Abstract
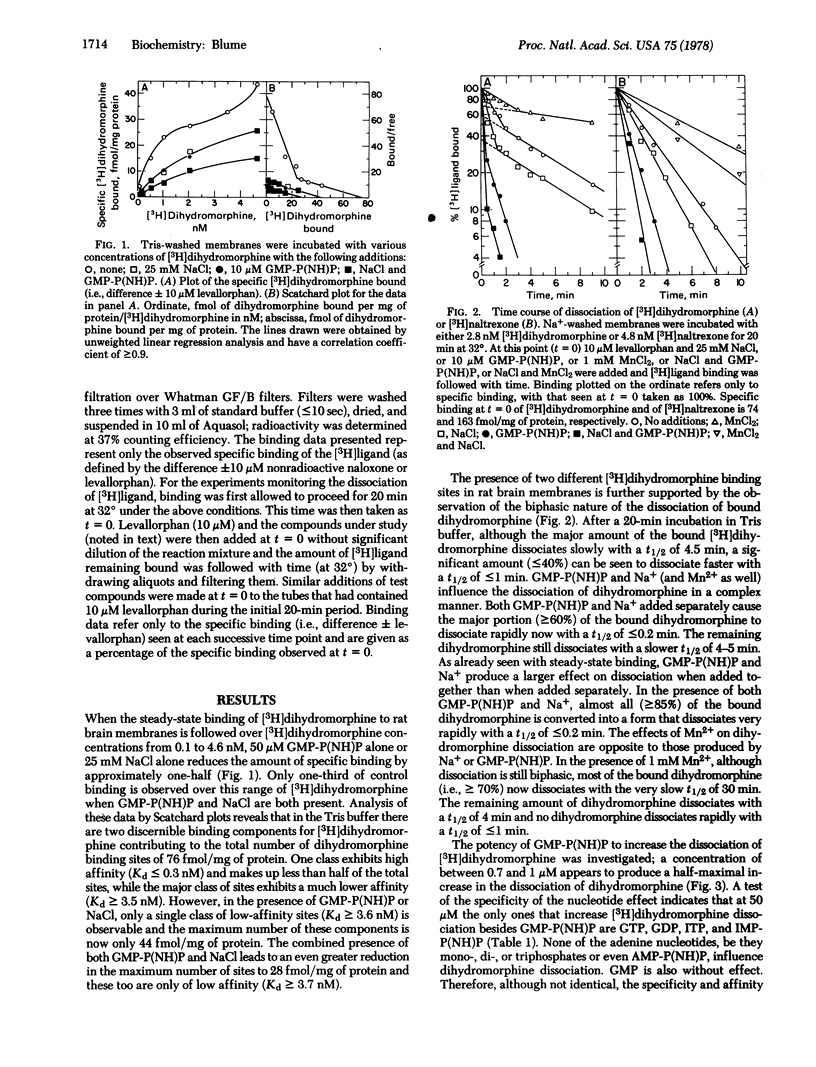
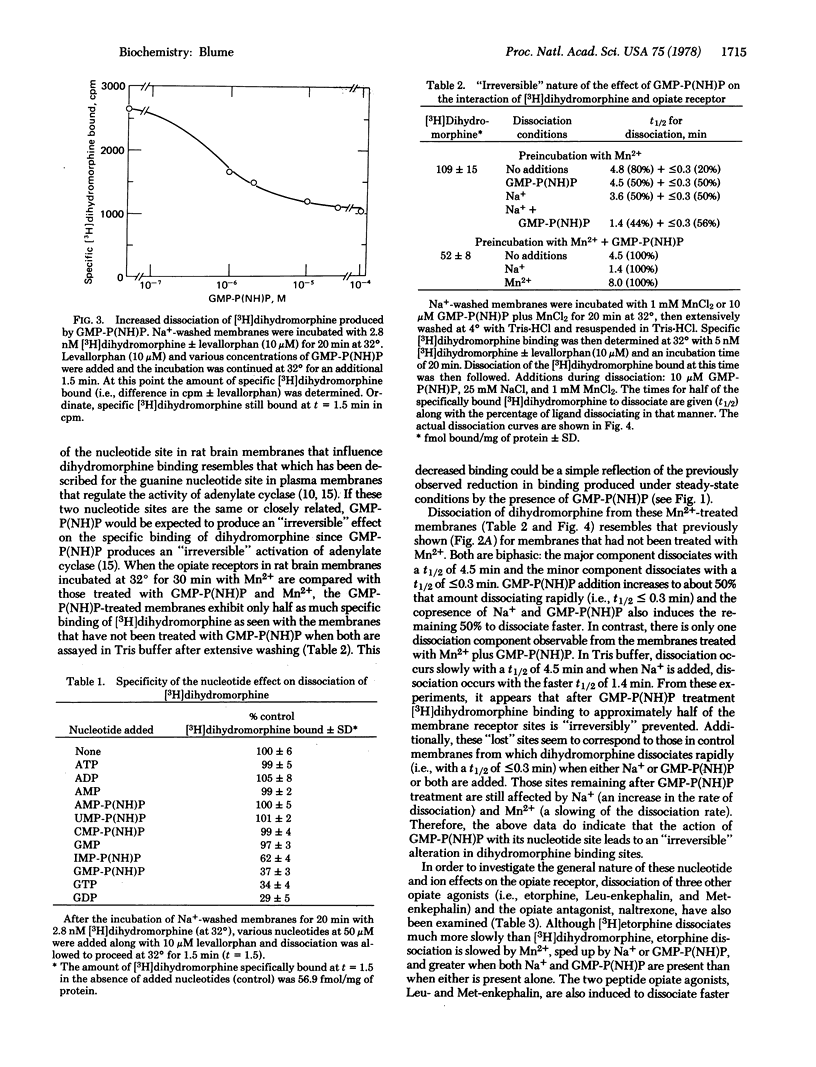
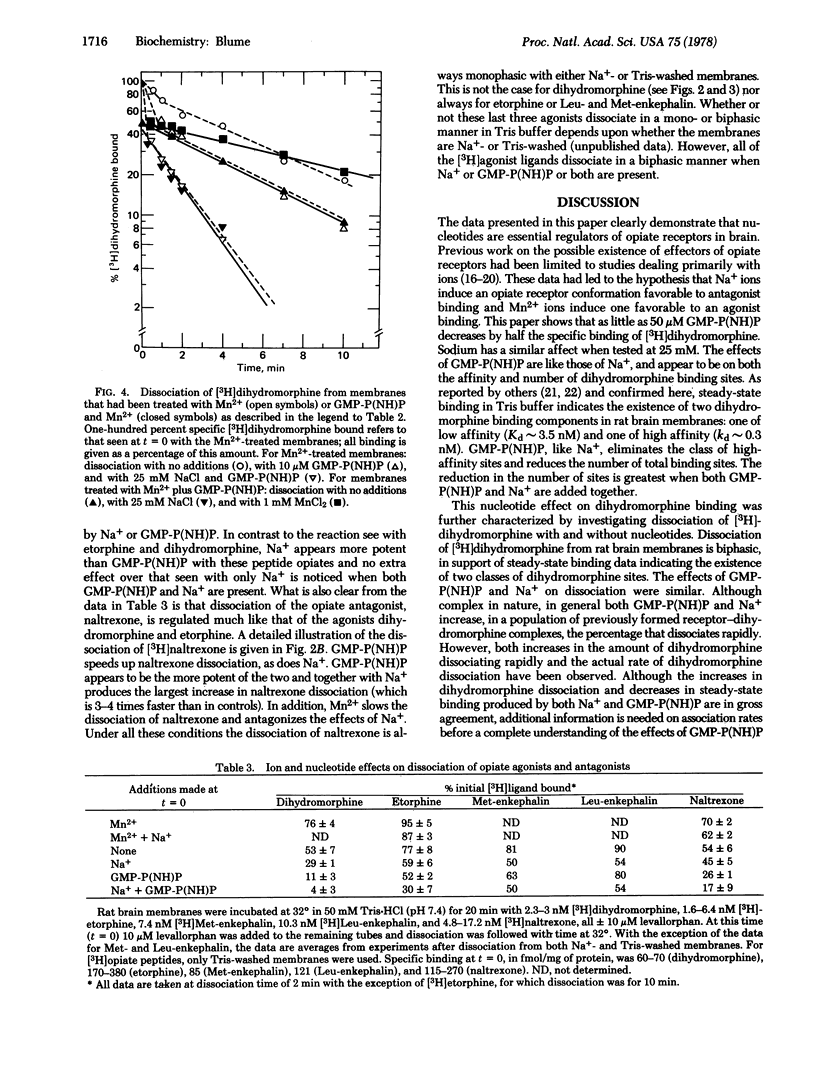
This study shows that nucleotides, as well as ions, regulate the opiate receptors of brain. GMP-P(NH)P and Na+ reduce the amount of steady-state specific [3H]dihydromorphine binding and increase the rate of dissociation of the ligand from the opiate receptor. In contrast, Mn2+ decreases the rate of ligand dissociation and antagonizes the ability of Na+ to increase dissociation. The effects of GMP-P(NH)P on steady-state binding and dissociation are not reversed by washing. Only GTP, GDP, ITP, and IMP-P(NH)P, in addition to GMP-P(NH)P, increase the rate of dihydromorphine dissociation. The site of nucleotide action appears to have high affinity: <1 μM GMP-P(NH)P produces half-maximal increases in ligand dissociation. GMP-P(NH)P- and Na+-directed increases in dissociation have also been found for the opiate agonists [3H]etorphine, [3H]Leu-enkephalin, and [3H]Met-enkephalin and the opiate antagonist [3H]naltrexone. Mn2+-directed decreases in dissociation have been found for the agonist [3H]-etorphine and the antagonist [3H]naltrexone. Although the plasma membrane receptors for a number of other neuro-transmitters and hormones are also regulated by guanine nucleotides, the opiate receptors appear unique because only they show nucleotide regulation of both agonist and antagonist binding.
