Abstract
The main role of leaf venation is to supply water across the photosynthetic surface to keep stomata open and allow access to atmospheric CO2 despite evaporative demand. The optimal uniform delivery of water occurs when the distance between veins equals the depth of vein placement within the leaf away from the evaporative surface. As presented here, only angiosperms maintain this anatomical optimum across all leaf thicknesses and different habitats, including sheltered environments where this optimization need not be required. Intriguingly, basal angiosperm lineages tend to be underinvested hydraulically; uniformly high optimization is derived independently in the magnoliids, monocots and core eudicots. Gymnosperms and ferns, including available fossils, are limited by their inability to produce high vein densities. The common association of ferns with shaded humid environments may, in part, be a direct evolutionary consequence of their inability to produce hydraulically optimized leaves. Some gymnosperms do approach optimal vein placement, but only by virtue of their ability to produce thick leaves most appropriate in environments requiring water conservation. Thus, this simple anatomical metric presents an important perspective on the evolution and phylogenetic distribution of plant ecologies and further evidence that the vegetative biology of flowering plants—not just their reproductive biology—is unique.
Keywords: leaf venation, plant evolution, ferns, gymnosperms, angiosperms
1. Introduction
In vascular plants, assimilation of CO2 from the atmosphere is linked to transpirational loss of H2O. The leaf exchange ratio of CO2 for H2O is by no means constant and depends on a variety of leaf properties, including morphology, anatomy, physiological status and biochemistry. As such, photosynthetic activity of any particular plant must lie somewhere between the two extremes of either being optimized to limit water loss to the detriment of carbon gain, thereby achieving high water use efficiency (WUE), or of being optimized to maximize carbon gain and take full advantage of leaf photosynthetic capacity at the cost of substantial water loss, thereby resulting in low WUE. Thus, both the leaf hydraulic properties that determine transpirational capacity and the environmental properties that determine the need to limit water use (e.g. water availability, vapour pressure deficit, wind, temperature and solar radiation) must be considered in order to understand evolutionary trends in the structure and function of planar leaves.
The recognized positive relationship between leaf hydraulic properties and photosynthetic capacity found across extant taxa [1] underscores the role of hydraulics in plant performance. Because higher vein densities allow lower impedance of water delivery to the sites of gas exchange (stomata), vein density (VD) was recently used as a proxy for hydraulic capacity of extant species and even used to derive performance of extinct plants [2–5]. However, the experimentally determined relationship of VD to CO2 uptake and water loss is complex and nonlinear: as VD increases, CO2 assimilation rates plateau while maximum stomatal conductance (and thereby potential transpiration rate) continue to increase exponentially within the existing range of vein densities [6]. These diminishing CO2 gains at the expense of exponentially increasing water losses would require uniform water delivery and equal water potential across leaf lamina for the most effective use of the photosynthetic surface. However, leaf hydraulics is inherently a three-dimensional problem [6–9], whereas VD only reflects the planar (two-dimensional) structure of the leaf and relates only to maximization of transpirational capacity. As outlined below, inclusion in the analysis of the third dimension of depth of vein placement within the leaf would allow exploration and better understanding of the evolutionary trends and limits of species ability to adapt to changes in atmospheric gas composition, humidity, temperature and light.
Theoretical considerations as well as experimental tests performed on bioinspired, artificial leaves revealed that significant reduction in water potential variability across the leaf epidermis would be achieved when the distance between veins (d) is equal to the depth of the veins away from the leaf surface(s) that bear stomata, i.e. the abaxial surface in most cases (δ) [7] (figure 1). The violation of this relationship would not come without significant costs to photosynthetic rates and/or WUE. If veins were far apart from each other but close to the leaf surface (d > δ), then transpiration and assimilation rates would be large only near the veins (where higher gs would coincide with the shorter diffusion path), whereas leaf tissue further from the veins would experience water shortages resulting in a drop in gs and CO2 uptake. In this case, photosynthetic gains and losses would both occur across the leaf surface, however, they would not be balanced. Photosynthetic gains in gas exchange near the veins would be at the point of diminishing return for water used, whereas the photosynthetic losses would be at the point of greatest detriment (i.e. where the return in carbon gain for increased water use would have been at its greatest). Thus, having the distance between veins be too long for a given vein depth (d > δ) is disadvantageous, leading to an overall drop of leaf productivity and lower WUE. The opposite anatomical relationships (d < δ) would also be problematic. Simply adding more veins so that the distance between them would be less than the vein depth (d < δ) would not increase gs, which was already near its maximum at d = δ, in fact we can expect that the overall leaf productivity would decrease in such a situation as the space available for photosynthetic mesophyll cells is lost to non-productive veins (figure 1).
