Abstract
To generate realistic projections of species’ responses to climate change, we need to understand the factors that limit their ability to respond. Although climatic niche conservatism, the maintenance of a species’s climatic niche over time, is a critical assumption in niche-based species distribution models, little is known about how universal it is and how it operates. In particular, few studies have tested the role of climatic niche conservatism via phenological changes in explaining the reported wide variance in the extent of range shifts among species. Using historical records of the phenology and spatial distribution of British plants under a warming climate, we revealed that: (i) perennial species, as well as those with weaker or lagged phenological responses to temperature, experienced a greater increase in temperature during flowering (i.e. failed to maintain climatic niche via phenological changes); (ii) species that failed to maintain climatic niche via phenological changes showed greater northward range shifts; and (iii) there was a complementary relationship between the levels of climatic niche conservatism via phenological changes and range shifts. These results indicate that even species with high climatic niche conservatism might not show range shifts as instead they track warming temperatures during flowering by advancing their phenology.
Keywords: climate change, phenology, climatic niche conservatism, species distribution models
1. Introduction
Understanding the determinants of species’ niches in time and space is a critical challenge for ecologists in an era of global change [1,2]. With widespread concerns over global biodiversity loss, increasing importance has been placed on understanding spatial–temporal dynamics of species’ niches in relation to projected species’ responses to climate change [3] and the spread of invasive species [4]. In particular, a better mechanistic understanding of spatial-temporal niche dynamics has been sought to inform projections of species’ responses to novel environments [5].
There is now considerable evidence that recent climate change has caused a wide range of species to shift their spatial distribution and/or phenology [6]. Such phenomena are known to be the consequences of climatic niche conservatism, the maintenance of a species’s climatic niche over time [2]. Climatic niche conservatism constitutes a critical assumption in niche-based species distribution models [1], which have been widely used to project potential range shifts and extinction in response to climate change [3,7]. However, responses to climate change can vary greatly between species [8], and factors determining the strength and generality of climatic niche conservatism have rarely been explored [1,2] (but see [9]). Thus, there is an urgent need to identify those factors that contribute significantly to the strength of climatic niche conservatism (i.e. factors that explain variation between the responses of different species to climate change) in order to understand and project niche dynamics across time and space under changing climatic conditions.
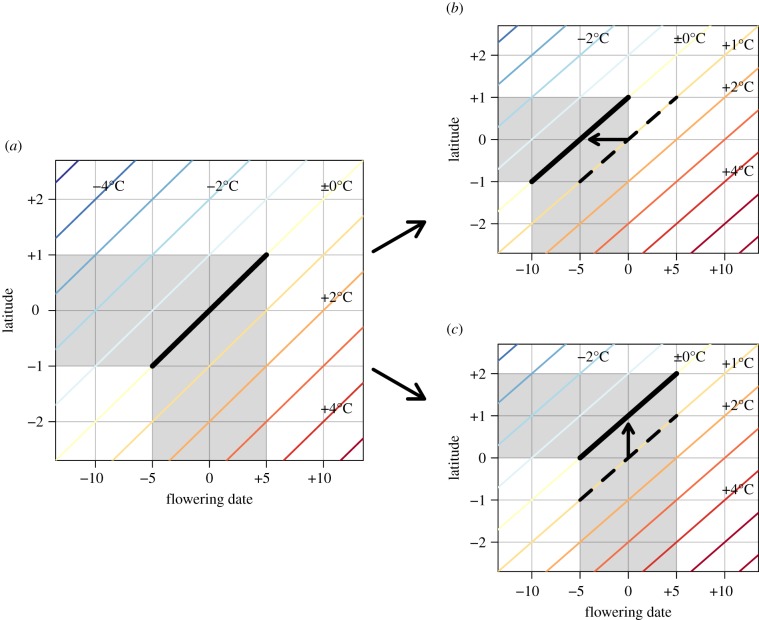
This study focused on the potential link between species’ spatial and temporal responses to conserve climatic niche. More specifically, we hypothesized that the level of species’ climatic niche conservatism through phenological changes explains, at least partly, the reported wide variance between species in the extent of range shifts and climatic niche conservatism via range shifts. Failure to track a changing climate during flowering has a detrimental effect, increasing the risk of frost and drought damage [10], reducing competitive ability [11] (particularly against species that are pre-adapted or more responsive [12]), lowering productivity [13] and disrupting temporal interactions between species [14], although climatic conditions can also affect the fitness of plant species outside the flowering season. Species can maintain climatic niche during flowering under a warming climate by two types of responses: range shift and phenological changes. For example, the successful tracking of a warming climate through phenological advances allows species to maintain climatic niche without changing their geographical range (figure 1a,b). By contrast, species that cannot track a warming climate by effectively advancing their phenology need to move to higher latitudes to maintain their climatic niche during flowering (figure 1a,c). Considering the reported wide variance in the extent of both range shifts and phenological advances among species [6,15], a complementary relationship would be expected between these two types of species’ responses for maintaining climatic niche under changing climate. Such a link between the magnitude of phenological responses and range shifts is expected across the geographical range of each species, given that different populations of the same species are adapted locally [16]. Some studies have emphasized the importance of phenology in determining species’ distributional range (e.g. through the effect of frost [17,18]). However, few studies to date have explicitly tested the link between species’ spatial and temporal responses to climate change [19].
Figure 1.
