Abstract
Correlations in family size across generations could have a major influence on human population size in the future. Empirical studies have shown that the associations between the fertility of parents and the fertility of children are substantial and growing over time. Despite their potential long-term consequences, intergenerational fertility correlations have largely been ignored by researchers. We present a model of the fertility transition as a cultural process acting on new lifestyles associated with fertility. Differences in parental and social influences on the acquisition of these lifestyles result in intergenerational correlations in fertility. We show different scenarios for future population size based on models that disregard intergenerational correlations in fertility, models with fertility correlations and a single lifestyle, and models with fertility correlations and multiple lifestyles. We show that intergenerational fertility correlations will result in an increase in fertility over time. However, present low-fertility levels may persist if the rapid introduction of new cultural lifestyles continues into the future.
Keywords: fertility, intergenerational transmission of fertility, differential fertility, demographic transition, cultural evolution, socialization
1. Introduction
During the past 200 years, fertility has declined all over the world. This decline has been dramatic in the developed world with fertility dropping by more than half between the mid-nineteenth century and the early twentieth century. This reduction in fertility, called the fertility transition, is well known and widely documented [1–3]. A slightly less well-known but related phenomenon is that the correlation between parent and child fertility has increased from insignificant levels prior to the fertility transition, to moderate and increasing levels in contemporary societies [4–6]. This correlation may be genetic, cultural or some combination of the two [7–13]. The increasing intergenerational correlation suggests that cultural or genetic inheritance has become an increasingly significant determinant of fertility. However, the rapidness of the diffusion of low fertility during the fertility transition precludes the possibility that genes and culture inherited from parents are the sole determinants of fertility [14–16]. Thus, cultural information acquired from non-parents must also affect fertility.
Intergenerational fertility correlations can be found consistently in developed countries and are increasing over time [4–6]. Most social scientists have explained these fertility continuities in terms of values transmitted from parents to children [8,17–20]. Fertility norms are transmitted via interaction along social networks [14,21,22]. Fertility has been shown to be deeply ingrained in the culture of a society and less associated with socioeconomic factors [2,23].
Twin studies have shown that both shared environment and genetic components play an important role in explaining fertility continuities [10,24]. Genetic effects are stronger in populations practising contraception, and are growing in strength over time and when individuals have agency over their fertility [25,26]. Intergenerational transmission of fertility was low or non-existent prior to and during the fertility transition, both in terms of population-level intergenerational correlations and measures of genetic heritability [25,27–29] (though see [11]).
2. Consequences of intergenerational fertility
Given that variation in fertility has become associated across generations, the basic premises of evolution suggest that the frequency of individuals with relatively more children will increase, and hence the overall level of fertility must also increase (a logical implication recognized by several studies, e.g. [26,30–35]). This raises the possibility that the recent global decline in fertility might be reversed. This reversal will occur regardless of whether intergenerational fertility is the result of cultural or genetic factors, provided intergenerational fertility correlations persist. Researchers have shown that even moderate intergenerational fertility associations could increase population size [32,34].
Any explanation of recent fertility trends must account for both the transition from high fertility to low fertility and the transition from no intergenerational associations to large associations. In this study, we propose a relationship between the fertility of parents, children and other role models within a society, and illustrate this relationship with an analytical model. Intergenerational fertility associations may be driven by children resembling their parents both culturally and genetically, and our model is agnostic to the particular mechanism. In this study, we examine how the fertility transition affects intergenerational fertility correlations and examine potential long-term developments in fertility and fertility correlations based on different assumptions concerning how fertility is transmitted across generations. We explore under which circumstances low fertility can persist and under which circumstances the fertility transition will be reversed.
We assume that an individual's fertility is influenced by its social environment, including both parents and a wider social group. To describe how these influences operate, we make a distinction between lifestyles and lifestyle preferences. Lifestyles are cultural behaviours that individuals copy or acquire from other individuals. Lifestyles may affect fertility both directly (e.g. the timing of first child and ideal family size) and indirectly (e.g. choices concerning education, religion and career paths) [36–40]. While lifestyles are cultural behaviours that can be copied from any individual, lifestyle preferences are traits that stem solely from the parents. Lifestyle preferences determine the choice of lifestyle in the case where individuals are exposed to more than one lifestyle. If this influence is genetic, it is obvious that it is inherited from the parents. However, our models also apply, without modifications, to cultural values or norms that are primarily inherited from parents because such cultural traits have an evolutionary dynamic similar to that of a genetically heritable trait (cf. [35,41]). Preferences may also be a combination of genetic and cultural information. To the extent that preferences are cultural, they can be viewed as deep-rooted values and aspirations acquired from parents early in life and transmitted across generations. It has been shown that many fundamental aspects of values and preferences are acquired from parents at a young age [42]. The separation of preferences and lifestyles has similarities with the difference between primary and secondary socialization [43,44].