Figure 1.
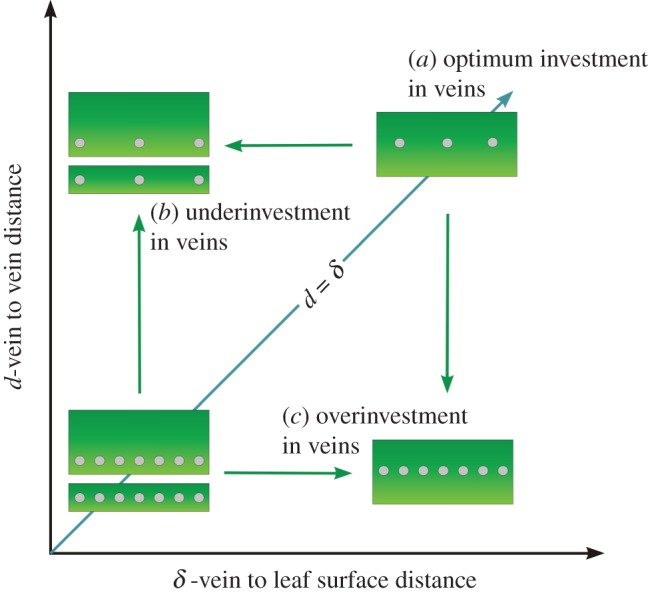
Morphospace of potential vein placement in leaves, shown in cross section. (a) Optimum designs for both thin and thick leaves lie along the line where the distance between veins (d) is equal to the distance from veins to evaporative surface (δ). Note that δ is not equivalent to leaf thickness; thick leaves may have either large or small values for δ depending on how deeply the veins are seated within the leaf away from the evaporative surface. (b) Underinvestment in vein density (VD) characterized by d > δ. (c) Overinvestment in VD where d < δ. Each of these three scenarios has significant physiological implications. In scenario (a) of optimum vein placement, stomata most likely act together, because the leaf surface experiences relatively homogeneous water potential. In scenario (b) of underinvestment in vein density (VD) the leaf would experience non-homogeneous water distribution across its surface that would lead to unevenly distributed stomatal closure with increasing evaporative demand, potentially leading to greater stress related damage in severe cases. Furthermore, overall transpiration capacity in scenario (b) would be less than in scenario (a) even for the same δ. In scenario (c) of overinvestment in VD, there would be no further increase in transpiration rate over scenario (a). The excessive VD would, however, lead to a loss of internal space that could be used for chlorophyll bearing cells and, thus, may result in a decrease in total leaf photosynthetic capacity. (Online version in colour.)
Given the above, it is not surprising that a strong 1 : 1 relation between d and δ was found across a large number of angiosperm species [7]. However, much remains unknown regarding this relationship, including what the d : δ relationships may be for basal angiosperms and non-angiosperms not included in that previous study, how far from the l : 1 relationship must a plant be before photosynthetic losses might be limiting, and how those potential losses would scale across different ecologies. For example, the above discussion of optimal vein placement is only valid when CO2 uptake is limited by hydraulics. In the case of low light and/or high humidity, an apparent disconnect between CO2 uptake and stomatal conductance could be predicted, allowing for lower vein densities to be viable for a given leaf thickness (d > δ). Furthermore, while some environmental conditions may be more permissive of departures from optimal vein placement (d = δ) some plants may be physiologically or developmentally constrained in ways that limit their potential for achieving hydraulic optimization. For example, only few non-angiosperms approach the high vein densities seen in the angiosperms [2], which might limit many lineages to either thick leaves or more sheltered environments where poorly optimized leaves can be sustainable. Thus, occupation of the (d,δ) morphospace (figure 1) could reflect both ecological and evolutionary heterogeneity. In this report, we take a closer look at the structure and evolution of the d to δ relationship across the ecological and phylogenetic diversity of vascular plants.
2. Methods
Mature, fully expanded leaves were collected from plants in their natural habitats or common gardens (all temperate and many tropical species) except for a few instances where greenhouse plants were used. Several individual plants were sampled per species, from separate locations where possible in order to account for site variability (total number of leaves analysed was 881, number of leaves per species available in the electronic supplementary material, table S1). Leaves were preserved in 70% alcohol in the short-term before analyses if immediate observation was not available. Vein-to-vein distance (d) and distance of vein-to-the stomata-bearing epidermis (δ) was determined directly from leaf cross-sections obtained either from freehand cuts or from a freezing microtome. Microscope images were taken at varying magnifications to assure adequate field of view for assessing variation in vein–vein distances. Measurements followed previous practice [7], i.e. vein–vein distance was measured between the edges of the xylem conduits in neighbouring veins and vein–epidermis was measured from the abaxial edge of the xylem conduits of a vein to the outer edge of the epidermis directly beneath the centre of the vein (figure 2).