Schematic of climatic niche conservatism through phenological changes and range shifts. Diagonal lines represent isothermal lines. Grey areas show a species’s geographical range and flowering periods, with black thick lines indicating flowering dates at different latitudes within the species’s range. (a) Before warming, this example species flowers between day −5 and day +5, consequently maintaining the temperature during flowering as ±0°C across its geographical range between latitude −1 and latitude +1. (b) Climatic niche conservatism via phenological changes. Owing to warming, with neither phenological changes nor range shifts, the species would experience increasing temperature during flowering (dashed line). Advancing flowering dates by 5 days (arrow) would enable this species to maintain the temperature during flowering without changing the geographical range. (c) Climatic niche conservatism via range shifts. If a species could not track a warming climate by advancing phenology like in (b), the species would need to move to higher latitudes (arrow) to maintain the temperature during flowering.
Britain is an ideal study system for this purpose because (i) historical changes in first flowering dates have been estimated for 405 plant species by applying a hierarchical model to almost 400 000 observation records throughout the country [20], and (ii) records on spatial distribution are available for 6669 higher plant taxa throughout Britain at two census periods [21]. These extensive data enabled us to explore the interacting dynamics of species’ realized niches over space and time. Therefore, this study first quantified the level of species’ spatial and temporal responses to a warming climate, and consequent levels of climatic niche conservatism. The following questions were then addressed (see also the electronic supplementary material, figure S1). (i) What species characteristics determine the level of climatic niche conservatism via phenological changes? (ii) Do species that cannot track a warming climate via phenological changes show more notable northward range shifts? (iii) As a consequence of (ii), is there a complementary relationship between the levels of climatic niche conservatism via phenological changes and that through range shifts?
2. Material and methods
(a). Climatic niche conservatism through phenological changes
The level of temporal climatic niche conservatism in each species was first quantified using data from the UK Phenology Network (www.naturescalendar.org.uk) supplied by the Woodland Trust. The data consisted of 395 466 records of first flowering dates collected at multiple sites throughout the UK for 405 species from 1753 to 2009. Amano et al. [20] applied a Bayesian analysis of a hierarchical model to the data to estimate (i) a community-level index, which summarizes a community-level nationwide temporal trend of first flowering dates, and (ii) species-level indices, which represent nationwide trends for each species. The model explicitly takes into account the latitudinal difference in first flowering dates between sites, but assumes the same phenological change across latitudes within the same species when estimating the species-level indices. The species-level index shows the first flowering dates of the species at the mean latitude of all records (i.e. irrespective of potential range shifts; see the electronic supplementary material, appendix A and [20] for further information on the data and model).
Next, using the species-level indices of first flowering dates and central England temperature (CET) [22], changes in temperatures experienced by the flowers of each species were estimated to represent the level of climatic niche conservatism achieved through the species’s phenological changes only, without range shifts (i.e. the effectiveness of the species’s response illustrated in figure 1b). The CET has been shown to be broadly representative of temperatures in other parts of Britain [23]. The daily mean CET was downloaded from the webpage of the Meteorological Office Hadley Centre (www.metoffice.gov.uk/hadobs/hadcet). Temperatures experienced by the flowers of each species in each year were defined as the mean daily CET in the week starting from the first flowering date estimated by the species-level index for that year and species. The change in temperatures experienced by the flowers of each species was estimated by the regression on years for the period between 1930 and 2009, as information on species’ range shifts is only available after 1930 (see §2c for more details). Only native species (native status = N or NH in the PLANTATT database [24]) whose indices exceeded 19 years were used in this analysis, based on an earlier study which reported that, in most species, relatively accurate estimates of the relationship between flowering dates and temperature can be obtained with 20-year data [25].
(b). Effect of species characteristics on climatic niche conservatism through phenological changes
Five species characteristics that can affect the level of climatic niche conservatism through phenological changes were explored: (i) species’ phenological responses to temperature, (ii) inter-annual autocorrelations in first flowering dates, (iii) mean first flowering dates, (iv) lag in phenological responses and (v) perennation. To quantify these characteristics, species-level indices were first regressed against the CET of one of the six months from the month of mean first flowering date and the preceding five months (see the electronic supplementary material, appendix B for the justification of this approach). The first-order autoregressive term was also included in each regression model. The month of the model with the highest R2 value was then defined as the month most responsible for the flowering time of that species. The estimated coefficients for temperature of this month were then used to represent species' phenological responses to temperature (days/°C) while the first-order autoregressive term in the same model was used for the inter-annual autocorrelations in first flowering dates. The lag in phenological responses was defined as the difference between the month most responsible for flowering time and the month of mean first flowering date. Only native species whose indices exceeded 19 years were used in this analysis. Information on the perennation (annual or perennial; biennial was included in perennial) of each species was derived from the PLANTATT database [24].
Regression analysis was conducted to investigate the effect of the five species characteristics on the level of climatic niche conservatism through phenological changes. The interaction term between responses to temperature and the response lag was also tested after centring both the variables. Models for all possible parameter subsets were compared in terms of parsimony and prediction through the calculation of Akaike's information criterion for small samples (AICc) using the package MuMIn [26] in R [27]. Of those species with estimates of responses to temperature, only those with information on all the explanatory variables were used for the analysis (n = 246).
(c). Range shifts and climatic niche conservatism through range shifts
The distribution records of British plant species, collected from various sources, including the Botanical Society for the British Isles’ vice-county recorder scheme, as well as dedicated surveys, have been published as an atlas [28]. The atlas was updated [29] when the original version was found to be outdated [30]. The distribution data were obtained from this atlas, which is now maintained online, as the vascular plant database, covering 6669 higher plant taxa in the British Isles [21]. Records in this database were collected between 1629 and 2006, and are standardized and presented in the form of distribution maps of species’ records at a 10 × 10 km resolution. The data for each species were sorted to include only the records from two time periods: 1930–1960 and 1987–1999. These intervals correspond with the full data collection periods for each edition of the atlas.