We model intergenerational fertility continuities as the product of the following social process. Inherited (either cultural or genetic) preferences influence which lifestyles an individual adopts from those available in their social environment. This broader social environment consists not only of parents but also other cultural sources, such as teachers, media, neighbours and friends. The fertility of individuals is a function of the fertility characteristics associated with the various lifestyles they have adopted. Thus, the preferences and values inherited from parents do not affect fertility directly, but rather influence fertility through the choices an individual makes between different lifestyles. This social process will cause evolution (biological and cultural) of fertility patterns, which we explore in this paper when cultural evolution introduces new lifestyles into the population.
From our perspective, the fertility transition can be viewed as the enthusiastic response to new low-fertility lifestyles, introduced as a result of the radical socioeconomic transformations of the nineteenth and twentieth centuries. During this time, a broader range of lifestyles became socially acceptable (cf. [1]), allowing latent lifestyle preferences to manifest. These newly adopted lifestyles were primarily associated with decreased fertility. The increasing fertility correlations after this time suggest that there is variation transmitted across generations in the preferences for these novel low-fertility lifestyles.
We make a distinction between lifestyles and preferences so that we can distinguish between genetic and cultural factors when genetic factors are involved. But the distinction is also a part of our explanation of the fertility transition, which requires that preferences exist for at least some novel low-fertility lifestyles. If individuals copied lifestyle without any bias with respect to lifestyle properties, low-fertility lifestyles could never invade.
3. Model 1: single low-fertility lifestyle
We aim to keep our analytical model as simple as possible while remaining consistent with both the observed decline in fertility and the increase in fertility correlations. Our model is embedded in a simple demographic process with discrete, non-overlapping generations. In each generation, individuals inherit the lifestyle preferences of their parents. For simplicity, we assume that there are only two possible lifestyles, one with low fertility (L) and the other with high fertility (H), with individuals inheriting a preference for either of the lifestyles. Individuals then acquire their preferred lifestyle if it is available within the social environment (i.e. a lifestyle displayed by at least one cultural role model, including parents), otherwise they acquire the other lifestyle. Individuals then produce a variable number of children based on their acquired fertility lifestyle. This process is then repeated every generation with new individuals acquiring the lifestyle preferences of their parents.
We model the change in relative proportions of the four possible types of mature individuals (HH, LH, LL, HL, where the first letter denotes lifestyle and the second letter inherited preference). In each generation t, some proportion of the population will have the preference for and have successfully acquired the high-fertility lifestyle trait. This proportion is denoted PHH(t). Similarly, the proportion of generation t which prefers and has the low-fertility lifestyle trait is denoted PLL(t). Some proportion of generation t will have the preference for the high-fertility trait but have failed to acquire it, while some other proportion will have the preference for the low-fertility trait but have failed to acquire it. These proportions are denoted PLH(t) and PHL(t), respectively.
We assume that each individual, both those with low- and high-fertility traits, has a discrete number of children given by the Poisson distribution. Those individuals with the high-fertility trait have rh children on average, while those with the low-fertility trait have rl children on average.
In model 1, we assume the following:
— Children perfectly inherit their parents' lifestyle preferences.
— The social environment of a child (those individuals from whom lifestyle traits can be acquired) consists of the child's parents and m (for models) other cultural role models chosen at random from the parents' generation.
— If a child has a preference for a given lifestyle and that lifestyle is displayed within the social environment of the child, then the child will acquire their preferred lifestyle trait. If the preferred lifestyle trait is not present in the social environment, which in our two-lifestyle model implies a homogeneous social environment, then the child acquires the only lifestyle available.
Consider children born to LH parents (i.e. parents with a preference for the high-fertility lifestyle who display the low-fertility lifestyle). These children inherit their parents' preferences for the high-fertility lifestyle; however, they only acquire the high-fertility lifestyle if it is present in their social environment (that is, if at least one of m cultural role models displays the high-fertility trait). This occurs with probability
The probability that all models display the low-fertility trait is (1 − p).
Similarly, children born to HL parents inherit their parents' preferences for the low-fertility lifestyle but only acquire the low-fertility lifestyle if it is present in their social environment (that is, if at least one of their cultural role models displays the high-fertility lifestyle). This occurs with probability
The probability that all cultural role models display the low-fertility trait is (1 − q).
By contrast, children born to HH parents and LL parents inherit their parents' lifestyle preferences, and then as their parents are a part of the social environment these children also acquire their preferred lifestyle traits.
Using the preceding considerations, the proportions of each the four types of individuals have the following dynamics from generation to generation:
In spite of its drastically simplifying assumptions, this model is able to capture the observed qualitative trends of decreasing fertility and increasing intergenerational fertility correlations, while clearly illustrating the cultural and evolutionary processes we believe are partially responsible for these empirical trends.
(a). Results from model 1
To examine the effect of intergenerational transmission of fertility on the development of fertility and fertility correlations over time, we initialize our model simulations in a high-fertility population where a new, low-fertility lifestyle has been introduced and where the preference for this new lifestyle is initially widespread. This disequilibrium state is our representation of the preconditions for the fertility transition, resulting from the social changes owing to rapid industrialization. We used a fertility difference between our low- and high-fertility lifestyles of 1.5 : 1 for most of our models. In contemporary societies, differential fertility up to 2 : 1 can frequently be observed between subpopulations with differing education [45] or ethnicity [46].
