Abstract
Current views about the impact of Wolbachia on Plasmodium infections are almost entirely based on data regarding artificially transfected mosquitoes. This work has shown that Wolbachia reduces the intensity of Plasmodium infections in mosquitoes, raising the exciting possibility of using Wolbachia to control or limit the spread of malaria. Whether natural Wolbachia infections have the same parasite-inhibiting properties is not yet clear. Wolbachia–mosquito combinations with a long evolutionary history are, however, key for understanding what may happen with Wolbachia-transfected mosquitoes after several generations of coevolution. We investigate this issue using an entirely natural mosquito–Wolbachia–Plasmodium combination. In contrast to most previous studies, which have been centred on the quantification of the midgut stages of Plasmodium, we obtain a measurement of parasitaemia that relates directly to transmission by following infections to the salivary gland stages. We show that Wolbachia increases the susceptibility of Culex pipiens mosquitoes to Plasmodium relictum, significantly increasing the prevalence of salivary gland stage infections. This effect is independent of the density of Wolbachia in the mosquito. These results suggest that naturally Wolbachia-infected mosquitoes may, in fact, be better vectors of malaria than Wolbachia-free ones.
Keywords: symbiont-mediated protection, vectorial competence, infection prevalence, infection intensity, oocysts, sporozoites
1. Introduction
Individual hosts are often simultaneously infected with more than one parasite species. Co-infections can impact both host fitness and parasite transmissibility, and can therefore have important evolutionary and epidemiological consequences [1,2]. Within a host, parasites may interact in different ways. They may suppress each other because they are in competition for a resource in limited supply, such as a particular nutrient or tissue, or because they stimulate the same branch of the immune system [3]. In the most extreme cases, parasites can excrete molecules that directly inhibit the growth of competitors [4]. Host sharing may also, however, facilitate parasite development, most notably when one of the parasites immunosupresses the host [2]. Co-infections have been intensely investigated in the biomedical literature, as several important human infections are known to be complicated by the arrival of secondary or opportunistic pathogens [3]. More recently, however, a great deal of attention has been drawn to the impact of co-infections on vector-transmitted diseases with the realization that, in the field, arthropod vectors are also often infected by multiple parasites [5–7].
A few years ago, two seminal papers showed that Wolbachia, a maternally transmitted bacterial endosymbiont of arthropods, protects Drosophila flies from several viral infections [8,9]. This stimulated a great deal of research into Wolbachia-mediated parasite interference in other insect systems (see the electronic supplementary material, table S1), and raised the exciting possibility of using Wolbachia to control or limit the spread of mosquito-transmitted diseases, such as dengue and malaria. Interestingly, although neither Aedes aegypti (vector of the dengue virus) nor Anopheles gambiae or Anopheles stephensi (vectors of Plasmodium falciparum) are naturally infected by Wolbachia, they can be successfully transfected in the laboratory using bacteria isolated from other insect species [10–12], although not always stably (in An. gambiae the infections are somatic and do not transmit vertically to the offspring [13,14]). As a consequence, in the past few years, a large number of studies have been conducted using transfected mosquitoes. These studies have largely confirmed the results obtained in naturally infected Drosophila: transfected Wolbachia exhibit considerable pathogen-interference properties against a wide range of parasite taxa (e.g. [12,13,15–17]; see also the electronic supplementary material, table S1). By contrast, studies of natural Wolbachia infections in mosquitoes have been much less conclusive; some studies have shown no effect of Wolbachia on pathogen development [17–19], while others have shown that Wolbachia facilitates [20] or blocks [21] pathogen replication (see the electronic supplementary material, table S1 for a summary). This raises the question of whether the Wolbachia-mediated parasite protection observed in recently transfected mosquitoes can be maintained across generations. Wolbachia–mosquito combinations with a long evolutionary history may be key for understanding what will happen with Wolbachia-transfected mosquitoes several generations down the line if, as has been shown in other systems [22,23], the novel Wolbachia–mosquito interactions evolve rapidly.
Here, we investigate whether a natural Wolbachia infection interferes with or facilitates Plasmodium development in mosquitoes. Previous work on the outcome of Plasmodium–Wolbachia coinfections has been carried out using transfected Wolbachia and/or mosquito–Plasmodium combinations that work well in the laboratory but do not exist in nature (see electronic supplementary material, table S1). The results obtained range from an increase [14,19] to a decrease [12–15] in Plasmodium parasitaemia in the presence of Wolbachia, depending on the particular Wolbachia–mosquito–Plasmodium combination used. Results from artificial mosquito–Plasmodium combinations are particularly difficult to interpret, because there is growing evidence that they do not behave in the same way as natural combinations [24,25]. One intriguing example from the Wolbachia literature is that of the human malaria vector, An. gambiae, transfected with the wAlbB strain of Wolbachia. This strain of Wolbachia decreases parasitaemia when mosquitoes are infected with a human (Plasmodium falciparum) malaria parasite [13], but has the opposite effect when mosquitoes are infected with a rodent (Plasmodium berghei) malaria parasite [14]. The reasons for these contrasting results are not yet known, but one possibility is that the disparity may be immune-mediated, as the natural (P. falciparum) and unnatural (P. berghei) parasites are controlled by different immune pathways in An. gambiae mosquitoes [25].