The distribution data were standardized to contain records corresponding to a grid of 2646 10 × 10 km squares, which are a comparable set of coordinates and do not include squares recorded only in the 2002 atlas [29], for example some coastal squares. The distribution data from Northern Ireland and the Isle of Man were not used in this study, as the new atlas used a different grid map for these areas, allowing no direct comparison with the first atlas. The mean latitude and temperature of the first flowering month of each species’s distribution were calculated from all 10 km square sites where the species was recorded during the first (1930–1960) and second (1987–1999) time periods. Note that first flowering months used to calculate the range-wide mean temperature for each species were not based on our own phenological data, but derived from an independent database (the Ecological Flora Database [31]). Thus, the calculated range-wide temperatures were independent of the estimated phenological changes between the two periods. The difference in the mean latitude between these two periods was used to define mean range shifts, while differences in mean temperature were used to assess the degree of climatic niche conservatism through range shifts only, without phenological changes (i.e. effectiveness of the species’s responses illustrated in figure 1c). Shifts in species’ northern and southern range margins were also assessed to explore potential mechanisms underlying the revealed mean range shifts. Species’ northern and southern range margins were defined as 10% of the most northern and southern 10 km squares occupied by each species, and the differences in the mean latitudes of these range margins between the two periods were used to define shifts in species’ northern and southern range margins.
(d). Effect of climatic niche conservatism through phenological changes on range shifts and the link between the two types of climatic niche conservatism
As well as the level of climatic niche conservatism via phenological changes, this study focused on traits that can affect species’ range shifts considered by other studies [32,33]: (i) dispersal abilities, (ii) niche breadth (reflecting ecological generalization) and (iii) perennation (reflecting generation time). Mean seed mass (mg) and seed releasing height (m), both measures of dispersal abilities, were derived from the LEDA Traitbase [34]. The number of main habitats (as a measure of niche breadth) and perennation were obtained from the PLANTATT database [24].
A regression analysis was conducted to investigate the effect of the level of climatic niche conservatism via phenological changes on species’ mean range shifts. In addition to the four explanatory variables described above, the initial mean latitude of each species’s range was also included in the regression. The interaction terms between the level of climatic niche conservatism via phenological changes and each of the other five explanatory variables were also included in the regression. All the explanatory variables were centred, apart from a binary variable, perennation, and the level of climatic niche conservatism via phenological changes, which naturally followed a near-normal distribution around zero. As for §2b, models for all possible parameter subsets were compared through the calculation of the AICc, using the package MuMIn in R. The same analysis was also conducted using shifts in species’ northern and southern range margins as the dependent variables.
Quantile regression was used to test a link between climatic niche conservatism through phenological changes and that through range shifts. When plotting changes in the range-wide mean temperature against changes in mean temperatures during flowering, the two variables are expected to be correlated particularly at the upper boundary, on the assumption that species need to conserve climatic niche at least either through phenological changes or through range shifts, while some species may potentially show niche conservatism via both the temporal and spatial responses. Thus, we adopted quantile regression, which is an effective approach for evaluating the magnitude of boundaries of the relationship between two variables (i.e. a correlation between two variables at the upper or lower boundaries of scatter plots) [35]. The choice of quantile to best represent the edge of a scatter plot can be subjective [35]; thus the 75th, 85th and 95th percentiles were used in this study. Quantile regression was implemented using the package quantreg [36] in R.
Only native species with information on all the explanatory variables were used for both of the two analyses above (n = 244).
(e). Testing phylogenetic dependence of model residuals
Pagel's λ [37] was used to test whether phylogenetic comparative methods were necessary in the analyses described in §2b,d above. The estimation of λ and the log-likelihood ratio test were performed in R using code written by R.P.F. based on [38] (see the electronic supplementary material, appendix C for more detail and results).
3. Results
(a). Climatic niche conservatism through phenological changes and range shifts
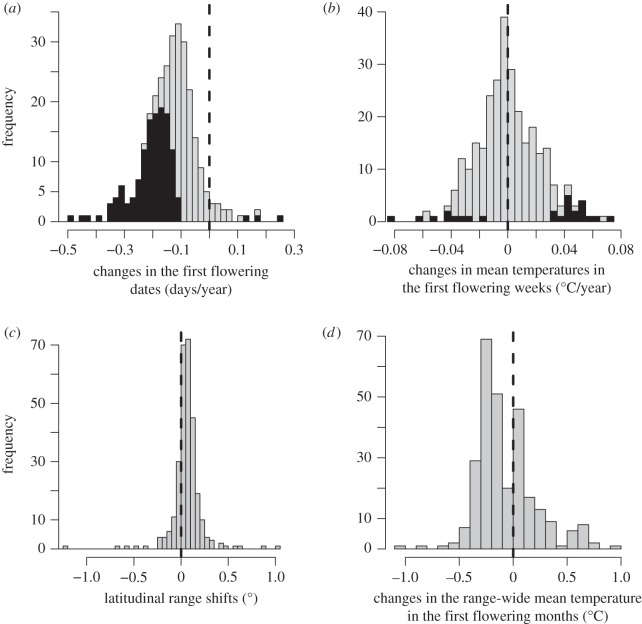
Of the 293 species investigated, 132 showed a significant p < 0.05) advance in first flowering dates during the 1930–2009 period, while only three species showed a significant delay (figure 2a). As a consequence of this phenological advance, 267 species did not show a significant change in mean temperature in the first flowering weeks, while 17 species showed a significant increase in mean temperature and nine species a decrease (figure 2b). Of the 284 species investigated, 225 species showed a northward mean range shift while 59 species showed a southward shift (figure 2c). Despite these range shifts, 103 species experienced an increase in mean temperature within their ranges, although only 17 species were exposed to an increase larger than 0.5°C (figure 2d). By contrast, 181 species experienced a decrease in mean temperature within their ranges but only five species were exposed to a decrease larger than 0.5°C (figure 2d).