The results of our model over 25 generations are illustrated in figures 1 and 2. Figure 1 shows the relative proportions of the population from generation to generation. Note that initially and very rapidly (over the course of three generations) the HL proportion of the population (purple, dotted) goes to zero, while the LL proportion of the population (red, dot-dashed) increases. However after this initial rapid increase in the LL type, the population shifts more gradually to being composed primarily of the HH type (blue, solid), as this type of individual has a higher fertility than the LL type, and both of these types reproduce themselves every generation.
Figure 1.
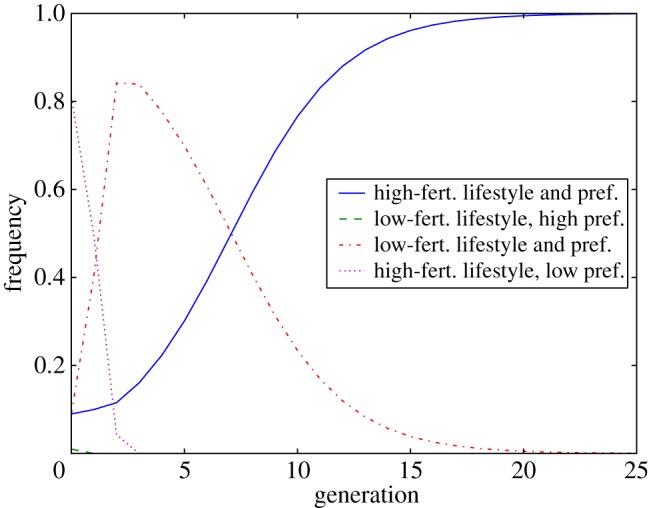
Relative frequencies of PHH (blue, solid), PLH (green, dashed), PHL (purple, dotted) and PLL (red, dot-dashed) over time, after the manifestation of a new low-fertility lifestyle (model 1). We use the following initial values at t = 0: PHH(0) = 0.09, PLH(0) = 0.01, PHL(0) = 0.81 and PLL(0) = 0.09. The code to produce this figure can be found in electronic supplementary material, appendix B. (Online version in colour.)
Figure 2.
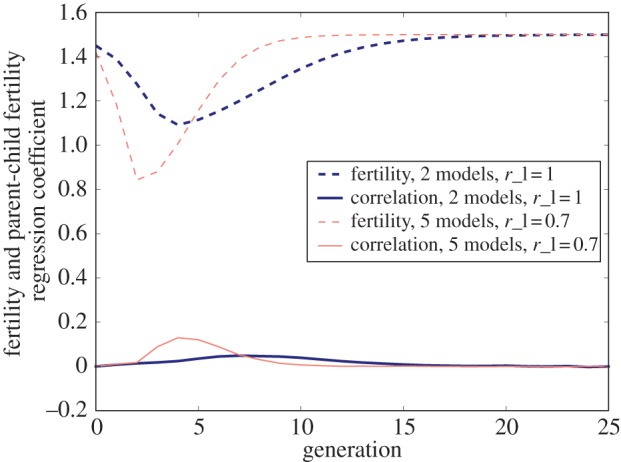
Fertility and fertility correlations over time after the manifestation of a new low-fertility lifestyle (model 1). Dashed lines show fertility, solid lines show observed fertility correlations. The code to produce this figure can be found in electronic supplementary material, appendix B. (Online version in colour.)
The effects on fertility and the intergenerational fertility correlations of these changes in population composition can be seen in figure 2. We show results for two different sets of parameters. The dark blue lines show a model with two cultural role models and with low and high mean fertility of 1 and 1.5, respectively. The light red lines show a model with five cultural role models and with low and high mean fertility of 0.7 and 1.5, respectively. The top dashed lines show the average fertility of the population. The initial sharp decrease in fertility is the result of the rapid decrease in HL types and the corresponding increase in LL types. After this initial drop in fertility, the relatively slow increase in fertility is the result of the HH subpopulation slowly out growing the LL subpopulation. The results for different parameters are qualitatively similar though more cultural role models increase the pace of the process, and the levels of differential fertility determine the magnitude of change in fertility. Our measure of the extent of intergenerational fertility is the correlation (Pearson's r) in fertility between the parent and child generations. The correlation is shown with a solid line for two different parameter choices. Initially, fertility correlations are low because almost all individuals have high fertility, as do their children. However, as the low-fertility lifestyle spreads variation in fertility increases and this variation is inherited. Fertility correlations reach the maximum roughly around the time that the population is composed of equal parts of LL and HH types, and hence variance in fertility is at the maximum. As the HH subpopulation dominates the whole population, there is less variance in the total population, and hence less fertility correlation. For parameters with high differential fertility, fertility correlations are higher.