We used an entirely natural system, consisting of the avian malaria parasite P. relictum, its natural vector, the mosquito Cx. pipiens, and its native (wPip) Wolbachia strain. The aim was to establish whether the infection with Wolbachia decreases the prevalence and/or intensity of Plasmodium infection. In contrast to most previous studies, which have been exclusively centred on the quantification of oocysts in the midgut of mosquitoes 7 days after the infection (but see [12]), we aimed to obtain a measurement of parasitaemia that would relate more directly to transmission by following the infections all the way to day 14, when the sporozoites have infected the salivary glands of the female. Indeed, the epidemiological significance of having more or fewer oocysts in the gut remains to be demonstrated: a single oocyst produces thousands of sporozoites, but as few as 10 of these sporozoites suffice to initiate a new infection in a host [26]. Thus, despite earlier studies showing a difference in Plasmodium oocystaemia in Wolbachia-infected mosquitoes, the question of whether natural Wolbachia infections can interfere with Plasmodium transmission in mosquitoes has not been entirely resolved.
2. Material and methods
(a). Mosquito lines
We used two isogenic lines of Cx. pipiens quinquefasciatus that share the same nuclear genome but differ in their Wolbachia infection. The first line (wSL) is naturally infected by the Wolbachia wPip(Sl) strain. The second line (w(−)) was generated by antibiotic treatment of wSL larvae to eliminate the Wolbachia infection (see [27] for details of the lines). The w(−) was reared for ca 30 generations before the experiment to eliminate side effects of the tetracycline. Both lines, wSL and w(−) were reared throughout under identical conditions. Newly hatched (L1) larvae from these two different lines were placed in plastic trays (34 × 23 × 7 cm) filled with 1 l of water at a constant density of 300 larvae per tray (n = 10 trays per line). The experiment took place under standard temperature (24±2°C), humidity (65 ± 5%) and photoperiod (12 L : 12 D) conditions. Larvae were fed ad libitum on brewer's yeast on the first day, and thereafter on ground Tetramin fish flakes. On day seven post hatching, each plastic tray was individually placed inside an ‘emergence cage’ (40 × 28 × 31 cm) and emerged adults were allowed to feed ad libitum on a 10% glucose water solution.
(b). Plasmodium strain and bird infections
We used a lineage of P. relictum known as SGS1. It is the most prevalent avian malaria lineage in Europe, both in wild Passeriformes birds and in Cx. pipiens mosquitoes (MalAvi database; see [28]). The strain used in the experiment was isolated from wild sparrows and has been since maintained in our animal house by carrying out regular passages between our stock canaries every ca three weeks [29]. Experimental canaries (n = 6) were haphazardly allocated to one of two treatments: half of them were experimentally infected with our SGS1 Plasmodium lineage (‘infected cages’) and the other half were left as uninfected controls (‘control cages’). Experimental infections took place by intraperitoneal injection of ca 50–100 µl of blood from our infected canary stock, and mosquito blood feeding took place 10 days after the infection, to coincide with the acute phase of the parasitaemia [29].
(c). Mosquito experimental infections and dissections
To estimate Plasmodium burden and Wolbachia density simultaneously, groups of 90 adult Cx. pipiens females (8–10 days old) from each line (wSL and w(−)) were haphazardly chosen from the different emergence cages and placed together to feed overnight inside an experimental cage (n = 3 infected cages, n = 3 control cages). After the blood meal, the birds were taken out and all the cages were supplied with ad libitum glucose water until the end of the experiment. Mosquitoes that had not taken a blood meal (less than 8%) were removed from the cages. To simplify the identification of the strains, three days before the blood meal, the mosquitoes were marked using a small amount (1 µg per female) of either pink or blue fluorescent powder (RadGlo JST) applied as a dust storm. Preliminary trials have shown that at this concentration the dust has no effect on mosquito survival or parasite burden [27]. The two colours were used in rotation to mark the two strains so that the strain-colour code was switched from cage to cage.
To count oocysts in the mosquito gut, 20 blood-fed females of each line were haphazardly chosen from each cage 7–8 days post blood meal (dpbm) and dissected under a binocular microscope in 100 μl of 0.01 M phosphate-buffered saline (PBS). One wing was also extracted and measured along its longest axis as an estimate of female size. The dissected midguts were stained with a 5% mercurochrome solution to assess infection rate (oocysts present/absent) and oocyst burden (number of oocysts) under a phase contrast microscope. The dissected abdomens (minus the midguts) were individually frozen at −20°C for the subsequent Wolbachia quantification. A similar procedure was carried out at day 14 pbm, when the sporozoites have migrated to the salivary glands. At this time, 40 blood-fed females from each mosquito line were haphazardly sampled from each of the cages. Females were first dissected to get rid of the midgut (at this stage, all oocysts in the midgut are expected to have burst), and then the mosquito was severed to separate the thorax (containing the salivary glands) and the abdomen, both of which were individually frozen at −20°C for the subsequent quantification of Plasmodium and Wolbachia infections, respectively.
(d). Wolbachia and Plasmodium sporozoite quantification
Real-time quantitative PCR was used to estimate the relative density of Wolbachia (abdomen) and Plasmodium sporozoites (thorax) in each mosquito. We carried out two PCRs on each of the body segments: one was specific for the Culex ace-2 locus [30], and the other was either specific for the Wolbachia wsp locus [31] or for the mtDNA cytb gene of Plasmodium. For the latter, we used the primers CytSPO7F (5′-AGTTTCATGGATATGTGGTGGA-3′) and CytSPO10R (5′-AAAGATTTGGATAGAAGGGTATTT-3′). For each of the genes under study, the 5 µl reaction mixture contained 1 µl of template DNA (thorax at 5 ng µl−1 and abdomens at 10 ng µl−1), 2.5 µl of 2X LightCycler DNA Master SYBR Green I (Roche Applied Science), 0.25 µl of primers at 10 µM and 1 µl of RNase-Free Water (QIAGEN). Amplification conditions were as follows: 8 min at 95°C, followed by 45 cycles of 95°C for 10 s, 58°C for 20 s, 65°C for 20 s. Standard curves were plotted using dilutions of a pBluescriptKS vector containing one copy of each of the ace-2, wsp and cytb gene fragments. Each abdomen (or thorax) DNA template was analysed in triplicate for ace-2 and wsp (or cytb) quantification. Assuming that each gene is present in a single copy per haploid genome, the ratio between the wsp (or cytb) and ace-2 provides the number of Wolbachia (or Plasmodium) genomes relative to the Culex genomes.
