ABSTRACT
The Hippo-YAP pathway mediates the control of cell proliferation by contact inhibition as well as other attributes of the physical state of cells in tissues. Several mechanisms sense the spatial and physical organization of cells, and function through distinct upstream modules to stimulate Hippo-YAP signaling: adherens junction or cadherin–catenin complexes, epithelial polarity and tight junction complexes, the FAT-Dachsous morphogen pathway, as well as cell shape, actomyosin or mechanotransduction. Soluble extracellular factors also regulate Hippo pathway signaling, often inhibiting its activity. Indeed, the Hippo pathway mediates a reciprocal relationship between contact inhibition and mitogenic signaling. As a result, cells at the edges of a colony, a wound in a tissue or a tumor are more sensitive to ambient levels of growth factors and more likely to proliferate, migrate or differentiate through a YAP and/or TAZ-dependent process. Thus, the Hippo-YAP pathway senses and responds to the physical organization of cells in tissues and coordinates these physical cues with classic growth-factor-mediated signaling pathways. This Commentary is focused on the biological significance of Hippo-YAP signaling and how upstream regulatory modules of the pathway interact to produce biological outcomes.
KEY WORDS: Hippo, YAP, Cadherin, Mechanotransduction, Mitogenesis, Polarity
Introduction
Contact inhibition of cell proliferation has been recognized for a long time to be an important phenomenon controlling tissue growth (Fagotto and Gumbiner, 1996; Perrais et al., 2007; McClatchey and Yap, 2012). Contact inhibition is thought to occur as cells in a tissue or in monolayer culture reach a high density. Other phenomena have also been invoked to explain density-dependent growth, including accessibility to growth factors and changes in cell shape and/or tension (Dupont et al., 2011; Halder et al., 2012; Aragona et al., 2013), but there is also direct evidence for a role of physical contact (Perrais et al., 2007; Nishioka et al., 2009; Kim et al., 2011; McClatchey and Yap, 2012). A number of signaling pathways have been implicated in contact inhibition, but the underlying mechanisms were still unclear. The Hippo pathway was found to mediate contact inhibition of growth (Zhao et al., 2007), and recent findings on Hippo-YAP signaling have provided mechanistic insights into contact inhibition as well as a way to understand how various aspects of the physical state and/or organization of cells in tissues are integrated to regulate tissue growth.
Contact inhibition is most frequently invoked in the context of cancer. Cancer cells are often thought to have lost contact inhibition of growth, and they proliferate despite the normal confines of the tissue structure they reside in. Contact inhibition has been considered an important regulator of tissue growth, but much less frequently in the context of development. Indeed, cells in rapidly growing tissues in embryos do not appear to be strongly contact inhibited, as there can be a very rapid proliferation of cells that are completely surrounded by neighbors. This indicates that contact inhibition must be a highly regulated process rather than a constitutive property of cells; often turned on in fully developed and differentiated tissues, and switched off or attenuated in normally growing cells during development, in tissues with high turnover or in regeneration. Therefore, we can expect to see considerable variations in the degree of contact inhibition in various tissues or developmental stages. In cancer cells, contact inhibition may be misregulated, causing differentiated cells to revert to a more embryonic and/or developmental state, just as they acquire other properties of embryonic cells such as enhanced cell migration.
Many different signaling pathways have been implicated in the contact inhibition of growth, and a number of pathways seem to be altered as a function of cell density (Polyak et al., 1994; Wieser et al., 1999; Heit et al., 2001; Faust et al., 2005; Li et al., 2012; McClatchey and Yap, 2012). However, the Hippo pathway has been found to have an important role in contact inhibition and growth regulation through physical properties of cells. Indeed, it may be the main locus where various pathways that sense cell contact, cell shape, cell density and tissue organization are integrated to control cell growth. The Hippo pathway was first discovered as a result of genetic studies of tissue growth in Drosophila, and this system is still a main engine of discovery for this pathway (Halder and Johnson, 2011; Tumaneng et al., 2012; Bossuyt et al., 2013). In Drosophila, the Hippo pathway is thought to mediate organ size control, because loss of Hippo pathway activity leads to enlarged but fairly normally organized tissues, such as imaginal disks. Many Hippo pathway components and genes are also expressed in mammalian cells and tissues, and play a similar role in tissue growth and organ size, as well as tumor growth. The core components of the Hippo pathway are shared between Drosophila and mammals, but there are some differences in the upstream regulators. As we will see below, these differences reveal interesting variations on growth regulation in different tissues.