An equilibrium analysis found in electronic supplementary material, appendix A-1, shows that regardless of the initial conditions, high fertility will eventually dominate the population. Specifically, any initial condition with PHH > 0 will converge to a population state satisfying PHH + PHL = 1. In this sense, the broad qualitative results of our models are robust to changes in parameters (so long as rl < rh), though details (for example, how long it takes to converge to an equilibrium population state, and what the balance of HH and HL types is at this equilibrium, are sensitive to parameter choice). Our model of the fertility transition is contingent upon a specific set of initial conditions (see the electronic supplementary material, appendix A-1), namely that a large share of the population has a latent preference for the low-fertility lifestyle (HL).
4. Model 2: multiple low-fertility lifestyles
Overall, the results of the first model show how basic evolutionary mechanisms and intergenerational fertility correlations result in the eventual dominance of high fertility. To examine the conditions under which low fertility may be sustainable, we develop the second model, exploring circumstances in which the outcome of model 1 is not inevitable. In this model, the continuous introduction of novel cultural traits allows for the possibility of sustained low fertility.
Given that fertility remains associated across generations, the only way for low fertility to be maintained is by the continuous introduction of novel lifestyles that reduce fertility (e.g. new leisure activities or professional opportunities). This seems possible if cultural diversity continues to increase. This process can be viewed as analogous to what population geneticists refer to as mutation–selection balance. Genetic mutations with significant fertility effects do not occur with the frequency required to sustain relatively low fertility. However, there is the possibility that cultural innovations, similar to those responsible for the fertility transition, may be introduced with much higher frequency than genetic mutations, perhaps even at the frequency required to maintain relatively low fertility. Implicit in this line of reasoning is that the rate at which novel lifestyles are introduced has increased dramatically since the time of fertility transition.
In this second model, in contrast to our basic model, we allow a person's lifestyle to consist of many binary lifestyle elements, each with its unique effect on fertility. In this model, individuals’ preferences are also, with some small probability, the opposite of their parents’ preferences. In model 2, individuals occasionally adopt a lifestyle element for which they have a preference, even when this lifestyle element has not been displayed by parents or other members in the social environment. This is in contrast to our basic model, where people only acquire lifestyle elements which are in the social environment and perfectly acquire preferences from their parents. In model 2, we assume that lifestyle preferences are independent of each other. If lifestyle preferences with a similar effect on fertility were correlated, the dynamics of such a model come to resemble those of model 1 if the correlation is high enough. For a detailed description of model 2, including parameter choices and equations, we refer to electronic supplementary material, appendix A-2. The Python code used to generate our results for model 2 is available in electronic supplementary material, appendix C.
(a). Results from model 2
The result of our second model is depicted in figure 3. We show results for two different sets of parameter choices (similar to figure 2). The early fertility dynamics of model 2 are broadly similar to those of model 1. However, dissimilar to model 1, fertility does not fully rebound to its initial high level. This stable intermediate level of fertility is the result of a cultural innovation process continuously introducing novel lifestyles associated with low fertility. Similarly, fertility correlations initially increase in both models. However in model 2, dissimilar to model 1, the fertility correlations persist over time. The reason for the decline of fertility correlations in the first model is that eventually there is no variation in lifestyles and thus no possibility of variation across generations. By contrast, in model 2, novel lifestyle elements are continuously introduced, maintaining variation and creating the possibility of variation across generations.
Figure 3.
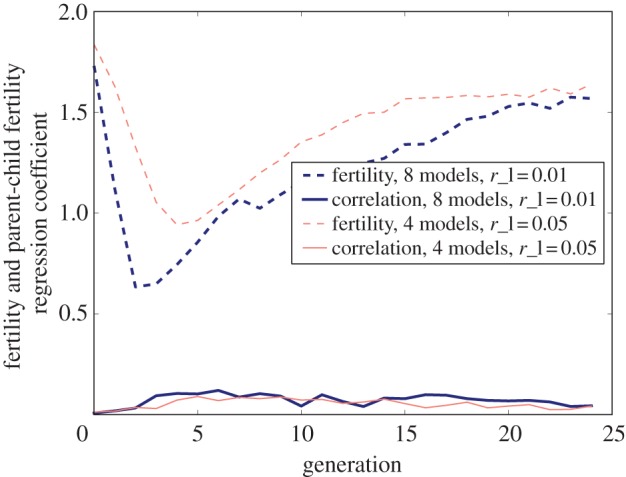
Fertility and fertility correlations over time after the manifestation of a new low-fertility lifestyle, followed by the continuous introduction of new lifestyles (model 2). The dashed lines show mean fertility and the solid lines show observed fertility correlations. The parameter choices and code used to produce this figure can be found in electronic supplementary material, appendix C. (Online version in colour.)
The results of model 2 show that the rapid introduction of novel lifestyles can maintain low fertility even when fertility is associated across generations. This effect is magnified when, as in model 2, novel lifestyle preferences are initially-fertility-neutral (and hence able to spread rapidly), and only later become associated with low fertility.