(e). Statistical analysis
Analyses were carried out using the R statistical package (v. 2.12.0). The different statistical models built to analyse the data are described in the electronic supplementary material, table S2. The general procedure for building the statistical models was as follows: mosquito lines (wSL and w(−)), dissection day (7–8 days pbm) and mosquito wing size were fitted as fixed explanatory variables, whereas bird and qPCR plate were fitted as random explanatory variables. Plasmodium infection prevalence (proportion of mosquitoes containing at least one parasite; models 1–5, electronic supplementary material, table S2) was analysed using generalized linear mixed models with a binomial error distribution (lmer, lme4 package). Plasmodium infection intensity (oocyst and sporozoite loads) was analysed by including only individuals that became infected. As found in other systems [32], oocyst count data were greatly overdispersed. One way of handling this overdispersion is by using negative binomial pseudo distributions [32]. However, to our knowledge, it is not currently possible to account for negative binomial distributions within a mixed model lmer procedure. For this reason, we used instead a glm model with a negative binomial error distribution (glm.nb, MASS package; models 6 and 8; electronic supplementary material, table S2) and we fitted bird and qPCR plate as fixed factors, next to our variables of interest (i.e. mosquito strain, dissection day, mosquito wing size). Using fixed rather than mixed models results in some loss of statistical power, but the results are likely to be conservative [33]. Sporozoite load data were analysed using a glm model with a quasi-error distribution and a log link with a variance equal to μ² to correct for overdispersion (models 7 and 9). Wolbachia density was Box-Cox transformed [34] (models 10 and 11) and subsequently analysed using linear mixed-effect models (lme, nlme package). Differences in wing size between the lines were analysed using an ANOVA (aov). Maximal models, including all higher-order interactions, were simplified by sequentially eliminating non-significant terms and interactions to establish a minimal model [34]. The significance of the explanatory variables was established using a likelihood ratio test (LRT), which is approximately distributed as a χ2 distribution [33]. The significant χ2 values given in the text are for the minimal model [34]. Full dataset has been deposited in the Dryad Digital Repository (doi.org/10.5061/dryad.m3752).
3. Results
During the blood meal, one infected canary died for an unknown reason, so this replicate was eliminated from all subsequent analyses. The percentages of mosquitoes that did not blood feed or died before the dissections are detailed in the electronic supplementary material, table S3. In the end, a total of 77 wSL and 79 w(−) mosquitoes and 81 wSL and 83 w(−) mosquitoes were dissected at the oocyst (day 7–8 pbm) and sporozoite (day 14 pbm) stages, respectively. Overall, w(−) females were smaller than wSL ones (mean ± se, w(−) 3.52 ± 0.01 mm, wSL 3.62 ± 0.01 mm, 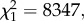 p < 0.0001).
p < 0.0001).
We first analysed whether Wolbachia influences Plasmodium prevalence. Our results show that the probability of becoming infected with P. relictum is significantly higher when Wolbachia is present (wSL). This effect is consistent across the oocyst (probability of infection in wSL is on average 15.9 ± 7.1% higher than in w(−), 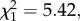 p = 0.02, model 1) and the sporozoite (20.6 ± 7.7% higher,
p = 0.02, model 1) and the sporozoite (20.6 ± 7.7% higher, 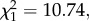 p = 0.001, model 2) stages (figure 1). The combined analysis of the two measurement times revealed a mean (±s.e.) decrease of 26.2 (± 5.3) % in the Plasmodium prevalence between 7–8 and 14 dpbm (Plasmodium stage effect:
p = 0.001, model 2) stages (figure 1). The combined analysis of the two measurement times revealed a mean (±s.e.) decrease of 26.2 (± 5.3) % in the Plasmodium prevalence between 7–8 and 14 dpbm (Plasmodium stage effect: 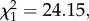 p < 0.0001, model 3), irrespective of the presence of Wolbachia (Wolbachia × Plasmodium stage interaction:
p < 0.0001, model 3), irrespective of the presence of Wolbachia (Wolbachia × Plasmodium stage interaction: 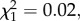 p = 0.88, model 3; figure 1). In wSL females, the probability of becoming infected by Plasmodium when exposed to an infected bird is independent of the density of Wolbachia (oocysts:
p = 0.88, model 3; figure 1). In wSL females, the probability of becoming infected by Plasmodium when exposed to an infected bird is independent of the density of Wolbachia (oocysts: 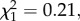 p = 0.64, model 4; sporozoites:
p = 0.64, model 4; sporozoites: 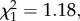 p = 0.28, model 5). Reciprocally, the Wolbachia density in female abdomens did not differ between mosquitoes fed on a Plasmodium-infected or uninfected bird either at 7–8 dpbm (
p = 0.28, model 5). Reciprocally, the Wolbachia density in female abdomens did not differ between mosquitoes fed on a Plasmodium-infected or uninfected bird either at 7–8 dpbm (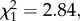 p = 0.09, model 10) or at 14 dpbm (
p = 0.09, model 10) or at 14 dpbm (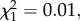 p = 0.91, model 11; figure 2).
p = 0.91, model 11; figure 2).