Figure 2.
Histograms of (a) changes in the first flowering dates (days/year) between 1930 and 2009 (n = 293), (b) changes in mean temperatures in the first flowering weeks (°C/year) between 1930 and 2009 as a measure of climatic niche conservatism via phenological changes (n = 293), (c) latitudinal mean range shifts (degrees) (n = 284) and (d) changes in the range-wide mean temperature in the first flowering months (°C) between two atlas survey periods (1930–1960 and 1987–1999) as a measure of climatic niche conservatism via range shifts (n = 284). In (a,b), shaded bars show species with significant changes.
(b). Effect of species characteristics on climatic niche conservatism through phenological changes
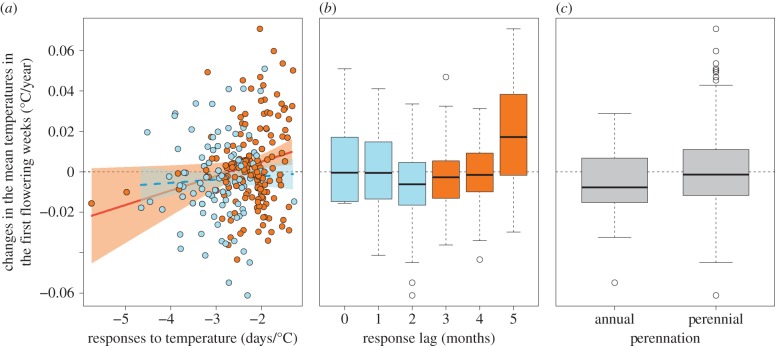
The model selection procedure showed that six models with the smallest AICc had Δi below 2.0 (AICc of the best model = −1210.23, null model = −1199.96; see the electronic supplementary material, table S1), which provides substantial evidence that these are the best models of those tested [39]. A response lag was included in all six models, indicating its importance in explaining the level of climatic niche conservatism through phenological changes, while species’ responses to temperature were included in four of the six models and perennation in three models (see electronic supplementary material, table S1). Overall, perennial species, as well as those with weaker or lagged phenological responses to temperature, were particularly likely to experience a greater increase in mean temperatures in their first flowering weeks (figure 3a–c). The interaction term between responses to temperature and response lag was also included in two of the six models with the smallest AICc (see electronic supplementary material, table S1), indicating a stronger effect of responses to temperature on the level of climatic niche conservatism via phenological changes in species with a larger response lag (figure 3a). Consequently, in species with a large response lag (shown in orange in figure 3a), strong and weak responses to temperature both caused climatic niche shifts, each associated with a decrease and increase in mean temperatures in their first flowering weeks, respectively (figure 3a).
Figure 3.
The effect of (a) species’ responses to temperature (days/°C), (b) lag (months) in responses to temperature (i.e. the difference between the first flowering month and month most responsible for flowering time) and (c) perennation on changes in mean temperatures in the first flowering weeks (°C/year, 1930–2009) as a measure of climatic niche conservatism via phenological changes. Regression lines are based on the estimated coefficients in the best model in the electronic supplementary material, table S1. In (a), blue and orange dots show species with response lag within and exceeding two months, respectively (as shown in (b)), and regression lines with 95% CI are shown for each group: lag of two months (first quartile, blue dashed line) and four months (third quartiles, orange line).
(c). Effect of climatic niche conservatism through phenological changes on range shifts
In the analysis of mean range shifts, 10 models with the smallest AICc had Δi below 2.0 (AICc of the best model = −246.44, null model = −222.41; see the electronic supplementary material, table S2). Initial latitude and perennation were included in all 10 models, while the degree of climatic niche conservatism through phenological changes was included in 8 of the 10 models and its interaction with perennation in 7 (see electronic supplementary material, table S2). The estimated coefficients indicated that annual species, as well as those that were originally distributed at lower latitudes and experienced greater increases in mean temperatures in their first flowering weeks, showed greater northward mean range shifts (see electronic supplementary material, table S2). The interaction term indicated that the effect of climatic niche conservatism via phenological changes on mean range shifts was particularly prominent in annual species (figure 4).
Figure 4.
The effect of changes in mean temperatures in the first flowering weeks (°C/year, 1930 to 2009), as a measure of climatic niche conservatism via phenological changes, on species’ mean range shifts (degree latitude change, 1930–1960 to 1987–1999). Annual and perennial species are shown as orange and blue dots, respectively. Regression lines with 95% CI are based on the estimated coefficients in the best model in the electronic supplementary material, table S2 for annual (orange line) and perennial (blue dashed line) species, respectively.