Figure 2.
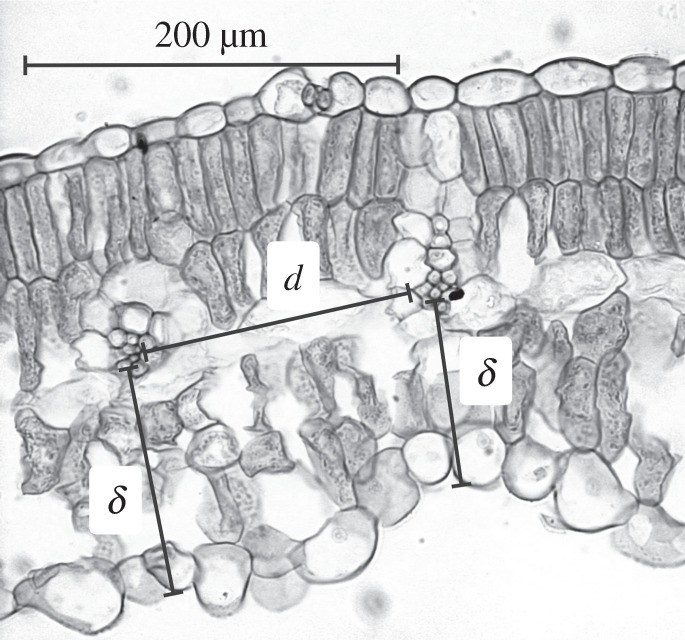
Example of vein-to-vein and vein-to-epidermis distance determination on soya bean leaf cross section. Average value of the measurements for d and for δ was taken.
Sampling of leaf anatomy via sectioning was dependent on the venation type. For leaves with parallel venation, cross sections were taken perpendicular to the veins. In the case of angiosperms and other taxa with more complex reticulate venation, cross sections were taken in multiple random directions with the final reported vein distance per leaf being the mean of 20 or more individual measurements (with more than 15 000 total individual measurements for d and for δ ). In a few angiosperms with very high vein densities and narrow veins, VD was determined from the surface image and then average vein–vein distance was determined as the inverse of density.
The gymnosperms bearing broad leaves with multiple veins—Ginkgo, Gnetum, Welwitschia, a variety of cycads, some conifers in the Araucariaceae—were supplemented with some sampling of flattened, laminate single-veined leaves (e.g. Taxus, some Cupressaceae), for which d was assumed to be twice the distance from the vein to the lateral margin of the lamina. Cylindrical or polygonal leaves (e.g. some Pinaceae) were excluded, as were those with accessory transfusion tissue (e.g. Podocarpus, Cycas). The phylogenetic inclusiveness of this study was further supplemented via measurements of figured specimens in the literature for some cycads with relatively simple leaf vein patterns for which the availability of fresh material was limited as well as for a variety of non-angiosperm fossil leaf taxa.
3. Results
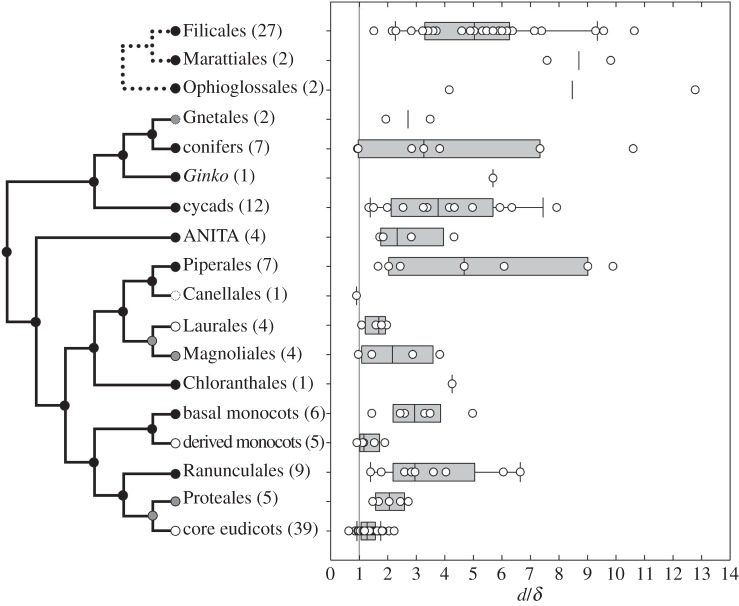
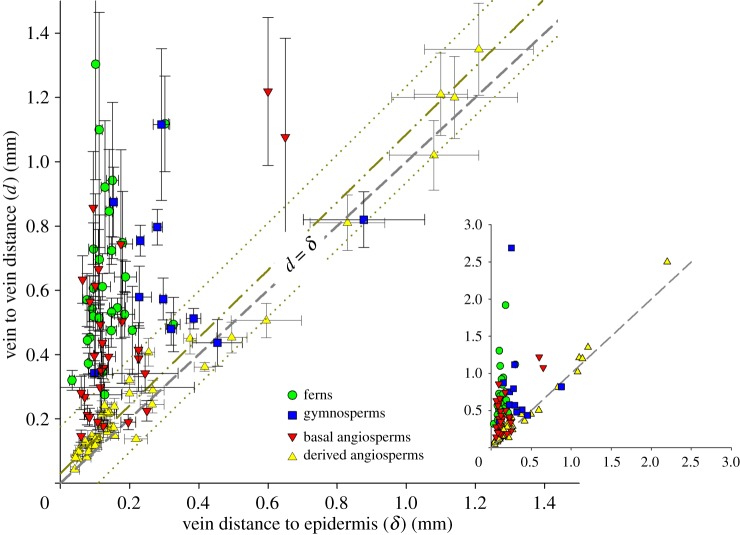
Comparison of the d with δ ratio across the vascular plants (135 species; see the electronic supplementary material, table S1 for complete list) revealed significant phylogenetic differences (figure 3). As seen previously [7], most angiosperms are tightly clustered near a d : δ ratio of 1. In that previous study, shade-demanding taxa were sparsely represented, and it is here shown that a distances ratio close to 1 is exhibited even by these angiosperms for which tolerance of higher d : δ ratios might be expected (see electronic supplementary material, figure S1). Broader phylogenetic sampling, however, reveals that the high degree of optimization seen in the angiosperms is unique to the angiosperms. Sampling is incomplete, but many early diverging angiosperm lineages—ANITA-grade taxa, Chloranthales, most magnoliids, the basal Acorales and Alismatales among monocots, and the basal Ranunculales and Proteales among eudicots—are the most poorly optimized