Figure 1.
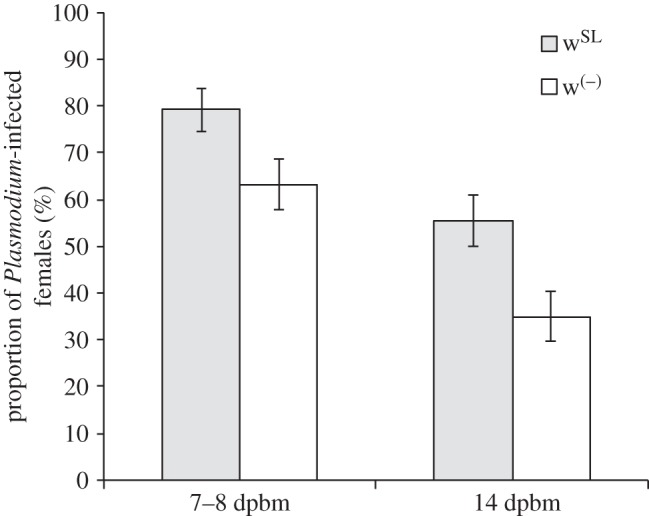
Effect of Wolbachia on the prevalence of Plasmodium infection 7 days (oocyst stage) and 14 days post blood meal (sporozoite stage). Bars represent means (±s.e.) for Wolbachia-carrying females (grey bars) and Wolbachia-free ones (white bars). dpbm, days post blood meal.
Figure 2.
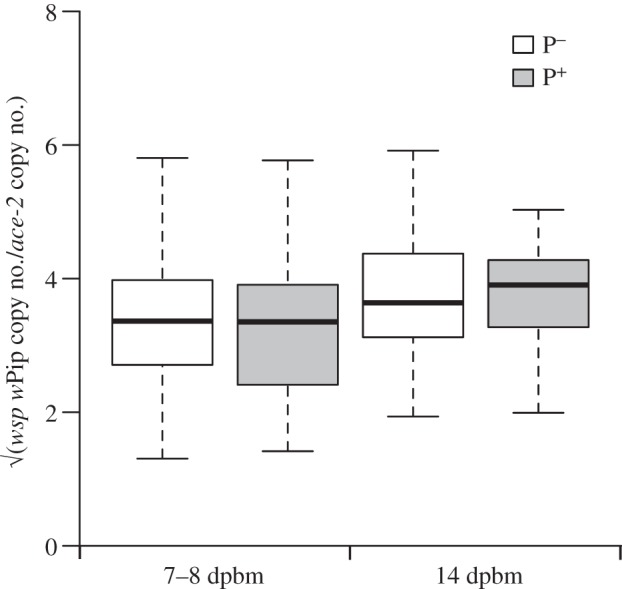
Boxplot of the Wolbachia density in wSL females according to the Plasmodium infection status at 7–8 days (oocysts) and 14 days (sporozoites) post blood meal. White boxes: Plasmodium uninfected mosquitoes (includes females fed on a control bird and females that did not become infected after feeding on a Plasmodium-infected bird) and grey boxes: Plasmodium infected mosquitoes. Wolbachia densities were Box-Cox transformed to linearize the data for the graphic representation.
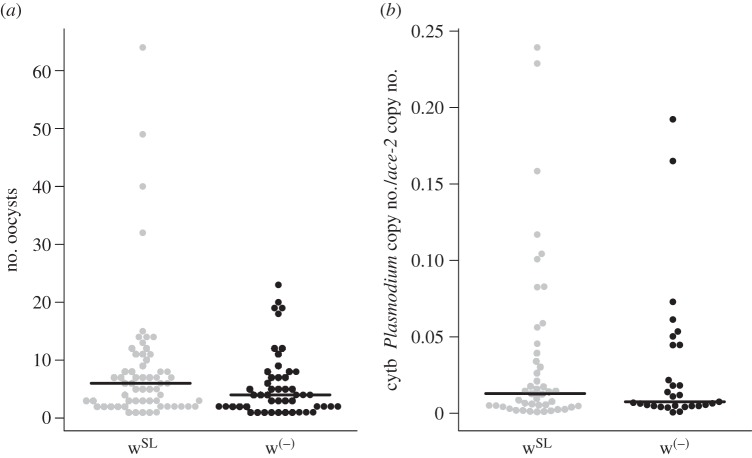
We then analysed whether Wolbachia influences intensity of the Plasmodium infection. The number of oocysts that successfully developed in the mosquito midgut is significantly higher in wSL than in w(−) females (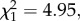 p = 0.03, model 6, figure 3a). wSL females have on average three more oocysts than w(−) ones (mean ± s.e., 8.4 ± 1.4 and 5.7 ± 0.8 oocysts, respectively). By contrast, the relative quantity of sporozoites present in infected mosquito thoraxes is independent of the presence of Wolbachia (
p = 0.03, model 6, figure 3a). wSL females have on average three more oocysts than w(−) ones (mean ± s.e., 8.4 ± 1.4 and 5.7 ± 0.8 oocysts, respectively). By contrast, the relative quantity of sporozoites present in infected mosquito thoraxes is independent of the presence of Wolbachia (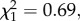 p = 0.55, model 7; figure 3b). As above, neither oocyst nor sporozoite load are correlated with Wolbachia density (oocyst:
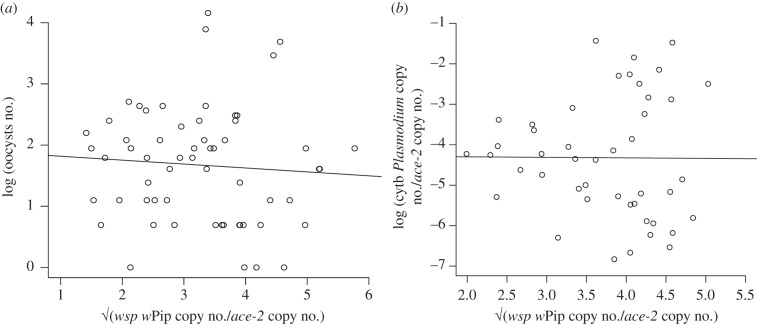
p = 0.55, model 7; figure 3b). As above, neither oocyst nor sporozoite load are correlated with Wolbachia density (oocyst: 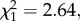 p = 0.10, model 8; sporozoite:
p = 0.10, model 8; sporozoite: 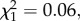 p = 0.84, model 9; figure 4).
