Abstract
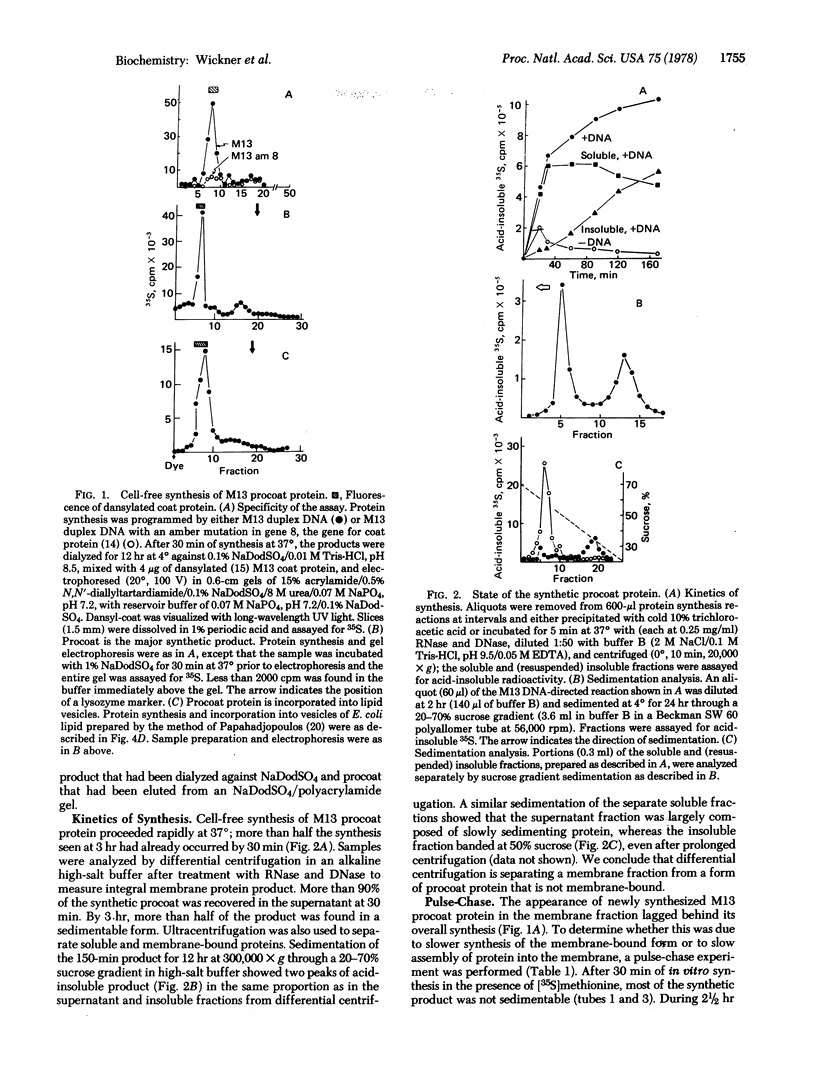
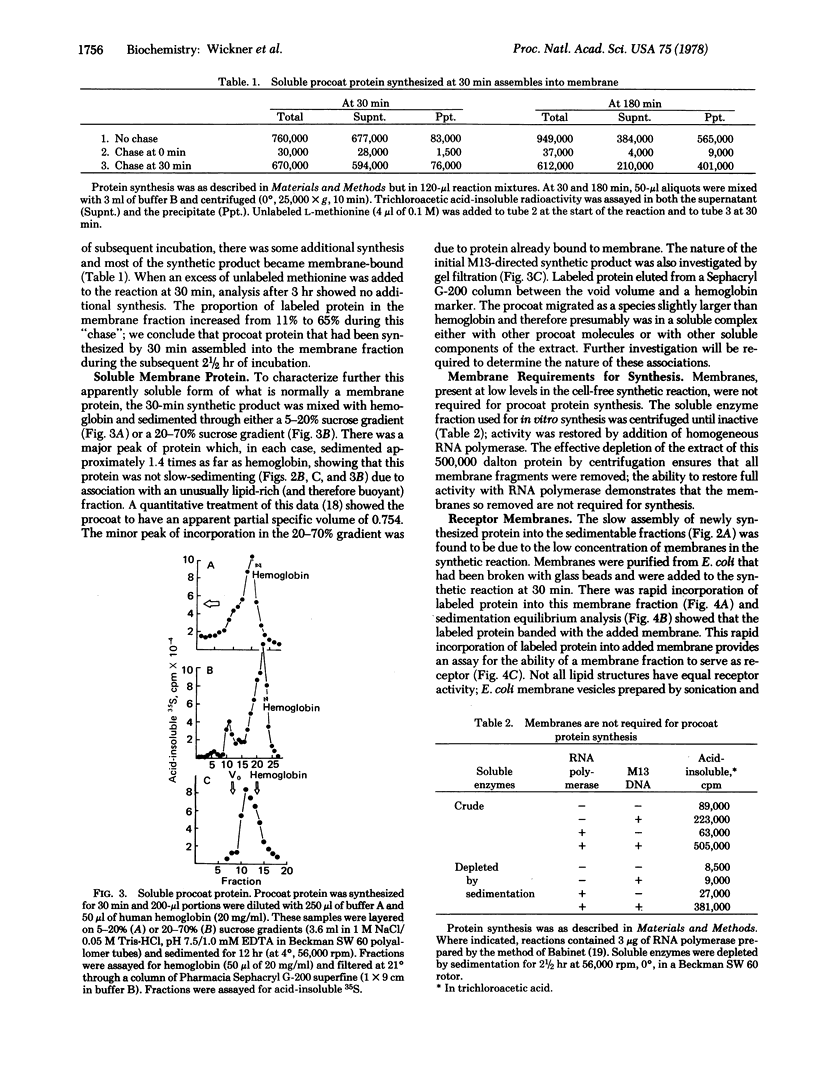
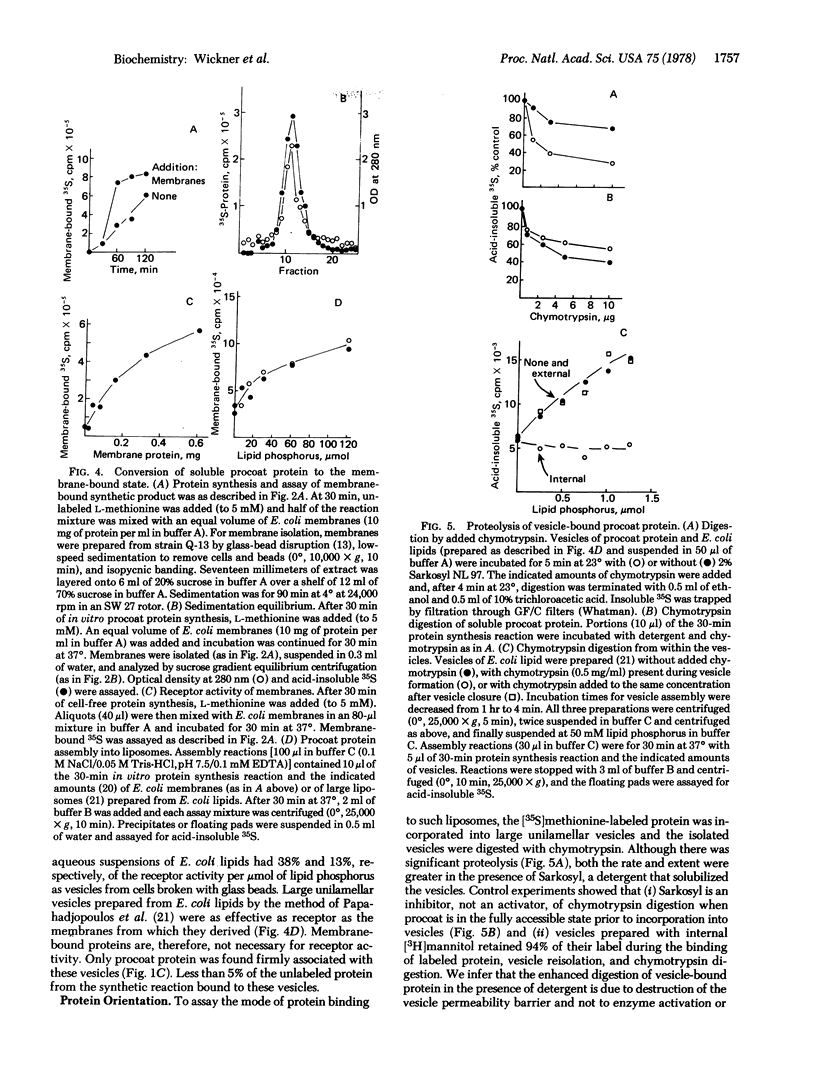
The coat protein (gene 8 product) of coliphage M1O is an integral protein of the host cell membrane at all stages of virus infection. This protein, when made in a cell-free reaction, has been shown by others to have an additional NH2-terminal peptide region and is referred to as "procoat." It is initially not membrane-bound but, upon exposure to Escherichia coli membrane vesicles or to liposomes prepared from E. coli lipids, it assembles into the bilayer in an integral fashion. Much of this protein is shown to be exposed on the inner surface of the liposome. We suggest that refolding of procoat as it encounters the bilayer is sufficient to transport large segments of the peptide chain through the apolar hydrocarbon core.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Babinet C. A new method for the purification of RNA-polymerase. Biochem Biophys Res Commun. 1967 Mar 21;26(6):639–644. doi: 10.1016/s0006-291x(67)80119-0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings R. N., Hulsebos T., Van den Hondel C. A. Identification and characterization of the in vitro synthesized gene products of bacteriophage M13. J Virol. 1975 Mar;15(3):570–584. doi: 10.1128/jvi.15.3.570-584.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N. S., Pratt D. Bacteriophage M 13 gene 2 protein: increasing its yield in infected cells, and identification and localization. Virology. 1974 Oct;61(2):334–342. doi: 10.1016/0042-6822(74)90271-2. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Konigsberg W. Reinvestigation of a region of the fd bacteriophage coat protein sequence. J Mol Biol. 1974 Sep 25;88(3):598–600. doi: 10.1016/0022-2836(74)90410-0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Smigel M., Fleischer S. Characterization of triton X-100-solubilized prostaglandin E binding protein of rat liver plasma membranes. J Biol Chem. 1977 Jun 10;252(11):3689–3696. [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. doi: 10.1016/s0022-2836(77)80065-x. [DOI] [PubMed] [Google Scholar]
- Wickner W. T. Role of hydrophobic forces in membrane protein asymmetry. Biochemistry. 1977 Jan 25;16(2):254–258. doi: 10.1021/bi00621a015. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of a phage coat protein in cytoplasmic membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4749–4753. doi: 10.1073/pnas.72.12.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Killick T. Membrane-associated assembly of M13 phage in extracts of virus-infected Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):505–509. doi: 10.1073/pnas.74.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Wickner W. Studies of asymmetric membrane assembly. Biochim Biophys Acta. 1977 Dec 1;471(2):169–176. doi: 10.1016/0005-2736(77)90247-4. [DOI] [PubMed] [Google Scholar]