flowering plants. Individual members of those lineages may be well optimized, but the groups are not consistently so. It is specifically the Laurales among Magnoliids, derived monocots and the core eudicots that each independently shows uniformly high levels of anatomical optimization with tight clustering near ratios of 1 : 1 (figure 3 and the electronic supplementary material, table S1). In ferns, the median d : δ ratio of 5.3 is higher than all but the most extreme basal angiosperm outliers and the maximum ratios seen among ferns is greater than 10. Gymnospermous seed plants with laminate leaves present only modest improvements over ferns, having a median ratio of 3.3 and a maximum also greater than 10. Several important relationships are seen when d and δ are presented simultaneously (figure 4). Most fundamentally, the prediction was correct that no plants would occupy the theoretical morphospace where d < δ. Modelling and experimental results both suggest that such leaves—although developmentally possible—would be overbuilt, i.e. the high investment in veins would serve no hydraulic purpose and might be detrimental to leaf photosynthetic capacity. Thus, the general absence of d < δ leaves suggests that the hypothesized physiological relationship between these parameters really does reflect a fundamental reality for the plants. Hydraulic underinvestment—the area above the 1 : 1 line in figure 4—was expected to be permissible, but only in sheltered environments. This area of figure 4 is, indeed, well represented, but not evenly so. That underinvestment in veins is much less common in thick leaves than in thin leaves is likely to reflect that thick leaves are most relevant to more exposed environments where an inadequate hydraulic supply would not be viable.
Figure 3.
Box and whisker graph of the phylogenetic distribution of d/δ ratios across the extant vascular plants, including separate evolutions of laminate leaves in seed plants and ferns. Early branching lineages of flowering plants are parsed out to highlight that the most parsimonious interpretation is that well-optimized leaves arose multiple times in different angiosperm lineages. Each nested lineage is labelled according to composition: black circles indicate lineages that include poorly optimized taxa, grey circles indicate lineages that maintain at least moderate optimization (d/δ < 4 for all individuals sampled), white circles indicate lineages that exclusively maintain high degrees of optimization (d/δ < 2.5 for all individuals sampled). Dashed circles indicate lineages that show some degree of optimization, but with poor sampling. Line represents the optimal d/δ ratio of 1 : 1. (Online version in colour.)
Figure 4.