Mean range shifts were significantly associated with shifts in both northern and southern range margins (linear regression: R2 = 0.50; slope for northern margins = 0.42, t = 13.82, p < 0.001; slope for southern margins = 0.87, t = 9.22, p < 0.001). However, shifts in northern range margins were not explained effectively by the explanatory variables in this study; the null model had the smallest AICc and no variable was included in more than one of the six models with Δi below 2.0 (see the electronic supplementary material, table S3 and figure S2). By contrast, in the analysis of shifts in southern range margins, three models with the smallest AICc had Δi below 2.0 (AICc of the best model = −596.92, null model = −569.09; electronic supplementary material, table S4). The estimated coefficients in these three models indicated that annual species, as well as those that (i) experienced greater increases in mean temperatures in their first flowering weeks, (ii) were distributed at lower latitudes in the first place and (iii) were associated with a smaller number of habitats, showed greater northward shifts in their southern range margins (see electronic supplementary material, table S4). The interaction between climatic niche conservatism via phenological changes and perennation was also included in the second best model (see electronic supplementary material, table S4), indicating that the effect of climatic niche conservatism through phenological changes on shifts in southern range margins was particularly prominent in annual species (see electronic supplementary material, figure S3).
(d). Link between climatic niche conservatism through phenological changes and range shifts
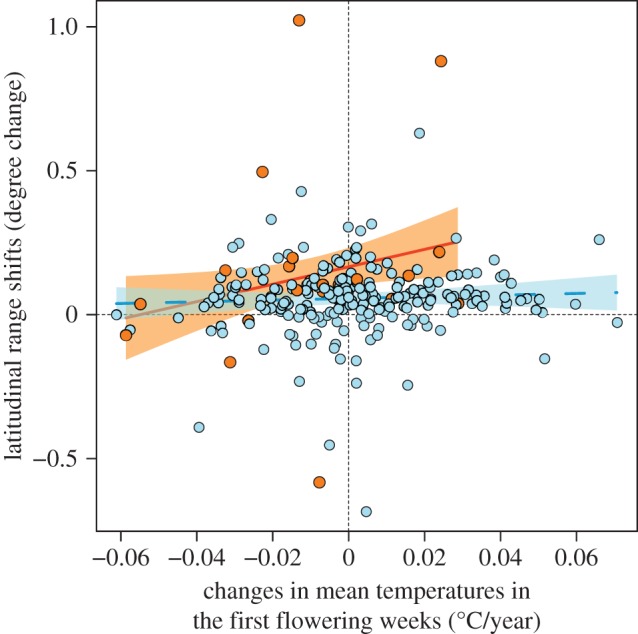
The relationship between changes in mean temperatures in the first flowering weeks and those in range-wide mean temperature was significant for the 85th percentile (slope (s.e.) = −2.913 (1.297), t = −2.247, p = 0.026), but not for the 95th (slope (s.e.) = −2.788 (3.912), t = −0.713, p = 0.477) and 75th (slope (s.e.) = −1.232 (0.978), t = −1.260, p = 0.209) percentiles (figure 5). There was also a moderate number of species that experienced increases both in mean temperatures in the first flowering weeks and in range-wide mean temperature in the first flowering months (i.e. those shown in the upper right region of figure 5). However, all but one of the annual species were not located in this region; they had conserved climatic niche at least either through phenological changes or range shifts (orange dots in figure 5).
Figure 5.
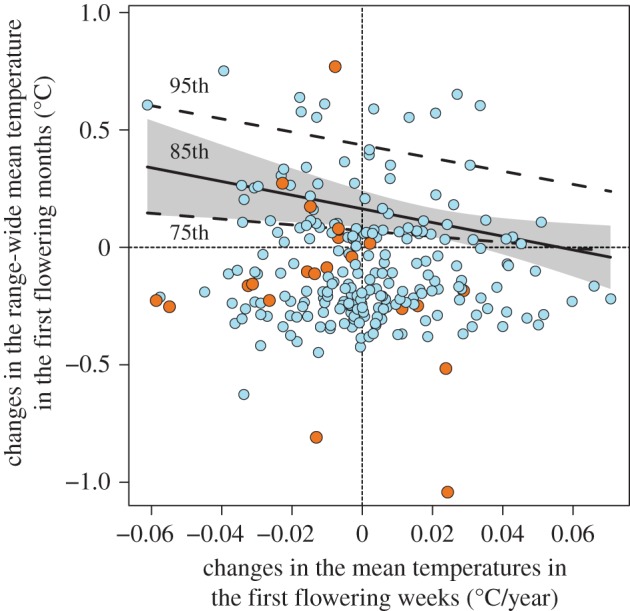
The relationship between changes in mean temperatures in the first flowering weeks (°C/year, 1930–2009), as a measure of climatic niche conservatism via phenological changes, and changes in the range-wide mean temperature in the first flowering months (°C, 1930–1960 to 1987–1999) as a measure of climatic niche conservatism via range shifts. Annual and perennial species are shown as orange and blue dots, respectively. Regression lines are based on the estimated slopes in quantile regressions for 75th, 85th (with 95% CI in grey) and 95th percentiles.
4. Discussion
The results of this study reveal the interacting dynamics of species’ spatial and temporal responses for maintaining climatic niche, and highlight key species characteristics that drive niche dynamics in British plants.
Most importantly, we found a link between the level of climatic niche conservatism through phenological changes and the degree of northward range shifts. Species that experienced greater increases in temperature during flowering time (i.e. those that failed to maintain climatic niche through phenological changes) showed greater northward mean range shifts, causing a complementary relationship between the levels of climatic niche conservatism via phenological changes and that through range shifts. Plant flowering phenology is shaped by the interacting dynamics of both biotic and abiotic factors affecting species’ fitness [14,40]. However, accumulated evidence has shown that advancing flowering time with warming maintains, or even increases, fitness [41], particularly in temperate regions [42], causing directional selection favouring earlier flowering [43]. Thus, the northward mean range shifts in species experiencing warming temperatures during flowering have presumably been caused by local extirpation of such species failing to advance their phenology sufficiently. Our analysis shows that the level of climatic niche conservatism through phenological changes was also associated with shifts in southern range margins. This result suggests that such local extirpation has been occurring particularly at species’ southern range margins. By contrast, shifts in northern range margins were not associated with climatic niche conservatism through phenological changes, which indicates that new colonization at northern range margins might be explained by other factors, such as more direct measures of dispersal abilities (e.g. dispersal distances), though such data are not available for most species and thus are not used in this study.