5. Discussion
The fall in human fertility over the last two centuries is still a puzzle to demographers. The decline in fertility is at most weakly correlated with socioeconomic determinants and mortality patterns [2]. Nor can fertility decline be explained by changes in demand for children [47]. Human behaviour ecologists have largely failed to show that the demographic transition is consistent with fitness-maximizing strategies [48,49]. Recent explanations of the fertility transition have instead increasingly relied on explanations focusing on the role of cultural diffusion [16,25,35,41,50]. The last century has been characterized by the spread of new cultural norms and new technology that have reduced fertility all over the world. One example of such a norm is the increase in individual agency relative to broader societal norms over family formation [38,51]. One way to understand this is that both the number of lifestyles and the freedom to choose cultural lifestyles have increased, creating greater potential correlations in lifestyle choices across generations [26]. The spread of lifestyles associated with low fertility is then the cause of the fertility decline.
Boyd and Richerson's ‘parent–teacher model’ [41] shows how low fertility can spread in a population and work counter to genetic evolution. As in our model, when lifestyle traits are acquired from people other than parents and when those lifestyle traits associated with lower fertility are favoured by cultural learning, a decrease in fertility owing to cultural processes is possible.
The purpose of our models is to study the short- and long-term consequences of the introduction of new cultural lifestyles on human fertility, paying particular attention to the role of parent–child correlations in fertility. Our models provide a parsimonious account of interrelated phenomena, namely that parent–child fertility became increasingly correlated while fertility rates simultaneously declined. The existence of intergenerational fertility correlations and basic evolutionary logic together suggest that lifestyles with higher fertility will increase over time. On the other hand, low-fertility lifestyles favoured by cultural transmission could potentially have an opposite effect. Using our simple model, we are able to examine the circumstances that will determine which of these processes dominate in the short and long term.
Our models are limited to discrete lifestyles, non-overlapping generations, a single sex, near-perfect intergenerational correlations in preferences and simplified cultural learning. We ignore factors that could potentially influence fertility over time exogenous to our models, for example technological or cultural transformations with near-universal impact across society (e.g. government institutions, mass media or technological change). We also ignore the role of resource constraints on fertility. If human populations reach their carrying capacity, this will effectively cap population growth in a way unaccounted for in our models. Similarly, future changes in the cost of rearing children could affect fertility. Thus, future fertility developments are affected by a large number of demographic and socioeconomic aspects unaccounted for in our models. These other factors may be at least as important as intergenerational fertility correlations.
Central to our models is a separation between lifestyles and lifestyle preferences. It is by assuming that lifestyle preferences are acquired not only primarily from parents, but that lifestyles are also acquired culturally from non-parents, that we can simultaneously have both an increase in fertility continuities and a decline in fertility. The models allow for these preferences acquired from parents to be either genetic or cultural (or some combination of the two). The distinction between preferences and lifestyles is less clear in the cultural case. It may not always be possible to make this distinction when social experiences are diverse and nested. However, and in spite of this, if there are social experiences that stem mainly from parents and influence lifestyle choices, then cultural evolution of the kind depicted in our models will nonetheless take place. Thus, while this distinction is a simplification of reality, we believe that it provides valuable insight into the fertility transition and the associated rise in fertility continuities.
6. Will low fertility persist?
To answer this question, we distinguish three different scenarios for future fertility patterns given different assumptions on the availability of lifestyles and intergenerational fertility associations. Each scenario begins with an initial decline in fertility similar to empirical patterns of the last century. However, after this initial decline these scenarios diverge, in terms of both trends in fertility and observed fertility correlations. These scenarios are illustrated in figure 4. We believe that contemporary societies are approaching, or have recently reached, the fertility minimum of these scenarios. We have marked the approximate temporal location of contemporary societies in figure 4.
Figure 4.
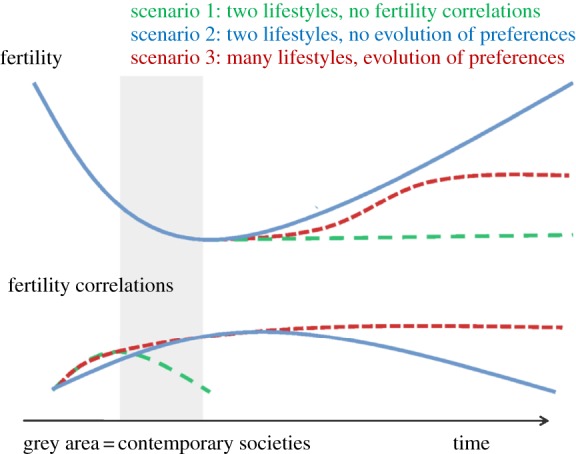
Sketch of three different scenarios, each with different assumptions on fertility correlations across generations, showing implications for fertility (population size) and fertility correlations. (Online version in colour.)