p = 0.84, model 9; figure 4).
Figure 3.
Effect of the presence of Wolbachia on Plasmodium burden in mosquitoes. (a) Distribution of the number of oocysts in the midgut of Plasmodium-infected females 7–8 days post blood meal, and (b) distribution of the relative quantity of sporozoites in the thorax of Plasmodium-infected females 14 days post blood meal, for Wolbachia-carrying females (grey circles) and Wolbachia-free ones (black circles). Horizontal lines represent medians.
Figure 4.
Correlation between the density of Wolbachia and the intensity of Plasmodium infection at the (a) oocyst and (b) sporozoite stages (7–8 days and 14 days post blood meal, respectively). Both Wolbachia and Plasmodium densities were Box-Cox transformed to linearize the data for the graphic representation.
4. Discussion
Current views about the impact of Wolbachia on Plasmodium infections are almost entirely based on data regarding artificially transfected mosquitoes. This work has shown that Wolbachia reduces the number of Plasmodium oocysts in the midgut of mosquitoes. By contrast, and probably because of the difficulty in finding natural Wolbachia infections in epidemiologically significant malaria vectors, the role of natural Wolbachia infections in Plasmodium development has either been ignored entirely or been given only cursory attention. Wolbachia–mosquito combinations with a long evolutionary history may, however, be key for understanding what will happen with Wolbachia-transfected mosquitoes several generations down the line if, as has been shown in other systems [22,23], the novel Wolbachia–host interaction evolves rapidly. The number of generations needed for such evolutionary change can be between 20 [22] and 200 [23,35]. To our knowledge, the only previous studies carried out using natural Wolbachia infections involve the mosquito Aedes fluviatilis and the Asian avian malaria parasite P. gallinaceum. This work has shown that, far from decreasing parasitaemia, Wolbachia either has no effect [17,19] or increases [19] the number of Plasmodium oocysts in the midgut of the mosquito. Aedes fluviatilis is, however, a South American mosquito that serves as a convenient laboratory host for P. gallinaceum, but it is not its natural vector. Previous work has indeed shown that Wolbachia can render contrasting results on natural [23] and artificial [17,19] Plasmodium combinations, so the question that is relevant for the long-term success of malaria control programmes—of whether Wolbachia can interfere with Plasmodium transmission in an entirely natural system—is still unresolved.
Here, we used an entirely natural mosquito–Wolbachia–Plasmodium combination to investigate whether Wolbachia increases or decreases the parasitaemia of mosquitoes. In contrast to most previous studies, which have been centred on the quantification of oocysts in the midgut of mosquitoes, we aimed to obtain a measurement of parasitaemia that would relate more directly to transmission by following the infections all the way to the sporozoites stage, as recently done in An. stephensi [12]. We found that Wolbachia increases marginally, albeit statistically significantly, the oocyst load of mosquitoes. However, the difference in oocyst load found in the midguts on day 7 was not sufficiently marked to translate into a difference in sporozoite load in the salivary glands 7 days later. One potential explanation for these results is that since a single oocyst can produce thousands sporozoites, beyond a certain oocyst threshold the salivary glands of mosquitoes may have become saturated by sporozoites [36]. Alternatively, the drastic loss of parasites that inevitably takes place between the midgut and the salivary stages in any Plasmodium infection [32] may upstage the marginal differences in oocystaemia that exist early on. Proof of the inefficient migration from the midgut to the salivary glands is the significant (26%) decrease in Plasmodium prevalence that we observed between the oocyst and the sporozoite stages, which was independent of the presence of Wolbachia.
Irrespective of the underlying mechanism, we believe that the epidemiological significance of having more or fewer Plasmodium parasites in the gut or even in the salivary glands remains to be demonstrated. As stated above, a single oocyst can produce between 2000 and 8000 sporozoites [37], and as few as 10 sporozoites suffice to start a new infection [26]. There is also no consistent evidence that the density of sporozoites in the salivary glands correlates with the number of infecting sporozoites [38], or that this correlates with the probability of a successful infection in the host (but see [39]). Mosquito infection intensity is, indeed, conspicuously absent from current models of malaria transmission and epidemiology [26,40]. Infection intensity may, however, bear on epidemiology if it correlates negatively with key life-history traits of the vector, such as longevity, but the evidence for this is sparse and comes from unrealistically high infections [41]. By contrast, infection prevalence, i.e. the number of infectious mosquitoes in a population, is the keystone of epidemiological models [26]. The proportion of infectious mosquitoes in a population, sometimes called the sporozoite rate, is a key determinant of the rate at which hosts are bitten in a population [26,40]. Here, we show that the presence of Wolbachia increases sporozoite prevalence by as much as 21%. Wolbachia does therefore play a major role in the transmission of Plasmodium in the avian malaria system.
