treatment (table 2). There was a trend that spiderlings of the 100% foster treatment had a lower mortality compared with the 66% foster treatment, albeit not significant (table 2 and figure 4).
, p = 0.008). Spiderlings belonging to the 0% foster treatment had a significantly lower mortality than spiderlings belonging to the 66% foster treatment (table 2). There was a trend that spiderlings of the 100% foster treatment had a lower mortality compared with the 66% foster treatment, albeit not significant (table 2 and figure 4).
Table 2.
Wald statistics (W) obtained from the GEE showing the differences in mortality between the four treatments.
| treatments | estimate | s.e. | W | p |
|---|---|---|---|---|
| 33% foster/0% foster | 0.86 | 0.52 | 2.76 | 0.096 |
| 66% foster/0% foster | 1.25 | 0.42 | 8.53 | 0.0035 |
| 66% foster/33% foster | 0.39 | 0.51 | 0.57 | 0.45 |
| 100% foster/0% foster | 0.44 | 0.42 | 1.12 | 0.3 |
| 33% foster/100% foster | 0.42 | 0.51 | 0.69 | 0.4 |
| 66% foster/100% foster | 0.81 | 0.44 | 3.4 | 0.065 |
Figure 4.
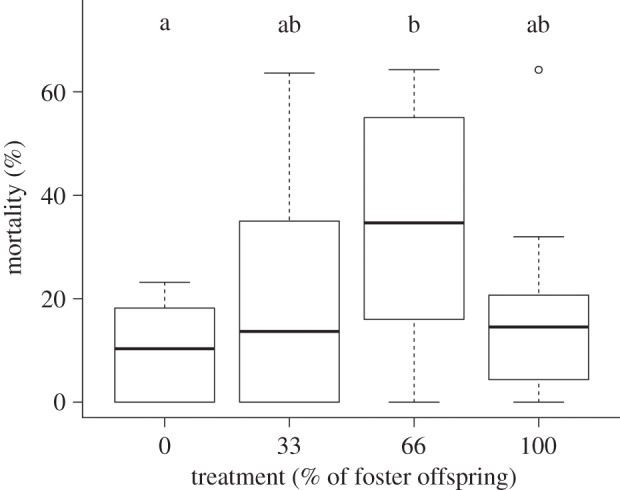
Differences in offspring mortality between the four treatments (0, 33, 66 and 100% foster treatment; a and b express the statistical difference, Wald test p < 0.05).
4. Discussion
We experimentally manipulated the group composition of D. ergandros broods to test how the presence of unrelated spiderlings affects dynamics between female and brood as well as within broods. We found that broods consisting of siblings grew better compared with mixed broods, independent of their relatedness to the caring female. Our results are consistent with a scenario where resource distribution is more strongly influenced by interactions between offspring than by female interests. Contrary to predictions of the maternal control hypothesis, females lost mass when caring for broods consisting of siblings only (0 and 100% foster treatment). This suggests that females shared more food with siblings than with mixed broods. Indeed females caring for 66% foster offspring (mixed broods) gained mass and spiderlings in this treatment had the highest mortality. These results indicate that (i) females do vary the amount of prey they share with offspring groups, although the pattern of food sharing does not consistently decrease with decreasing relatedness to the brood; and (ii) offspring dynamics may have a signalling function that affects the food-provisioning behaviour even of foster mothers.
A previous study of the same species concluded that females provide more care for own offspring, as females caught more prey for own offspring than for foster offspring, and own offspring grew better than foster offspring [17]. These different findings may be due to different experimental procedures. In our experiment, we provided a single large fly per female and all females were equally likely to capture prey regardless of the relatedness to their brood. Evans [17], on the other hand, offered two slightly smaller flies, which may have resulted in a different hunting and food-sharing pattern. The contrasting results indicate the presence of a flexible hunting behaviour depending on the available prey type. Moreover, we did not find matriphagy, suggesting that these spiders may be plastic in both their hunting behaviour and whether matriphagy occurs or not. In fact, these two may be linked: in situations where exclusively large prey items are available and offspring are not able to overwhelm them, the presence of a hunting mother may be more beneficial than consuming her. In situations where small prey items are dominating, spiderlings may be able to hunt on their own and would have an additional nutritional benefit by consuming the mother [26]. Plasticity in matriphagy has been demonstrated in another subsocial spider. In Stegodyphus lineatus, matriphagy occurred significantly later or not at all when females were caring for an experimentally reduced number of offspring [26]. Plasticity in brood-caring behaviour is also common in birds, and several factors, including prey availability [27] and parents’ personalities [28], may affect the amount of care.