Morphospace (d : δ) of vein placement in leaves of ferns, gymnosperms and angiosperms. Flowering plants are subdivided into derived angiosperms (Laurales, core eudicots, monocots except Acorales and Alismatales) and basal angiosperms (ANITA-grade taxa, all magnoliids except Laurales, the basal eudicot Ranunculales and Proteales, and the basal monocot Acorales and Alismatales). Values of d and δ are species averages (see the electronic supplementary material, table S1), not individual measurements. Standard deviations reported reflect variation of d and δ between leaves within each species, not between individual measurements within each leaf. Dashed line represents the optimal d/δ ratio of 1 : 1. Dashed double-dotted line is a linear fit of derived angiosperm plants only with dotted line representing 95% CI. Main graph axes are limited to 1.5 mm distances. Inset graph includes all sampled plants. (Online version in colour.)
Ferns and gymnosperms both occupy the area of morphospace described as underinvestment in VD (d > δ), although they exhibit different occupations of this space (figure 4). Both groups only produce leaves with low-to-moderate vein densities (relatively high vein–vein distances, d). However, ferns only have relatively thin leaves and, thus, would be susceptible to non-uniform water loss under condition of high evapo-transpirational demand. This could be avoided only in sheltered environments where shade and humidity can allow stomata to remain open despite deficiencies in equitable water distribution. Gymnosperms, on the other hand, tend to be only moderately underinvested (d > δ), because they produce thicker leaves than are seen among ferns. Thus, gymnosperms are shifted towards a 1 : 1 relationship in more exposed environments where their thick leaves would be appropriate for water conservation, i.e. high WUE but overall lower photosynthetic capacity. Inclusion of fossil leaves (see electronic supplementary material, table S2 for the list of specimens) suggests that the range of anatomies seen among living ferns and gymnosperms (low VD leaves that are either thin and poorly optimized or thick and relatively well optimized) is the space that has been occupied by non-flowering plants throughout the evolutionary history of vascular plants (figure 5).
Figure 5.
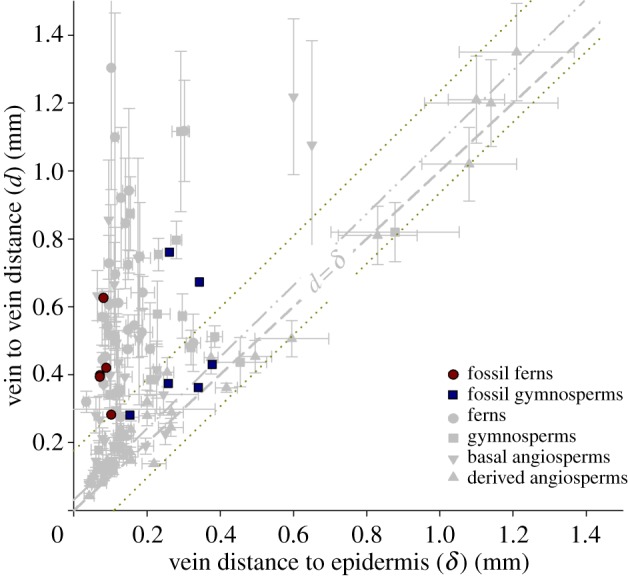
Morphospace (d : δ) of fossil ferns and gymnosperms against a background of values from all sampled extant plants. Fossil ‘ferns’ here includes Equisetales and Sphenophyllales (see the electronic supplementary material, table S2). (Online version in colour.)
Derived angiosperms are seen to cluster along the 1 : 1 line (figure 4). Thin angiosperm leaves have high VD, and any increases in leaf thickness that result in deeper vein placement also are met with a parallel increase in the distance between veins, i.e. a decrease in VD. Thus, angiosperms are well optimized hydraulically (from the perspective of uniform water supply to the leaf surface) across a broad range of habitats. However, basal angiosperms are more variable, they overlap with the area of hydraulic underinvestment occupied by the ferns while also ranging down to the thin, well-optimized leaves found only among angiosperms.
4. Discussion
(a). Limits on the ecological possibilities of ferns and gymnosperms
All ferns couple thin leaves with low vein densities (figure 4 and the electronic supplementary material, table S1), resulting in poor hydraulic optimization being a universal characteristic across at least three independent evolutions of laminate leaves [10] in the distinct ophioglossalean, marattialean and leptosporangiate lineages of ‘ferns’. In the d : δ morphospace, they occupy the most underinvested anatomies that would lead to a highly non-homogeneous distribution of water across the leaf surface and loss of photosynthetic capacity with any increase in evaporative demand. Whether cause or effect, this limitation of ferns to thin leaves of low VD is also associated with a limitation to sheltered environments—humid, shaded or both—where leaves with such poor hydraulic characteristics can best be tolerated.