It is also noteworthy that some species seem to have experienced a decrease in temperature during the same period. The decrease in temperature during flowering has been partly due to the over-tracking of a warming climate through phenological changes in some species, caused by a large response lag combined with strong responses to temperature (figure 3a). These decreases in temperature during flowering, however, seem to have been compensated by southward mean range shifts, particularly in annual species (species shown in the lower left region of figure 4). This study could not find evidence that climatic niche conservatism through phenological changes is important in determining species’ northern range margins. However, such over-tracking of a warming climate might cause local extirpation of species at their northern range margins, for example through a higher risk of frost damage [44], and thus is worth exploring further in future studies. On the other hand, the decline in the range-wide mean temperature (figure 2d) can be at least partly explained because regions at higher latitudes in Britain are generally associated with higher mean altitudes. Species are most likely to start colonizing from low-altitude sites when moving northwards, in which case the mean temperature calculated in each grid cell at a 10 km resolution might underestimate the mean temperature actually experienced by the species, particularly at higher latitudes associated with higher mean altitudes. Data at a finer resolution would enable a more accurate assessment of climatic niche conservatism through range shifts.
The results also showed that even species with high climatic niche conservatism might not move to higher latitudes, as they instead track warming temperatures during flowering by advancing their phenology. Thus, we need to be careful about the extent to which we rely upon climate envelope models that focus only on climatic niche conservatism through range shifts when projecting future demands on species to shift ranges. Although climatic niche conservatism through phenological changes has received little attention in earlier studies on species’ range shifts [2,19], our study suggests that it is one of the key processes that can improve our ability to project species-specific range shifts, if included in mechanistic models. This is also supported by earlier modelling studies that suggest that accounting for phenology can have a significant consequence for projections of species’ range shifts [17,18]. Our study highlights that simple climate envelope models will be ineffective in projecting spatial responses of species that (i) show high climatic niche conservatism through phenological changes and thus may not move to higher latitudes, and (ii) do not move to higher latitudes despite failure to maintain climatic niche through phenological changes.
This study also reveals key species characteristics that should be of help in identifying such species. Species with strong, immediate phenological responses to temperature have shown high climatic niche conservatism through phenological changes. In particular, the response lag was among the most important drivers of climatic niche conservatism via phenological changes. As the rate of temperature increase varies between months (see the electronic supplementary material, figure S4), response lag can easily lead to the mistracking of actual temperature changes during flowering time, when combined not only with weak responses to temperature but also with strong responses, as shown in figure 3a. Although the presence of such a response lag, particularly in late-flowering species, has been reported by earlier studies [45], its ecological consequence has attracted little attention. The importance of temperature in explaining inter-annual variations in flowering dates was not dependent on flowering time (see electronic supplementary material, figure S5), indicating that temperature can be important even for late-flowering species, but some of those species could not track warming temperatures effectively because of the response lag.
The perennation of species was another key trait that affected climatic niche conservatism through both phenological changes and range shifts. In contrast to the perennial species studied here, 38 of which have tracked warming temperatures neither spatially nor temporally, all but one of the annual species have successfully conserved their climatic niche at least either by advancing flowering time or by moving northward. Furthermore, in the analysis of both mean range shifts and shifts in southern range margins, the interaction term between climatic niche conservatism through phenological changes and perennation seemed to be influential, suggesting that annual species have a stronger link between spatial and temporal niche dynamics. Lifetime fitness in annual plants may be more affected by the flowering time in any particular year compared with perennial species, as their fitness relies on only one reproductive season, and flowering during times of favourable conditions may be a more critical issue [42]. This means that the selection on flowering time tends to be stronger in annual plants [42]. Annual plants also have greater potential to adapt to changes in climate because of their short generation time [46]. In fact, larger range shifts in response to climate change have previously been reported for species with shorter life-cycles in plants [47] and fish [48]. Our study has provided additional evidence on the importance of species’ generation time in governing the interactive dynamics of spatial and temporal climatic niche.
The findings in this study, however, need to be carefully interpreted, as our models generally had a low explanatory power. Also, only one of the three models with different quantiles was significant in the analysis testing the link between climatic niche conservatism through phenological responses and range shifts. This may be partly due to the methodological limitations of this study. For example, this study simply defined species’ climatic niche on a single dimension (i.e. mean temperature), which represents a simpler quantification of species’ niche than, for example, parameters defined on multiple dimensions [49]. Considering that niche defined on a single dimension usually appears to be conserved more than those defined on multiple dimensions [50], we anticipate that a more detailed definition of species’ climatic niche would lead to a greater variation in the level of conservatism among species, potentially allowing us to detect a clearer link between spatial and temporal climatic niche dynamics. The use of the first flowering dates and coarse-resolution data on temperature within species’ range, though inevitable due to the limited availability of other data, might have also impeded the accurate evaluation of species’ climatic niche. Northward range shifts may have also been underestimated for species whose ranges exceed Britain. Thus, using data from the species’ entire range (unavailable for this study) might also enable the detection of a clearer relationship between species’ spatial and temporal responses. Species’ range shifts were estimated by comparing distribution records in 1930–1960 and 1987–1999, assuming that among-species patterns have remained the same after 1999, so estimates of range shifts would also be improved by using more recent records, when available. Incorporating the effect of altitudinal range shifts would also improve the estimates of species’ spatial responses. Meanwhile, there may be other factors that were not considered in this study but could be important in regulating species’ range shifts. For example, the fitness of plant species could also be affected by climatic conditions during the non-flowering season [17], potentially leading to a weak relationship between the maintenance of climatic niche during flowering through phenological changes and range shifts. Environmental changes in the more human-populated southern part of Britain might have caused range shifts even in species that did not experience an increase in temperature during flowering.