The first scenario (green, long-dashed) approximates the mainstream interpretation of the demographic transition in which the fertility transition is a one-way process from stable high to stable low fertility. This is analogous to the previously discussed parent–teacher model, in which a culturally attractive low-fertility lifestyle spreads until it is almost universal. In this scenario, fertility correlations will disappear at about the same time as the low-fertility lifestyle becomes universal. We believe that this scenario is implausible because it is not consistent with the empirical trend of stable or increasing fertility correlations. Not only does it appear to be empirically incorrect, but this scenario also suggests that future intergenerational fertility correlations will be trivial, which we argue is inconsistent with social scientists' understanding of parent–child socialization.
In the second scenario (blue, solid), which is analogous to our first model with a single lifestyle, an initial decline in fertility is followed by an increase in initial levels, reversing the initial fertility transition. In this scenario, fertility correlation initially increases, peaks after fertility reaches the minimum and slowly declines thereafter. In contrast to the first scenario, this scenario is consistent with current empirical trends in fertility correlations. This scenario implies that the fertility transition is the result of a distinct historical transformation, perhaps an increase in individual agency over fertility, associated with major socioeconomic transitions. If this is the case, mechanisms producing fertility associations across generations will eventually result in a substantial increase in fertility. In both our first and second scenarios, the predictions are identical if preferences are genetic or cultural.
In the third scenario (red, short-dashed), which is analogous to our second model, an initial decline in fertility is followed by a slight increase in fertility, partially reversing the initial fertility transition. In this scenario, fertility correlations reach a stable level. The third scenario is similar to the second scenario, except that it implies that the cultural changes associated with the fertility transition are not singular historical events, but rather the beginning of a rapid and ongoing increase in cultural diversity. The third scenario is consistent with model 2, which assumes a large number of potential lifestyles, each independently associated with fertility, and that preferences for these lifestyles are primarily acquired by children from their parents. The final fertility outcome in this scenario will depend on the long-term equilibrium of a mixed evolutionary process in which cultural processes both introduce novel lifestyle elements of varying effect on fertility and alter the reproductive effect of existent lifestyles, and in which a process similar to natural selection removes preference for lifestyles, reducing fertility from the population.
Regardless of the nature of preferences acquired from parents (cultural or genetic), if the number of possible lifestyles is large and cultural processes continuously introduce novel low-fertility lifestyles then a population with intermediate fertility can be maintained. If preferences are primarily cultural, new lifestyle preferences can be introduced more rapidly, but there is potentially a less clear distinction between lifestyles and lifestyle preferences (cf. [52], where preferences fluctuate owing to purely cultural processes). Without a separation of inherited preferences and culturally acquired lifestyles (as in models 1 and 2), scenarios 2 and 3 are not possible. While all three scenarios are consistent with the fertility transition, only the last two scenarios are consistent with current trends in intergenerational fertility, providing empirical motivation for the separation of lifestyles and lifestyle preferences. At present, we are unable to distinguish between scenarios 2 and 3.
The fertility transition can be explained as a cultural process whereby lifestyles are introduced in a population. This introduces correlations in fertility across generations, absent in societies that lack such choices. Our models suggest a mechanism in which the recent fertility decline may be reversed in the long run. Intergenerational fertility correlations create cultural and genetic selection processes that favour lifestyles with higher fertility. Only through continuous cultural change, introducing novel lifestyles associated with reduced child-bearing, can low fertility persist.
Acknowledgements
We particularly thank Pontus Strimling for helping to develop our initial analytical models, which were a key influence on our eventual models. We also want to acknowledge the insightful suggestions provided by two anonymous peer reviewers.
Data sharing
This article contains no empirical data. The code to produce the figures in the study is available as Python files in electronic supplementary material, appendices B and C.
Funding statement
This study is supported by the following grants from the Swedish Research Council, Vetenskapsrådet: 2009-2390, 2009-2678 and 839-2008-7495.