In several host species, Wolbachia density can fluctuate both between individuals [31,42] and within individuals over time [42,43], and several Wolbachia-induced phenotypes, such as cytoplasmic incompatibility [42] (but see [43]), longevity curtailment [44] or host resistance to viruses [45], have been shown to depend on the density of infecting bacteria. The correlation between Wolbachia density and parasite density can provide interesting insights as to the mechanisms underlying the interaction. For example, a strong negative correlation was found between Wolbachia density and dengue virus load in Ae. agypti and Aedes albopictus cell lines [45], whereas in Ae. albopictus infected with the chikungunya virus, the intensive phase of the viral replication is concomitant with a significant decrease in Wolbachia load [20,46,47], leading the authors to suggest immune competition and resource competition, respectively, as the mechanisms driving the interaction between these two players. Here, however, neither the probability nor the intensity of Plasmodium infection at either the oocyst or sporozoite stages are explained by the density of Wolbachia. It would therefore appear that it is the presence of Wolbachia, irrespective of its density, that determines the increase in prevalence and intensity observed, as previously found in An. gambiae with both P. falciparum and P. berghei [13,14]. In addition, the density of bacteria did not differ depending on whether the mosquitoes were infected by Plasmodium or not, suggesting that the Wolbachia–Plasmodium interaction only works one way.
With this in mind, several different, but non-exclusive, mechanisms may be envisaged to explain our results. First, we found that Wolbachia-infected mosquitoes were significantly bigger than Wolbachia-free ones and may thus have simply taken larger blood meals, thereby increasing their intake of Plasmodium gametocytes (the stage that is transmissible to mosquitoes). We have previously shown that the number of P. relictum oocysts is significantly correlated with the amount of blood ingested by the mosquitoes, albeit in a nonlinear way [29]. Second, Wolbachia may facilitate the successful establishment of Plasmodium within the mosquito tissues. One obvious way in which this could happen is through a Wolbachia-induced downregulation of the non-specific arm of the mosquito immune system, a form of self-protection that has been observed both in pill bugs (or woodlice) [48] and parasitoids [49]. In this respect, these natural Wolbachia infections would behave in a drastically different way to artificial infections, which are often found to upregulate the immune system when introduced into a novel host [12,13,15,17,45].
Third, the differences observed between our Wolbachia-infected and -free mosquito lines could be mediated by differences in their midgut microbiota, which have been recently shown to play a key role in mosquito resistance to Plasmodium infection [50,51]. Using tetracycline to eliminate Wolbachia is standard practice, the consensus being that mosquitoes recover their microbial flora over a certain number of generations, a premise that, to our knowledge has never been explicitly tested. Therefore, the possibility that the antibiotic treatment may have irreversibly altered the midgut microbiota of mosquitoes, and therefore the resistance to Plasmodium infection, cannot be totally eliminated. More interesting from a biological point of view, but to our knowledge also hitherto unexplored, is the possibility that Wolbachia itself may modify (through competition, or facilitation) the density and composition of the microbial flora of their hosts.
Finally, w(−) was reared for ca 30 generations before the experiment to eliminate side effects of the tetracycline. Although the wSL and w(−) were kept throughout under identical culturing conditions, we cannot entirely exclude the possibility that the two lines may have diverged and that the results we obtain are due to different genetic backgrounds. Further work should replicate these results with, if possible, several Wolbachia-infected and -uninfected lines.
Previous work in this system has shown that Plasmodium-infected females suffer lower mortality rates if they are also infected with Wolbachia [27]. We had originally advanced two potential explanations for these results: Wolbachia-infected mosquitoes could be either more resistant or more tolerant to a Plasmodium infection. Under the first (resistance) scenario, Wolbachia would limit or inhibit parasite development, thereby reducing overall parasitaemia. Dawes et al. [41] have indeed shown that in rodent malaria the number of oocysts in the mosquito midgut is correlated with mosquito longevity, but their evidence comes from extremely high (100–2000) oocyst burdens. Under the second (tolerance) scenario, Wolbachia would limit or compensate for the damage incurred by the parasite, without necessarily altering the within-host growth rate of the parasite [52]. An increase in tolerance to pathogens has been previously observed with native Wolbachia strain of Drosophila flies when challenged with viruses [9,53]. Elucidating which of these mechanisms is at play is essential from a transmission perspective because parasite-resistant vectors are expected to be worse vectors of diseases, while the opposite will be true for parasite-tolerant ones (the ‘tragedy of tolerance’ [54]). The results of the present experiments show that Wolbachia-infected mosquitoes are in fact less resistant to Plasmodium, leaving a higher Wolbachia-associated tolerance to Plasmodium as the only potential explanation for the longevity results, the mechanisms underlying which remain to be explored.
In conclusion, we show that Wolbachia increases the susceptibility of Cx. pipiens mosquitoes to P. relictum, significantly increasing the prevalence of salivary gland stage infections. Previous work on this same system has shown that Wolbachia also protects mosquitoes against a Plasmodium-induced mortality [27]. As both mosquito mortality and infection prevalence are two key determinants of Plasmodium epidemiology, these results suggest that naturally Wolbachia-infected mosquitoes may, in fact, be better vectors of malaria than Wolbachia-free ones.