Ferns do present some informative caveats to the well-known generality of their being limited to wet and shaded environments. The ferns sampled here that experienced the greatest light exposure—the emergent leaves of the aquatic Marsilea, tree ferns including one grown in full sun—also possess the lowest d : δ ratios of any ferns (see the electronic supplementary material, table S1 for these values and all other specific measurements discussed below). These ferns still have d : δ ratios higher than any angiosperm other than the most poorly optimized outliers (figure 3), but they nonetheless demonstrate that plants with intermediate ratios can survive in more exposed environments. However, it is perhaps instructive that, though such plants may persist in more exposed environments, tree ferns only form the canopy where productivity is limited by nutrient availability or other stressors [11]—otherwise the more favourable gas exchange and photosynthetic characteristics of angiosperms will dominate. Some epiphytes can be relatively well optimized by virtue of having thicker leaves (e.g. Pyrrosia), but greater sampling of epiphytic ferns would presumably present additional examples of relatively poor hydraulic optimization persisting in high-light environments where productivity is nutrient limited. Bracken fern, Pteridium aquilinum, can form open stands and yet it is poorly optimized based on the sample included here. Perhaps the well-documented density of bracken thickets, sufficient to exclude establishment of seed plant seedlings [12] means that the leaves are all somewhat sheltered by self-shading even when there is no overhanging tree canopy. Alternatively, the highly cosmopolitan bracken may display a fair amount of ecophenotypic variation. In that case, it is important to note that the lone sample included here comes from a wet, semi-shaded roadside; sampling of more exposed bracken habitats may reveal leaves with a lower d : δ ratio. Although not available for the current study, anatomical measurements of Dipteris may also be informative as a stand-forming plant of sunny habitats in southeast Asia that possesses the highest measured leaf vein densities of any fern [2].
Like ferns, non-angiosperm seed plants possess low vein densities, but they often achieve d : δ ratios that approach 1 : 1 due to having leaves much thicker than any fern (figure 4). These characteristics—low vein densities in thick leaves—contribute to high WUE and, thus, make gymnosperms well adapted to environments where productivity is limited at least seasonally by water and/or nutrient availability. Although this is certainly consistent with modern gymnosperm ecologies [13], the results presented here suggest gymnosperms may have been similarly constrained in the geological past because they never possessed high vein densities [2]. Three-dimensional preservation of leaf internal anatomy is limited, but the available fossil fern and gymnosperm leaves occupy the same range of anatomies as their modern relatives (figure 5). Thus, their anatomical differences may have been consistent throughout their evolutionary histories, as has been seen previously with other aspects of leaf morphology with the more complete sampling available from leaf compression fossils that lack internal anatomy [14]. Perhaps the most interesting departure from this gymnosperm tendency of thick leaves with high WUE are the scale-leaved stems of some Cupressaceae conifers. Leaves with such reduced area are often assumed to reflect water conservation, however, these leaves are thin and all of their limited surface area is close to a vein. Counterintuitively, they may provide instead the closest approximation to angiosperm-like high gas exchange potential (see also [15])—although it is important to note that they are still thick by angiosperm standards. As with tree ferns, Ginkgo demonstrates that high d : δ ratios can persist in full sun and even can support relatively rapid increases in tree height. However, individual Ginkgo leaves have low gas exchange capacities and that rapid growth of the few long shoots is sustained by a large number of low productivity leaves per stem on persistent short shoots [16]. Ultimately, the growth potentials of tree ferns and Ginkgo both represent horticultural possibilities, not native ecologies; Ginkgo is extinct in the wild.
(b). The evolution of angiosperm leaf physiology
Different viewpoints are available regarding how angiosperm physiology fits into the overall spectrum of vascular plant physiology. The vegetative biology of angiosperms is often treated as end members on a physiological continuum shared by other vascular plants and as if angiosperm advantages can be neutralized under the right environmental conditions, such as greatly elevated levels of atmospheric CO2, with other plants being elevated to comparably high levels of productivity [3,9,17–19]. Alternatively, the angiosperms can be viewed as presenting fundamentally new ecological strategies founded upon novel physiological innovations enabling uniquely high growth rates [2,6,13,20,21].