Despite these methodological limitations, this study, using historical records of changes in the spatial distribution and phenology of British plant species, has successfully detected a link between species’ spatial and temporal dynamics of climatic niche, and species characteristics affecting the dynamics. Although the low explanatory power of the analysis indicates that knowledge obtained in this study needs to be explored further before generalization, it should be of use in efforts to project climate-change-induced range shifts in plants. For example, annual species, as well as those with strong, immediate phenological responses to temperature, showed high climatic niche conservatism through phenological changes, and thus may require phenological processes to be incorporated in mechanistic models if better projections of climate change impacts are to be produced. However, even if such mechanistic models include phenological processes, it might be difficult to project range shifts more accurately in perennial species, as a substantial number were revealed to conserve climatic niche neither spatially nor temporally. Such knowledge obtained by linking species characteristics and spatial and temporal dynamics of climatic niche in plants also needs to be secured for other taxa showing variations in phenological changes and range shifts, in order to assess the complex consequences of climate change for biodiversity.
Acknowledgements
We thank the more than 100 000 volunteers for collecting the data, the Woodland Trust for supplying the UK Phenology Network dataset and M. Amano for all her support. We thank M. Vellend and two referees for their comments on an earlier version of this paper.
Data accessibility
All data are uploaded as the electronic supplementary material.
Funding statement
T.A. is financially supported by the European Commission's Marie Curie International Incoming Fellowship Programme (PIIF-GA-2011-303221) and W.J.S. by Arcadia. T.H.S. acknowledges the support of the Technische Universität München—Institute for Advanced Study, funded by the German Excellence Initiative. S.A.Q., S.W.D. and R.P.F. acknowledge funding from a Leverhulme Trust Research Leadership Award.
References
- 1.Pearman PB, Guisan A, Broennimann O, Randin CF. 2008. Niche dynamics in space and time. Trends Ecol. Evol. 23, 149–158 (doi:10.1016/j.tree.2007.11.005) [DOI] [PubMed] [Google Scholar]
- 2.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (doi:10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 3.Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN. 2004. Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 4.Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH. 2010. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 25, 310–318 (doi:10.1016/j.tree.2009.12.003) [DOI] [PubMed] [Google Scholar]
- 5.Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 6.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 7.Warren R, et al. 2013. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Climate Change 3, 678–682 (doi:10.1038/nclimate1887) [Google Scholar]
- 8.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872 (doi:10.1111/j.1365-2486.2007.01404.x) [Google Scholar]
- 9.Cooper N, Freckleton RP, Jetz W. 2011. Phylogenetic conservatism of environmental niches in mammals. Proc. R. Soc. B 278, 2384–2391 (doi:10.1098/rspb.2010.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuine I. 2010. Why does phenology drive species distribution? Phil. Trans. R. Soc. B 365, 3149–3160 (doi:10.1098/rstb.2010.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suttle KB, Thomsen MA, Power ME. 2007. Species interactions reverse grassland responses to changing climate. Science 315, 640–642 (doi:10.1126/science.1136401) [DOI] [PubMed] [Google Scholar]
- 12.Willis CG, Ruhfel BR, Primack RB, Miller-Rushing AJ, Losos JB, Davis CC. 2010. Favorable climate change response explains non-native species’ success in Thoreau's Woods. PLoS ONE 5, e8878 (doi:10.1371/journal.pone.0008878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steltzer H, Post E. 2009. Seasons and life cycles. Science 324, 886–887 (doi:10.1126/science.1171542) [DOI] [PubMed] [Google Scholar]
- 14.Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G. 2007. Time after time: flowering phenology and biotic interactions. Trends Ecol. Evol. 22, 432–439 (doi:10.1016/j.tree.2007.05.006) [DOI] [PubMed] [Google Scholar]
- 15.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 16.Phillimore AB, Hadfield JD, Jones OR, Smithers RJ. 2010. Differences in spawning date between populations of common frog reveal local adaptation. Proc. Natl Acad. Sci. USA 107, 8292–8297 (doi:10.1073/pnas.0913792107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuine I, Beaubien EG. 2001. Phenology is a major determinant of tree species range. Ecol. Lett. 4, 500–510 (doi:10.1046/j.1461-0248.2001.00261.x) [Google Scholar]
- 18.Morin X, Augspurger C, Chuine I. 2007. Process-based modeling of species’ distributions: what limits temperate tree species’ range boundaries? Ecology 88, 2280–2291 (doi:10.1890/06-1591.1) [DOI] [PubMed] [Google Scholar]
- 19.Hulme PE. 2011. Contrasting impacts of climate-driven flowering phenology on changes in alien and native plant species distributions. New Phytol. 189, 272–281 (doi:10.1111/j.1469-8137.2010.03446.x) [DOI] [PubMed] [Google Scholar]