Acknowledgements
We are grateful to S. Alizon and F. Vavre and the three anonymous referees for useful discussions and comments on the manuscript. We also thank N. Barougier, P. Boutinaud, J. Denoyelle, P. Perret and G. Sorci, for their help at different stages of the experiments.
Animal experiments were carried out in strict accordance with the ‘National Charter on the Ethics of Animal Experimentation’ of the French Government, and all efforts were made to minimize suffering. Experiments were approved by the Ethical Committee for Animal Experimentation established by the authors' institution (CNRS) under the auspices of the French Ministry of Education and Research (permit number CEEA- LR-1051).
Funding statement
This project is funded by the French ANR program (ANR ‘IRMAL’) to AR. AN was partly funded by an ERC starting grant to Sylvain Gandon, FZ was funded by a PhD grant from the CNRS and the Languedoc-Roussillon Region. This is contribution ISEM 2013-208 of the Institut des Sciences de l'Evolution de Montpellier (UMR 5554 CNRS – Université Montpellier 2).
References
- 1.Alizon S, van Baalen M. 2008. Multiple infections, immune dynamics, and the evolution of virulence. Am. Nat. 172, E150–E168 (doi:10.1086/590958) [DOI] [PubMed] [Google Scholar]
- 2.Pedersen AB, Fenton A. 2007. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 22, 133–139 (doi:10.1016/j.tree.2006.11.005) [DOI] [PubMed] [Google Scholar]
- 3.Graham AL. 2008. Ecological rules governing helminth-microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570 (doi:10.1073/pnas.0707221105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley MA, Wertz JE. 2002. Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56, 117–137 (doi:10.1146/annurev.micro.56.012302.161024) [DOI] [PubMed] [Google Scholar]
- 5.Swanson SJ, Neitzel D, Reed KD, Belongia EA. 2006. Coinfections acquired from Ixodes ticks. Clin. Microbiol. Rev. 19, 708–727 (doi:10.1128/cmr.00011-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes T, Irwin P, Hofmeister E, Paskewitz SM. 2010. Occurrence of avian Plasmodium and West Nile virus in Culex species in Wisconsin. J. Am. Mosq. Control Assoc. 26, 24–31 (doi:10.2987/09-5893.1) [DOI] [PubMed] [Google Scholar]
- 7.Vazeille M, Mousson L, Martin E, Failloux A-B. 2010. Orally co-infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl. Trop. Dis. 4, e706 (doi:10.1371/journal.pntd.0000706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322, 702 (doi:10.1126/science.1162418) [DOI] [PubMed] [Google Scholar]
- 9.Teixeira L, Ferreira A, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, 2753–2763 (doi:10.1371/journal.pbio.1000002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi ZY, Khoo CCH, Dobson SL. 2005. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310, 326–328 (doi:10.1126/science.1117607) [DOI] [PubMed] [Google Scholar]
- 11.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, O'Neill SL. 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323, 141–144 (doi:10.1126/science.1165326) [DOI] [PubMed] [Google Scholar]
- 12.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, Xu Y, Dimopoulos G, Xi Z. 2013. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340, 748–751 (doi:10.1126/science.1236192) [DOI] [PubMed] [Google Scholar]
- 13.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. 2011. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 7, e1002043 (doi:10.1371/journal.ppat.1002043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes GL, Vega-Rodriguez J, Xue P, Rasgon JL. 2012. Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl. Environ. Microbiol. 78, 1491–1495 (doi:10.1128/aem.06751-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kambris Z, Blagborough AM, Pinto SB, Blagrove MSC, Godfray HCJ, Sinden RE, Sinkins SP. 2010. Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog. 6, e1001143 (doi:10.1371/journal.ppat.1001143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kambris Z, Cook PE, Phuc HK, Sinkins SP. 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326, 134–136 (doi:10.1126/science.1177531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreira LA, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (doi:10.1016/j.cell.2009.11.042) [DOI] [PubMed] [Google Scholar]
- 18.Blagrove MSC, Arias-Goeta C, Failloux A-B, Sinkins SP. 2012. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl Acad. Sci. USA 109, 255–260 (doi:10.1073/pnas.1112021108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baton LA, Pacidonio EC, Goncalves DdS, Moreira LA. 2013. wFlu: Characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS ONE 8, e59619 (doi:10.1371/journal.pone.0059619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousson L, Martin E, Zouache K, Madec Y, Mavingui P, Failloux AB. 2010. Wolbachia modulates Chikungunya replication in Aedes albopictus. Mol. Ecol. 19, 1953–1964 (doi:10.1111/j.1365-294X.2010.04606.x) [DOI] [PubMed] [Google Scholar]
- 21.Glaser RL, Meola MA. 2010. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5, e11977 (doi:10.1371/journal.pone.0011977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGraw EA, Merritt DJ, Droller JN, O'Neill SL. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl Acad. Sci. USA 99, 2918–2923 (doi:10.1073/pnas.052466499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5, 997–1005 (doi:10.1371/journal.pbio.0050114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripet F. 2009. Ecological immunology of mosquito–malaria interactions: of non-natural versus natural model systems and their inferences. Parasitology 136, 1935–1942 (doi:10.1017/s0031182009006234) [DOI] [PubMed] [Google Scholar]