The results presented here support the view that angiosperm physiology—at least among more derived angiosperms—is fundamentally distinct. That angiosperm leaves remain well-optimized even in sheltered environments is particularly telling. Ferns demonstrate that high d : δ ratios can be viable in shaded, humid environments. The leaves of angiosperm shade plants, however, maintain ratios close to 1 : 1 even though it is not strictly necessary for those environments (see electronic supplementary material, figure S1). In this crucial respect, the leaf anatomy of shade angiosperms resembles sun angiosperms rather than the anatomy of non-angiosperms of similarly shaded environments, suggesting the angiosperms are doing something different than non-angiospermous plants. Consistent with existing work [22–25], a possible explanation for this disparity would be if shaded angiosperms are generally more dependent on maximizing photosynthesis from transient flecks of sunlight as opposed to fern dependence on low levels of diffuse skylight. Thus, d : δ optimization is required even in shade angiosperms, because nearly all flowering plant leaves are ‘sun’ leaves in a way that may not be true of other lineages.
As has been found for other aspects of their vegetative biology [26–28], the leaves of basal ANITA-grade angiosperms differ from more derived angiosperms in possessing d : δ ratios higher than other angiosperms. These plants live in shaded environments of the humid tropics and are intolerant of drought or high vapour pressure deficits [27]. As with ferns, their leaves are thin and have low vein densities. Sampling of basal extant angiosperms and fossil leaves suggests the high leaf vein densities more generally characteristic of flowering plants first appeared in more derived lineages, perhaps independently in the monocots, eudicots and magnolliids and perhaps along with the first angiosperm exploration of more light intensive environments [2–4,29]. Anatomical sampling of crucial intermediate taxa such as the Chloranthaceae is limited, but the results here suggest the parallel evolution of hydraulic optimization of leaf vein placement in the angiosperms may have been at the same time and in the same flowering plant lineages as the initial evolution of high vein densities.
Angiosperm leaf vein densities average between 8 and 10 mm mm−2 and cover the entire spectrum from less than 1 to more than 25 mm mm−2, but leaf vein densities of non-angiosperms are low, averaging only about 2 mm mm−2 and rarely straying outside of the range of 1–4 mm mm−2 [2]. These uniformly low vein densities impose high values for d, making leaf thickness—and, thereby, δ—the only variable available to non-angiosperm leaves. Thus, only two basic strategies are available among most non-angiosperms: thin leaves where sheltered environments are more permissive of poorly optimized d : δ ratios and thick leaves in more exposed environments that require optimization. Thin leaves allow high gas exchange rates, but are at increased risk of desiccation outside of the sheltered environments in which they are universally common. In a basic sense, the uniquely high productivity of flowering plants has been founded on their unique capacity to export thin leaves from the shadows to the exposed top of the canopy.
Acknowledgement
The authors thank Ramona Hihn for help with leaf material collection.
Funding statement
Work was supported by the National Science Foundation (grant no. EAR-1024041).
References
- 1.Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 144, 1890–1898 (doi:10.1104/pp.107.101352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce CK, Brodribb T, Feild TS, Zwieniecki MA. 2009. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. R. Soc. B 276, 1771–1776 (doi:10.1098/rspb.2008.1919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodribb TJ, Feild TS. 2010. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13, 175–183 (doi:10.1111/j.1461-0248.2009.01410.x) [DOI] [PubMed] [Google Scholar]
- 4.Feild TS, et al. 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl Acad. Sci. USA 108, 8363–8366 (doi:10.1073/pnas.1014456108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce CK, Leslie AB. 2012. The paleontological context of angiosperm vegetative evolution. Int. J. Plant Sci. 173, 561–568 (doi:10.1086/665820) [Google Scholar]
- 6.Boyce CK, Zwieniecki MA. 2012. Leaf fossil record suggests limited influence of atmospheric CO2 on terrestrial productivity prior to angiosperm evolution. Proc. Natl Acad. Sci. USA 109, 10 403–10 408 (doi:10.1073/pnas.1203769109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noblin X, Mahadevan L, Coomaraswamy IA, Weitz DA, Holbrook NM, Zwieniecki MA. 2008. Optimal vein density in artificial and real leaves. Proc. Natl Acad. Sci. USA 105, 9140–9144 (doi:10.1073/pnas.0709194105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niklas KJ, Cobb ED, Spatz H-C. 2009. Predicting the allometry of leaf surface area and dry mass. Am. J. Bot. 96, 531–536 (doi:10.3732/ajb.0800250) [DOI] [PubMed] [Google Scholar]