- 20.Amano T, Smithers RJ, Sparks TH, Sutherland WJ. 2010. A 250-year index of first flowering dates and its response to temperature changes. Proc. R. Soc. B 277, 2451–2457 (doi:10.1098/rspb.2010.0291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botanical Society for the British Isles 2008. Vascular plant database See http://data.nbn.org.uk (accessed 22 April 2013)
- 22.Manley G. 1974. Central England temperatures: monthly means 1659–1973. Q. J. R. Meteorol. Soc. 100, 389–405 (doi:10.1002/qj.49710042511) [Google Scholar]
- 23.Croxton PJ, Huber K, Collinson N, Sparks TH. 2006. How well do the central England temperature and the England and Wales precipitation series represent the climate of the UK? Int. J. Climatol. 26, 2287–2292 (doi:10.1002/joc.1378) [Google Scholar]
- 24.Hill MO, Preston CD, Roy DB. 2004. PLANTATT: attributes of British and Irish plants: status, size, life history, geography and habitats. Huntingdon, UK: Biological Records Centre, NERC Centre for Ecology and Hydrology [Google Scholar]
- 25.Bolmgren K, Vanhoenacker D, Miller-Rushing AJ. 2013. One man, 73 years, and 25 species: evaluating phenological responses using a lifelong study of first flowering dates. Int. J. Biometeorol. 57, 367–375 (doi:10.1007/s00484-012-0560-8) [DOI] [PubMed] [Google Scholar]
- 26.Bartoń K. 2012. MuMIn: multi-model inference: R package See http://cran.r-project.org/web/packages/MuMIn/index.html
- 27.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 28.Perring FH, Walters SM. 1962. Atlas of the British flora. London, UK: Thomas Nelson and Sons [Google Scholar]
- 29.Preston CD, Pearman DA, Dines TD. 2002. New atlas of the British and Irish Flora. Oxford, UK: Oxford University Press [Google Scholar]
- 30.Rich TCG, Woodruff ER. 1996. Changes in the vascular plant floras of England and Scotland between 1930–1960 and 1987–1988: the BSBI monitoring scheme. Biol. Conserv. 75, 217–229 (doi:10.1016/0006-3207(95)00077-1) [Google Scholar]
- 31.Fitter AH, Peat HJ. 1994. The ecological flora database. J. Ecol. 82, 415–425 (doi:10.2307/2261309) [Google Scholar]
- 32.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689 (doi:10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 33.Broennimann O, Thuiller W, Hughes G, Midgley GF, Alkemade JMR, Guisan A. 2006. Do geographic distribution, niche property and life form explain plants’ vulnerability to global change? Glob. Change Biol. 12, 1079–1093 (doi:10.1111/j.1365-2486.2006.01157.x) [Google Scholar]
- 34.Kleyer M, et al. 2008. The LEDA traitbase: a database of life-history traits of the Northwest European flora. J. Ecol. 96, 1266–1274 (doi:10.1111/j.1365-2745.2008.01430.x) [Google Scholar]
- 35.Scharf FS, Juanes F, Sutherland M. 1998. Inferring ecological relationships from the edges of scatter diagrams: comparison of regression techniques. Ecology 79, 448–460 (doi:10.1890/0012-9658(1998)079[0448:IERFTE]2.0.CO;2) [Google Scholar]
- 36.Koenker R. 2013. quantreg: quantile regression: R package See http://cran.r-project.org/web/packages/quantreg/index.html
- 37.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 38.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 39.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer [Google Scholar]
- 40.Augspurger CK. 2013. Reconstructing patterns of temperature, phenology, and frost damage over 124 years: spring damage risk is increasing. Ecology 94, 41–50 (doi:10.1890/12-0200.1) [DOI] [PubMed] [Google Scholar]
- 41.Cleland EE, Allen JM, Crimmins TM, Dunne JA, Pau S, Travers S, Zavaleta ES, Wolkovich EM. 2012. Phenological tracking enables positive species responses to climate change. Ecology 93, 1765–1771 (doi:10.1890/11-1912.1) [DOI] [PubMed] [Google Scholar]
- 42.Munguía-Rosas MA, Ollerton J, Parra-Tabla V, De-Nova JA. 2011. Meta-analysis of phenotypic selection on flowering phenology suggests that early flowering plants are favoured. Ecol. Lett. 14, 511–521 (doi:10.1111/j.1461-0248.2011.01601.x) [DOI] [PubMed] [Google Scholar]
- 43.Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T. 2012. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. R. Soc. B 279, 3843–3852 (doi:10.1098/rspb.2012.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inouye DW. 2008. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353–362 (doi:10.1890/06-2128.1) [DOI] [PubMed] [Google Scholar]
- 45.Fitter AH, Fitter RSR, Harris ITB, Williamson MH. 1995. Relationships between first flowering date and temperature in the flora of a locality in central England. Funct. Ecol. 9, 55–60 (doi:10.2307/2390090) [Google Scholar]
- 46.Jump AS, Peñuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 (doi:10.1111/j.1461-0248.2005.00796.x) [DOI] [PubMed] [Google Scholar]
- 47.Lenoir J, Gegout JC, Marquet PA, de Ruffray P, Brisse H. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771 (doi:10.1126/science.1156831) [DOI] [PubMed] [Google Scholar]
- 48.Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (doi:10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- 49.Petitpierre B, Kueffer C, Broennimann O, Randin C, Daehler C, Guisan A. 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science 335, 1344–1348 (doi:10.1126/science.1215933) [DOI] [PubMed] [Google Scholar]
- 50.Peterson AT. 2011. Ecological niche conservatism: a time-structured review of evidence. J. Biogeogr. 38, 817–827 (doi:10.1111/j.1365-2699.2010.02456.x) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are uploaded as the electronic supplementary material.