- 25.Cohuet A, Osta MA, Morlais I, Awono-Ambene PH, Michel K, Simard F, Christophides GK, Fontenille D, Kafatos FC. 2006. Anopheles and Plasmodium: from laboratory models to natural systems in the field. EMBO Rep. 7, 1285–1289 (doi:10.1038/sj.embor.7400831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DL, McKenzie FE. 2004. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria J. 3, 13 (doi:10.1186/1475-2875-3-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zélé F, Nicot A, Duron O, Rivero A. 2012. Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J. Evol. Biol. 25, 1243–1252 (doi:10.1111/j.1420-9101.2012.02519.x) [DOI] [PubMed] [Google Scholar]
- 28.Bensch S, Hellgren O, Perez-Tris J. 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Res. 9, 1353–1358 (doi:10.1111/j.1755-0998.2009.02692.x) [DOI] [PubMed] [Google Scholar]
- 29.Vézilier J, Nicot A, Gandon S, Rivero A. 2010. Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malaria J. 9, 379 (doi:10.1186/1475-2875-9-379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weill M, Berticat C, Raymond N, Chevillon C. 2000. Quantitative polymerase chain reaction to estimate the number of amplified esterase genes in insecticide-resistant mosquitoes. Anal. Biochem. 285, 267–270 (doi:10.1006/abio.2000.4781) [DOI] [PubMed] [Google Scholar]
- 31.Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M. 2002. High Wolbachia density in insecticide-resistant mosquitoes. Proc. R. Soc. Lond. B 269, 1413–1416 (doi:10.1098/rspb.2002.2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughan JA. 2007. Population dynamics of Plasmodium sporogony. Trends Parasitol. 23, 63–70 (doi:10.1016/j.pt.2006.12.009) [DOI] [PubMed] [Google Scholar]
- 33.Bolker BM. 2008. Ecological models and data in R. Princeton, NJ: Princeton University Press [Google Scholar]
- 34.Crawley MJ. 2007. The R book. Chichester, UK: John Wiley & Sons, Ltd [Google Scholar]
- 35.Carrington LB, Hoffmann AA, Weeks AR. 2010. Monitoring long-term evolutionary changes following Wolbachia introduction into a novel host: the Wolbachia popcorn infection in Drosophila simulans. Proc. R. Soc. B 277, 2059–2068 (doi:10.1098/rspb.2010.0166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinden RE, et al. 2007. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog. 3, 2005–2016 (doi:10.1371/journal.ppat.0030195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Fujioka H, Nussenzweig V. 2005. Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog. 1, e9 (doi:10.1371/journal.ppat.0010009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beier JC. 1998. Malaria parasite development in mosquitoes. Ann. Rev. Entomol. 43, 519–543 (doi:10.1146/annurev.ento.43.1.519) [DOI] [PubMed] [Google Scholar]
- 39.Kebaier C, Voza T, Vanderberg J. 2009. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 5, e1000399 (doi:10.1371/journal.ppat.1000399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DL, Dushoff J, Snow RW, Hay SI. 2005. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438, 492–495 (doi:10.1038/nature04024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basanez MG. 2009. Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malaria J. 8, 228 (doi:10.1186/1475-2875-8-228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark ME, Veneti Z, Bourtzis K, Karr TL. 2003. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech. Dev. 120, 185–198 (doi:10.1016/s0925-4773(02)00424-0) [DOI] [PubMed] [Google Scholar]
- 43.Duron O, Fort P, Weill M. 2007. Influence of aging on cytoplasmic incompatibility, sperm modification and Wolbachia density in Culex pipiens mosquitoes. Heredity 98, 368–374 (doi:10.1038/sj.hdy.6800948) [DOI] [PubMed] [Google Scholar]
- 44.Min KT, Benzer S. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl Acad. Sci. USA 94, 10 792–10 796 (doi:10.1073/pnas.94.20.10792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu P, Bian G, Pan X, Xi Z. 2012. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 6, e1754 (doi:10.1371/journal.pntd.0001754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tortosa P, Courtiol A, Moutailler S, Failloux AB, Weill M. 2008. Chikungunya–Wolbachia interplay in Aedes albopictus. Insect. Mol. Biol. 17, 677–684 (doi:10.1111/j.1365-2583.2008.00842.x) [DOI] [PubMed] [Google Scholar]
- 47.Zouache K, Michelland RJ, Failloux A-B, Grundmann GL, Mavingui P. 2012. Chikungunya virus impacts the diversity of symbiotic bacteria in mosquito vector. Mol. Ecol. 21, 2297–2309 (doi:10.1111/j.1365-294X.2012.05526.x) [DOI] [PubMed] [Google Scholar]
- 48.Sicard M, Chevalier F, De Vlechouver M, Bouchon D, Greve P, Braquart-Varnier C. 2010. Variations of immune parameters in terrestrial isopods: a matter of gender, aging and Wolbachia. Naturwissenschaften 97, 819–826 (doi:10.1007/s00114-010-0699-2) [DOI] [PubMed] [Google Scholar]
- 49.Fytrou A, Schofield PG, Kraaijeveld AR, Hubbard SF. 2006. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc. R. Soc. B 273, 791–796 (doi:10.1098/rspb.2005.3383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong YM, Manfredini F, Dimopoulos G. 2009. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 5, e1000423 (doi:10.1371/journal.ppat.1000423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cirimotich CM, Dong YM, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. 2011. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858 (doi:10.1126/science.1201618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raberg L, Sim D, Read AF. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814 (doi:10.1126/science.1148526) [DOI] [PubMed] [Google Scholar]
- 53.Osborne SE, Leong YS, O'Neill SL, Johnson KN. 2009. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 5, e1000656 (doi:10.1371/journal.ppat.1000656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vale PF, Wilson AJ, Best A, Boots M, Little TJ. 2011. Epidemiological, evolutionary, and coevolutionary implications of context-dependent parasitism. Am. Nat. 177, 510–521 (doi:10.1086/659002) [DOI] [PMC free article] [PubMed] [Google Scholar]