When feeding on a communal prey item, spiderlings in mixed broods may behave differently compared with siblings, because spiderlings of the mixed treatments are only related to a part of the group. In addition, genetic variation within the brood might lead to phenotypic variation in foraging efficiency, which may result in some individuals foraging better than others [29]. Direct competitive interactions between spiderlings may be more frequent in mixed broods, for example by excluding unrelated and/or unfamiliar group members from foraging, while siblings may cooperate and thus gain inclusive fitness when the direct costs of sharing are lower than the benefits [3,12]. An important next step to test this mechanism is to individually mark spiderlings and observe their foraging behaviour as well as interactions between female and offspring more closely. In barn swallows, unrelated nestlings were competing more intensely, and it was suggested that kin selection may be the mechanism to resolve this conflict [30]. Unrelated spiderlings might also be more reluctant than highly related broods to contribute their digestive enzymes to a common prey item, which ultimately reduces feeding efficiency and growth rates. This was found in the subsocial spider S. lineatus, where related spiders extracted more mass out of a common prey and grew better than unrelated spiders [31]. Similarly, differences in extracting prey mass may explain the overall reduced growth and higher mortality within mixed broods in our experiment. However, even though we assorted the groups at a very early stage in their life, we cannot distinguish whether effects of relatedness or familiarity cause the differences in our study. Diaea ergandros individuals are able to recognize kin [32], thus kin discrimination could potentially cause these differences. In other subsocial spiders, sibling-specific cuticular hydrocarbons are possible kin recognition cues [20] and these might exist with a similar function in D. ergandros as well.
In our experiment, sibling broods that were fed by an unrelated foster mother did not differ in growth from those sibling broods with genetic mothers. This result suggests that offspring dynamics as described above may be a signal that prompts even unrelated foster mothers to leave more of the liquefied prey for the brood. Offspring dynamics may for example affect the conflict over food provisioning in birds [10]. In great tits (Parus major), females and males provide food differently depending on the social network structure of offspring. Females provide more food to small and medium-sized offspring groups, which show a stronger social network structure than large groups, while the amount of male care is negatively correlated with a strong network structure and thus males provide more food when caring for large groups [10]. Mixed broods in our experiment may have sent a weaker signal due to a lower network structure and thus females caring for mixed broods may have ingested more food themselves. Contrary to our prediction, however, this did not result in the production of a second clutch. Even though we observed that females can produce a second clutch shortly after the first one failed, it seems that females are unable to produce another clutch after the first one has hatched. Evans et al. [19] described that the ovaries of D. ergandros degrade to produce trophic eggs after oviposition and there seems to be no plasticity even in cases where all offspring are removed.
The idea that food-provisioning behaviour of females may be more dependent on offspring dynamics than on female offspring discrimination is supported by the finding that all females were equally likely to hunt and share the prey item. Such a lack of discrimination has also been shown in birds, where parents of a semi-colonial swallow species do not discriminate between calls of genetically related or foster nestlings [33], and in burying beetles (Nicrophorus vespilloides), where females care for unrelated larvae [14]. An explanation may be that the costs of alloparental care could be relatively low compared with the cost of a rejection error [34]. Costs of alloparental care may further be outbalanced when an increased group size has positive effects [35], for example enhanced defence against predators [36].
In conclusion, we showed that immigrating spiderlings have a negative effect on spider group dynamics. This effect might be imposed by the non-relatedness and/or unfamiliarity of immigrant spiderlings. The challenge for future research is to identify the mechanism that causes the differences and also to investigate the interactions of immigrating individuals with the family group more closely.