References
- 1.Coale AJ. 1973. The demographic transition reconsidered. In Proc. of the Int. Population Conf., Liège, Belgium, 1973, pp. 53–71 Liège, Belgium: IUSSP [Google Scholar]
- 2.Coale AJ, Watkins SC. 1986. The decline of fertility in Europe: the revised proceedings of a Conference on the Princeton European Fertility Project. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Van de Walle E, Knodel J. 1980. Europe's fertility transition: new evidence and lessons for today's developing world. Popul. Bull. 34, 3–44 [PubMed] [Google Scholar]
- 4.Murphy M. 1999. Is the relationship between fertility of parents and children really weak? Soc. Biol. 46, 122–145 (doi:10.1080/19485565.1999.9988991) [DOI] [PubMed] [Google Scholar]
- 5.Murphy M, Wang DL. 2001. Family-level continuities in childbearing in low-fertility societies. Eur. J. Popul. 17, 75–96 (doi:10.1023/A:1010744314362) [Google Scholar]
- 6.Bernardi L, White R. 2010. Close kin influences on fertility behaviour. In Family, kinship and state in contemporary Europe (eds Heady P, Kohli M.), pp. 177–202 Frankfurt, Germany: Campus [Google Scholar]
- 7.Johnson NE, Stokes CS. 1976. Family size in successive generations: the effects of birth order, intergenerational change in lifestyle, and familial satisfaction. Demography 13, 175–187 (doi:10.2307/2060799) [PubMed] [Google Scholar]
- 8.Preston SH. 1976. Family sizes of children and family sizes of women. Demography 13, 105–114 (doi:10.2307/2060423) [PubMed] [Google Scholar]
- 9.Kohler H-P, Rodgers JL, Christensen K. 1999. Is fertility behavior in our genes? Findings from a Danish twin study. Popul. Dev. Rev. 25, 253–288 (doi:10.1111/j.1728-4457.1999.00253.x) [Google Scholar]
- 10.Rodgers JL, Hughes K, Kohler HP, Christensen K, Doughty D, Rowe DC, Miller WB. 2001. Genetic influence helps explain variation in human fertility: evidence from recent behavioral and molecular genetic studies. Curr. Dir. Psychol. Sci. 10, 184–188 (doi:10.1111/1467-8721.00145) [Google Scholar]
- 11.Kosova G, Abney M, Ober C. 2010. Heritability of reproductive fitness traits in a human population. Proc. Natl Acad. Sci. USA 107, 1772–1778 (doi:10.1073/pnas.0906196106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pluzhnikov A, Nolan DK, Tan Z, McPeek MS, Ober C. 2007. Correlation of intergenerational family sizes suggests a genetic component of reproductive fitness. Am. J. Hum. Genet. 81, 165–169 (doi:10.1086/518446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolk M. In press. Multigenerational transmission of family size in contemporary Sweden. Popul. Stud. (doi:10.1080/00324728.2013.819112) [DOI] [PubMed] [Google Scholar]
- 14.Carlsson G. 1966. The decline of fertility: innovation or adjustment process. Popul. Stud. 20, 149–174 (doi:10.1080/00324728.1966.10406092) [DOI] [PubMed] [Google Scholar]
- 15.Lesthaeghe R, Neels K. 2002. From the first to the second demographic transition: an interpretation of the spatial continuity of demographic innovation in France, Belgium and Switzerland. Eur. J. Popul. 18, 325–360 (doi:10.1023/A:1021125800070) [Google Scholar]
- 16.Casterline J. 2001. Diffusion processes and fertility transition: introduction. In Diffusion processes and fertility transition: selected perspectives (ed. Casterline J.), pp. 1–38 Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- 17.Anderton DL, Tsuya NO, Bean LL, Mineau GP. 1987. Intergenerational transmission of relative fertility and life course patterns. Demography 24, 467–480 (doi:10.2307/2061386) [PubMed] [Google Scholar]
- 18.Duncan OD, Freedman R, Coble JM, Slesinger DP. 1965. Marital fertility and size of family of orientation. Demography 2, 508–515 (doi:10.2307/2060135) [Google Scholar]
- 19.Barber JS. 2001. The intergenerational transmission of age at first birth among married and unmarried men and women. Soc. Sci. Res. 30, 219–247 (doi:10.1006/ssre.2000.0697) [Google Scholar]
- 20.Kolk M. In press. Understanding transmission of fertility across multiple generations—socialization or socioeconomics? Res. Soc. Stratification Mobility. (doi:10.1016/j.rssm.2013.09.006) [Google Scholar]
- 21.Kohler H-P, Behrman JR, Watkins SC. 2001. The density of social networks and fertility decisions: evidence from South Nyanza District, Kenya. Demography 38, 43–58 (doi:10.1353/dem.2001.0005) [DOI] [PubMed] [Google Scholar]
- 22.Rindfuss RR, Choe MK, Bumpass LL, Tsuya NO. 2004. Social networks and family change in Japan. Am. Sociological Rev. 69, 838–861 (doi:10.1177/000312240406900605) [Google Scholar]
- 23.Bongaarts J, Watkins SC. 1996. Social interactions and contemporary fertility transitions. Popul. Dev. Rev. 22, 639–682 (doi:10.2307/2137804) [Google Scholar]