Keywords: GMP-P(NH)P, agonists, antagonists
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Goldstein A., Cox B. M., Klee W. A., Nirenberg M. Endorphin from pituitary inhibits cyclic AMP formation in homogenates of neuroblastoma X glioma hybrid cells. Nature. 1977 Jan 27;265(5592):362–363. doi: 10.1038/265362a0. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N., Nagata N. The requirement of guanine nucleotides for glucagon stimulation of adenylate cyclase in rat liver plasma membranes. J Biol Chem. 1977 Jun 10;252(11):3829–3835. [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. Mode of action of endogenous opiate peptides. Nature. 1976 Oct 14;263(5578):609–612. doi: 10.1038/263609a0. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Lad P. M., Welton A. F., Rodbell M. Evidence for distinct guanine nucleotide sites in the regulation of the glucagon receptor and of adenylate cyclase activity. J Biol Chem. 1977 Sep 10;252(17):5942–5946. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Wood C. L., Gore T. B., Mukherjee C. Regulation of prostaglandin receptors by prostaglandins and guanine nucleotides in frog erythrocytes. J Biol Chem. 1977 Aug 10;252(15):5295–5303. [PubMed] [Google Scholar]
- Lefkowitz R. J. Stimulation of catecholamine-sensitive adenylate cyclase by 5'-guanylyl-imidodiphosphate. J Biol Chem. 1974 Oct 10;249(19):6119–6124. [PubMed] [Google Scholar]
- Londos C., Rodbell M. Multiple inhibitory and activating effects of nucleotides and magnesium on adrenal adenylate cyclase. J Biol Chem. 1975 May 10;250(9):3459–3465. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M. E., Van Arsdale P. M., Gilman A. G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976 Mar;12(2):335–339. [PubMed] [Google Scholar]
- Mukherjee C., Lefkowitz R. J. Desensitization of beta-adrenergic receptors by beta-adrenergic agonists in a cell-free system: resensitization by guanosine 5'-(beta, gamma-imino)triphosphate and other purine nucleotides. Proc Natl Acad Sci U S A. 1976 May;73(5):1494–1498. doi: 10.1073/pnas.73.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak G. W., Snowman A. M., Snyder S. H. Selective enhancement of [3H]opiate agonist binding by divalent cations. Mol Pharmacol. 1975 Nov;11(6):735–744. [PubMed] [Google Scholar]
- Pasternak G. W., Snyder S. H. Identification of novel high affinity opiate receptor binding in rat brain. Nature. 1975 Feb 13;253(5492):563–565. doi: 10.1038/253563a0. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Pasternak G., Snyder S. H. Opiate agonists and antagonists discriminated by receptor binding in brain. Science. 1973 Dec 28;182(4119):1359–1361. doi: 10.1126/science.182.4119.1359. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Schreier M. H., Noll H. Conformational changes in ribosomes during protein synthesis. Proc Natl Acad Sci U S A. 1971 Apr;68(4):805–809. doi: 10.1073/pnas.68.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R., Snowman A. M., Snyder S. H. Temperature and ionic influences on opiate receptor binding. Mol Pharmacol. 1976 Nov;12(6):977–986. [PubMed] [Google Scholar]
- Simantov R., Snyder S. H. Morphine-like peptides, leucine enkephalin and methionine enkephalin: interactions with the opiate receptor. Mol Pharmacol. 1976 Nov;12(6):987–998. [PubMed] [Google Scholar]