Acknowledgements
We would like to thank Theo Evans for helpful discussions prior to the project and comments on the manuscript. The manuscript was improved thanks to valuable comments from Per Smiseth and an anonymous reviewer. We are grateful for constructive comments on the manuscript from Torben Riehl as well as from the Behavioural Ecology Lab in Hamburg, especially Stefanie Zimmer, Jannis Liedtke, Wiebke Schuett and Rainer Neumann.
Data accessibility
Data are available in Dryad: http://doi.org/10.5061/dryad.53942.
Funding statement
J.R. was financially supported by an international scholarship of Macquarie University.
References
- 1.Parker GA, Macnair MR. 1979. Models of parent–offspring conflict. IV. Suppression: evolutionary retaliation by the parent. Anim. Behav. 27, 1210–1235 (doi:10.1016/0003-3472(79)90068-X) [Google Scholar]
- 2.Trivers RL. 1974. Parent–offspring-conflict. Am. Zool. 14, 249–264 [Google Scholar]
- 3.Mock DW, Parker GA. 1997. The evolution of sibling rivalry. In Oxford series in ecology and evolution (eds May RM, Harvey PH.), pp. 1–11 Oxford, UK: Oxford University Press [Google Scholar]
- 4.Roulin A, Dreiss AN. 2012. Sibling competition and cooperation over parental care. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M.), pp. 133–149 Oxford, UK: Oxford University Press [Google Scholar]
- 5.Macnair MR, Parker GA. 1979. Models of parent–offspring conflict. III. Intra-brood conflict. Anim. Behav. 27, 1202–1209 (doi:10.1016/0003-3472(79)90067-8) [Google Scholar]
- 6.Godfray HCJ. 1991. Signalling of need by offspring to their parents. Nature 352, 328–330 (doi:10.1038/352328a0) [Google Scholar]
- 7.Godfray HCJ. 1995. Evolutionary-theory of parent–offspring conflict. Nature 376, 133–138 (doi:10.1038/376133a0) [DOI] [PubMed] [Google Scholar]
- 8.Andrews CP, Smiseth PT. 2013. Differentiating among alternative models for the resolution of parent–offspring conflict. Behav. Ecol. 24, 1185–1191 (doi:10.1093/beheco/art048) [Google Scholar]
- 9.Parker GA, Royle NJ, Hartley IR. 2002. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. Lond. B 357, 295–307 (doi:10.1098/rstb.2001.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royle NJ, Pike TW, Heeb P, Richner H, Kolliker M. 2012. Offspring social network structure predicts fitness in families. Proc. R. Soc. B 279, 4914–4922 (doi:10.1098/rspb.2012.1701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royle NJ, Hartley IR, Parker GA. 2002. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 17, 434–440 (doi:10.1016/s0169-5347(02)02565-x) [Google Scholar]
- 12.Hamilton WD. 1964. The genetical evolution of social behaviour I. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 13.Evans TA, Goodisman MAD. 2002. Nestmate relatedness and population genetic structure of the Australian social crab spider Diaea ergandros (Araneae: Thomisidae). Mol. Ecol. 11, 2307–2316 (doi:10.1046/j.1365-294X.2002.01623.x) [DOI] [PubMed] [Google Scholar]
- 14.Muller JK, Eggert AK, Dressel J. 1990. Intraspecific brood parasitism in the burying beetle, Nicrophorus vespilloides (Coleoptera, Silphidae). Anim. Behav. 40, 491–499 (doi:10.1016/s0003-3472(05)80529-9) [Google Scholar]
- 15.Godfray HCJ. 1995. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 146, 1–24 (doi:10.1086/285784) [Google Scholar]
- 16.Royle NJ, Hartley IR, Owens IPF, Parker GA. 1999. Sibling competition and the evolution of growth rates in birds. Proc. R. Soc. Lond. B 266, 923–932 (doi:10.1098/rspb.1999.0725) [Google Scholar]
- 17.Evans TA. 1998. Offspring recognition by mother crab spiders with extreme maternal care. Proc. R. Soc. Lond. B 265, 129–134 (doi:10.1098/rspb.1998.0273) [Google Scholar]