- 24.Kirk KM, Blomberg SP, Duffy DL, Heath AC, Owens IPF, Martin NG. 2001. Natural selection and quantitative genetics of life-history traits in western women: a twin study. Evolution 55, 423–435 (doi:10.1554/0014-3820(2001)055[0423:NSAQGO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 25.Rodgers JL, Kohler H-P, Kyvik KO, Christensen K. 2001. Behavior genetic modeling of human fertility: findings from a contemporary Danish twin study. Demography 38, 29–42 (doi:10.1353/dem.2001.0009) [DOI] [PubMed] [Google Scholar]
- 26.Udry JR. 1996. Biosocial models of low-fertility societies. Popul. Dev. Rev. 22, 325–336 (doi:10.2307/2061790) [Google Scholar]
- 27.Langford CM, Wilson C. 1985. Is there a connection between a woman's fecundity and that of her mother? J. Biosocial Sci. 17, 437–443 (doi:10.1017/S0021932000015947) [DOI] [PubMed] [Google Scholar]
- 28.Gagnon A, Heyer E. 2001. Intergenerational correlation of effective family size in early Quebec (Canada). Am. J. Hum. Biol. 13, 645–659 (doi:10.1002/ajhb.1103) [DOI] [PubMed] [Google Scholar]
- 29.Reher D, Ortega J, Sanz-Gimeno A. 2008. Intergenerational transmission of reproductive traits in Spain during the demographic transition. Hum. Nat. 19, 23–43 (doi:10.1007/s12110-008-9032-6) [DOI] [PubMed] [Google Scholar]
- 30.Fisher R. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press [Google Scholar]
- 31.Keyfitz N. 1986. The family that does not reproduce itself. Popul. Dev. Rev. 12, 139–154 (doi:10.2307/2807898) [Google Scholar]
- 32.Murphy M, Wang D. 2003. The impact of intergenerationally-transmitted fertility and nuptuality on population dynamics in contemporary populations. In Biodemography of human reproduction and fertility (eds Rodgers JL, Kohler H-P.), pp. 209–228 Boston, MA: Kluwer Academic Publishers [Google Scholar]
- 33.Foster C. 2004. The limits to low fertility: a biosocial approach. Popul. Dev. Rev. 26, 209–234 (doi:10.1111/j.1728-4457.2000.00209.x). [Google Scholar]
- 34.Collins J, Richards JO.2013. Evolution, fertility and the ageing population. UWA Economics Discussion Paper Series, no. 13.02. Perth, Australia: UWA Business School.
- 35.Cavalli-Sforza LL, Feldman MW. 1981. Cultural transmission and evolution: a quantitative approach. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 36.Becker GS. 1991. A treatise on the family. Cambridge, MA: Harvard University Press [Google Scholar]
- 37.Blossfeld HP, Huinink J. 1991. Human capital investments or norms of role transition? How women's schooling and career affect the process of family formation. Am. J. Sociol. 97, 143–168 (doi:10.1086/229743) [Google Scholar]
- 38.Lesthaeghe R. 1995. The second demographic transition in Western countries: an interpretation. In Gender and family change in industrialized countries (eds Mason KO, Jensen A-M.), pp. 17–62 Oxford, UK: Clarendon Press [Google Scholar]
- 39.Shanahan MJ. 2000. Pathways to adulthood in changing societies: variability and mechanisms in life course perspective. Annu. Rev. Sociol. 26, 667–692 (doi:10.1146/annurev.soc.26.1.667) [Google Scholar]
- 40.Kohler H-P. 2001. Fertility and social interaction: an economic perspective. New York, NY: Oxford University Press [Google Scholar]
- 41.Boyd RPD, Richerson PJ. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 42.Berk LE. 1997. Child development, 4th edn Boston, MA: Allyn & Bacon [Google Scholar]
- 43.Parsons T, Bales RF. 1955. Family, socialization and interaction process. Glencoe, IL: Free Press [Google Scholar]
- 44.Mortimer JT, Simmons RG. 1978. Adult socialization. Annu. Rev. Sociol. 4, 421–454 (doi:10.1146/annurev.so.04.080178.002225) [Google Scholar]
- 45.de la Croix D, Doepke M. 2004. Public versus private education when differential fertility matters. J. Dev. Econ. 73, 607–629 (doi:10.1016/j.jdeveco.2003.05.005) [Google Scholar]
- 46.Sobotka T. 2008. The rising importance of migrants for childbearing in Europe. Demogr. Res. 19, 225–248 (doi:10.4054/DemRes.2008.19.9) [Google Scholar]
- 47.Cleland J, Wilson C. 1987. Demand theories of the fertility transition: an iconoclastic view. Popul. Stud. 41, 5–30 (doi:10.1080/0032472031000142516) [Google Scholar]
- 48.Borgerhoff Mulder M. 1998. The demographic transition: are we any closer to an evolutionary explanation? Trends Ecol. Evol. 13, 266–270 (doi:10.1016/S0169-5347(98)01357-3) [DOI] [PubMed] [Google Scholar]
- 49.Barkow JH, Burley N. 1980. Human fertility, evolutionary biology, and the demographic transition. Ethol. Sociobiol. 1, 163–180 (doi:10.1016/0162-3095(80)90006-0) [Google Scholar]
- 50.Watkins SC. 1986. Conclusions. In The decline of fertility in Europe: the revised proceedings of a Conference on the Princeton European Fertility Project (eds Coale AJ, Watkins SC.), pp. 420–449 Princeton, NJ: Princeton University Press [Google Scholar]
- 51.van de Kaa DJ. 1987. Europe's second demographic transition. Popul. Bull. 42, 1–59 [PubMed] [Google Scholar]
- 52.Acerbi A, Ghirlanda S, Enquist M. 2012. The logic of fashion cycles. PloS ONE 7, e32541 (doi:10.1371/journal.pone.0032541) [DOI] [PMC free article] [PubMed] [Google Scholar]