- 9.de Boer HJ, Eppinga MB, Wassen MJ, Dekker SC. 2012. A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution. Nat. Commun. 3, 1221 (doi:10.1038/ncomms2217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce CK. 2010. The evolution of plant development in a paleontological context. Curr. Opin. Plant Biol. 13, 1–6 (doi:10.1016/j.pbi.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 11.Morley RJ. 2000. Origin and evolution of tropical rain forests. Chichester, UK: John Wiley & Sons [Google Scholar]
- 12.Grime JP. 2002. Plant strategies, vegetation processes, and ecosystem properties. Hoboken, NJ: Wiley [Google Scholar]
- 13.Bond WJ. 1989. The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 36, 227–249 (doi:10.1111/j.1095-8312.1989.tb00492.x) [Google Scholar]
- 14.Boyce CK. 2005. Patterns of segregation and convergence in the evolution of fern and seed plant leaf morphologies. Paleobiology 31, 117–140 (doi:10.1666/0094-8373(2005)031<0117:POSACI>2.0.CO;2) [Google Scholar]
- 15.Zwieniecki MA, Boyce CK, Holbrook NM. 2004. Functional design space of single veined leaves: role of tissue hydraulic properties in constraining leaf size and shape. Ann. Bot. 94, 507–513 (doi:10.1093/aob/mch173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leigh A, Zwieniecki MA, Rockwell FE, Boyce CK, Nicotra AB, Holbrook NM. 2011. Structural and hydraulic correlates of heterophylly in Ginkgo biloba L. New Phytol. 189, 459–470 (doi:10.1111/j.1469-8137.2010.03476.x) [DOI] [PubMed] [Google Scholar]
- 17.Beerling DJ, Woodward FI. 1997. Changes in land plant function over the Phanerozoic: reconstructions based on the fossil record. Bot. J. Linn. Soc. 124, 137–153 [Google Scholar]
- 18.McElwain JC, Willis KJ, Lupia R. 2005. Cretaceous CO2 decline and the radiation and diversification of angiosperms. In A history of atmospheric CO2 and its effects on plants, animals, and ecosystems (eds Ehleringer JR, Cerling TE, Dearing MD.), pp. 133–166 New York, NY: Springer [Google Scholar]
- 19.Assouline S, Or D. 2013. Plant water use efficiency over geological time: evolution of leaf stomata configurations affecting plant gas exchange. PLoS ONE 8, e67757 (doi:10.1371/journal.pone.0067757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bond WJ, Scott AC. 2010. Fire and the spread of flowering plants in the Cretaceous. New Phytol. 188, 1137–1150 (doi:10.1111/j.1469-8137.2010.03418.x) [DOI] [PubMed] [Google Scholar]
- 21.Royer DL, Chernoff B. 2013. Diversity in neotropical wet forests during the Cenozoic is linked more to atmospheric CO2 than temperature. Proc. R. Soc. B 280, 20131024 (doi:10.1098/rspb.2013.1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suetsugu N, Mittman F, Wagner G, Hughes J, Wada M. 2005. A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc. Natl Acad. Sci. USA 102, 13 705–13 709 (doi:10.1073/pnas.0504734102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watkins JE, Cardelús CL. 2012. Ferns in an angiosperm world: Cretaceous radiation in the epiphytic niche and diversification on the forest floor. Int. J. Plant Sci. 173, 695–710 (doi:10.1086/665974) [Google Scholar]
- 24.Gago J, Coopman RE, Cabrera HM, Hermida C, Molins A, Conesa MÀ, Galmés J, Ribas-Carbó M, Flexas J. 2013. Photosynthesis limitations in three fern species. Physiol. Plant. 149, 599–611 (doi:10.1111/ppl.12073) [DOI] [PubMed] [Google Scholar]
- 25.Schymanski SJ, Or D, Zwieniecki MA. 2013. Stomatal control and leaf thermal and hydraulic capacitances under rapid environmental fluctuations. PLoS ONE 8, e54231 (doi:10.1371/journal.pone.0054231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feild TS, Brodribb T, Jaffré T, Holbrook NM. 2001. Acclimation of leaf anatomy, photosynthetic light use, and xylem hydraulics to light in Amborella trichopoda (Amborellaceae). Int. J. Plant Sci. 162, 999–1008 (doi:10.1086/322889) [Google Scholar]
- 27.Feild TS, Arens NC, Doyle JA, Dawson TE, Donoghue MJ. 2004. Dark and disturbed: a new image of early angiosperm ecology. Paleobiology 30, 82–107 (doi:10.1666/0094-8373(2004)030<0082:DADANI>2.0.CO;2) [Google Scholar]
- 28.Sperry JS, Hacke UW, Feild TS, Yano Y, Sikkema EH. 2007. Hydraulic consequences of vessel evolution in angiosperms. Int. J. Plant Sci. 168, 1127–1139 (doi:10.1086/520726) [Google Scholar]
- 29.Feild TS, Brodribb TJ. 2013. Hydraulic tuning of vein cell microstructure in the evolution of angiosperm venation networks. New Phytol. 199, 720–726 (doi:10.1111/nph.12311) [DOI] [PubMed] [Google Scholar]