- 18.Evans TA. 1995. Two new social crab spiders (Thomisidae: Diaea) from eastern Australia, their natural history and geographic range. Rec. West. Aust. Museum Suppl. 52, 151–158 [Google Scholar]
- 19.Evans TA, Wallis EJ, Elgar MA. 1995. Making meal of mother. Nature 376, 299 (doi:10.1038/376299a0)7630393 [Google Scholar]
- 20.Grinsted L, Bilde T, d'Ettorre P. 2011. Cuticular hydrocarbons as potential kin recognition cues in a subsocial spider. Behav. Ecol. 22, 1187–1194 (doi:10.1093/beheco/arr105) [Google Scholar]
- 21.Foelix RF. 1996. Biology of spiders. Oxford, UK: Oxford University Press [Google Scholar]
- 22.Schneider JM, Lubin Y. 1997. Does high adult mortality explain semelparity in the spider Stegodyphus lineatus (Eresidae)? Oikos 79, 92–100 (doi:10.2307/3546094) [Google Scholar]
- 23.Evans TA. 1997. Distribution of social crab spiders in eucalypt forests. Austr. J. Ecol. 22, 107–111 (doi:10.1111/j.1442-9993.1997.tb00646.x) [Google Scholar]
- 24.Evans TA. 1998. Factors influencing the evolution of social behaviour in Australian crab spiders (Araneae: Thomisidae). Biol. J. Linn. Soc. 63, 205–219 (doi:10.1111/j.1095-8312.1998.tb01514.x) [Google Scholar]
- 25.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 26.Salomon M, Schneider JM, Lubin Y. 2005. Maternal investment in a spider with suicidal maternal care, Stegodyphus lineatus (Araneae, Eresidae). Oikos 109, 614–622 (doi:10.1111/j.0030-1299.2005.13004.x) [Google Scholar]
- 27.Chiaradia A, Nisbet ICT. 2006. Plasticity in parental provisioning and chick growth in Little Penguins Eudyptula minor in years of high and low breeding success. Ardea 94, 257–270 [Google Scholar]
- 28.Westneat DF, Hatch MI, Wetzel DP, Ensminger AL. 2011. Individual variation in parental care reaction norms: integration of personality and plasticity. Am. Nat. 178, 652–667 (doi:10.1086/662173) [DOI] [PubMed] [Google Scholar]
- 29.Beauchamp G, Giraldeau LA, Ennis N. 1997. Experimental evidence for the maintenance of foraging specializations by frequency-dependent choice in flocks of spice finches. Ethol. Ecol. Evol 9, 105–117 (doi:10.1080/08927014.1997.9522890) [Google Scholar]
- 30.Boncoraglio G, Caprioli M, Saino N. 2009. Fine-tuned modulation of competitive behaviour according to kinship in barn swallow nestlings. Proc. R. Soc. B 276, 2117–2123 (doi:10.1098/rspb.2009.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider JM, Bilde T. 2008. Benefits of cooperation with genetic kin in a subsocial spider. Proc. Natl Acad. Sci. USA 105, 10 843–10 846 (doi:10.1073/pnas.0804126105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans TA. 1999. Kin recognition in a social spider. Proc. R. Soc. Lond. B 266, 287–292 (doi:10.1098/rspb.1999.0635) [Google Scholar]
- 33.Leonard ML, Horn AG, Brown CR, Fernandez NJ. 1997. Parent–offspring recognition in tree swallows, Tachycineta bicolor. Anim. Behav. 54, 1107–1116 (doi:10.1006/anbe.1997.0559) [DOI] [PubMed] [Google Scholar]
- 34.Keller L. 1997. Indiscriminate altruism: unduly nice parents and siblings. Trends Ecol. Evol. 12, 99–103 (doi:10.1016/s0169-5347(96)10065-3) [DOI] [PubMed] [Google Scholar]
- 35.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unglaub B, Ruch J, Herberstein M, Schneider J. 2013. Hunted hunters? Effect of group size on predation risk and growth in the Australian subsocial crab spider Diaea ergandros. Behav. Ecol. Sociobiol. 67, 785–794 (doi:10.1007/s00265-013-1502-0) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in Dryad: http://doi.org/10.5061/dryad.53942.