Hippo pathway basics
The core of the Hippo pathway consists of a serine kinase cascade with associated regulatory and/or scaffolding proteins that act on a transcriptional complex to regulate the expression of genes that control growth (Halder and Johnson, 2011; Halder et al., 2012; Tumaneng et al., 2012; Bossuyt et al., 2013; Harvey et al., 2013; Yu and Guan, 2013) (see Fig. 1). The Ser/Thr kinase Hippo in Drosophila (Mst 1 and Mst2 in mammals; officially known as STK4 and STK3, respectively), activates another kinase, Warts (Lats 1 and Lats2 in mammals), that phosphorylates the transcriptional activator Yorkie (YAP and TAZ in mammals), causing it to be excluded from the nucleus and retained in the cytoplasm; indeed, the localization and phosphorylation of Yorkie/YAP are often taken as a measure of the activity of the Hippo pathway. The scaffolding proteins that are required for Hippo pathway activity include Salvador/Sav1 and Mats/Mob1. Some membrane-associated proteins, including the Merlin/NF2 tumor suppressor and Kibra, also interact with the core kinase components and act as common upstream activators of the pathway. The Hippo signaling pathway has also been reported to lead to the degradation of TAZ or YAP under some circumstances (Liu et al., 2010; Zhao et al., 2010). In the nucleus, YAP interacts with the DNA-binding TEAD transcription factors (Scalloped in Drosophila) to turn on the expression of growth-promoting and apoptosis-inhibiting genes. Thus, the growth inhibitory activity of the Hippo pathway is to prevent the nuclear accumulation of YAP and activation of growth-promoting genes. Nuclear YAP and Yorkie have also been found to interact with other transcription factors, including Smads, PEBP2, tumor protein p73, ErbB4, and Traffic Jam/MAF to regulate various aspects of growth and differentiation (Yagi et al., 1999; Basu et al., 2003; Komuro et al., 2003; Varelas et al., 2010a; Jukam et al., 2013).
Fig. 1.
Control of the core Hippo signaling pathway through interacting upstream modules. (A) Overview of the interactions of various modules with the core pathway. The Hippo pathway consists of a core kinase cascade in which the transcriptional co-activators YAP/TAZ are phosphorylated and inactivated by either their exclusion from the nucleus or their enhanced degradation. The nuclear activity of YAP/TAZ promotes cell growth. (B) Upstream modules. (Panels i, ii) Two upstream cell surface regulators, epithelial polarity or tight junction (TJ) complexes (i) and adherens junction (AJ) or cadherin–catenin complexes may function together to sense the integrity of the epithelial layer. (Panel iii) Cell shape and mechanotransduction can regulate the activity of YAP/TAZ independently of Lats kinase, but Lats-dependent regulation of YAP/TAZ through the actin cytoskeleton has also been observed. (Panel iv) Extracellular soluble growth factors act reciprocally – with contact inhibition – through the Hippo pathway to integrate mitogenesis with growth inhibitory mechanisms. (Panel v) The atypical cadherins FAT and Dachsous set up a morphogen gradient to control the spatial patterning of both cell proliferation (through Hippo pathway signaling) and PCP. β-cat, β-catenin; α-cat, α-catenin, AP, apical polarity complexes; Dco, Discs overgrown; E-cad, E-cadherin; ECM, extracellular matrix; ex, Expanded; GPCRs, G-protein-coupled receptors; RTK, receptor tyrosine kinase; PCP, planar cell polarity.
In recent years there was a plethora of discoveries of molecules and pathways that act upstream of the core Hippo pathway, to either activate or inhibit YAP/TAZ, or the transcriptional activity of Yorkie. These include a variety of kinases (Harvey et al., 2013; Yu and Guan, 2013), other signaling pathways, such as the Wnt (Varelas et al., 2010b) and RTK pathways (Straßburger et al., 2012; Fan et al., 2013; Reddy and Irvine, 2013), cell adhesion and cell junction proteins, cell polarity proteins, and the state of the actin cytoskeleton (Nishioka et al., 2009; Kim et al., 2011; Zhao et al., 2011; Boggiano and Fehon, 2012; Halder et al., 2012; Aragona et al., 2013; Hirate et al., 2013). The reader is referred to the numerous recent review articles cited above that describe these mechanisms in detail; these descriptions will not be repeated here, except when used to illustrate certain points. A couple of general important issues should be noted, however. First, it has been claimed that, in some cases, the nuclear localization of YAP can be regulated by upstream components independent of the core Hippo cascade or YAP phosphorylation by Lats (Dupont et al., 2011; Silvis et al., 2011; Aragona et al., 2013). Ideally, these examples ought not be referred to as the Hippo pathway; however, it is common for YAP regulation to be generally discussed as being related to the Hippo pathway. A more minor exception is the finding that, in some cell types, regulation of the core Hippo complex and YAP phosphorylation by Lats can be controlled by kinases other than the Hippo homologues Mst1 and Mst2 (Zhou et al., 2009). Despite this variation, the core mechanism is typically still considered as the ‘Hippo pathway’. The second important issue is where in the cell this kinase cascade occurs. Originally, it was thought that it functioned in the cytosol, but several studies have discovered that many pathway components, including the downstream components Lats and YAP, interact with membrane-associated proteins, including Merlin/NF2 and proteins found in tight junctions, adherens junctions and apical polarity complexes (Silvis et al., 2011; Zhao et al., 2011; Boggiano and Fehon, 2012; Bossuyt et al., 2013; Hirate et al., 2013; Yin et al., 2013). Also, the scaffold protein Salvador interacts with both the membrane protein Echinoid and the Hippo kinase in Drosophila (Yin et al., 2013). One frequent interpretation of these findings in the literature is that YAP is simply sequestered out of the nucleus because it directly binds to these membrane-associated proteins (Schlegelmilch et al., 2011; Harvey et al., 2013). However, this explanation is likely to be incorrect because the vast majority of extranuclear YAP protein is present in the cytosol, with little if any accumulating at the membrane (Kim et al., 2011; Fan et al., 2013). A rather better explanation is that the membrane association of the Hippo pathway components is transient and catalytic, leading to phosphorylation or other modifications of YAP. How this occurs and how it is regulated by various cell junction and membrane proteins is presently not very well understood but will be key to furthering our understanding of the mechanisms that underly contact inhibition.
Interacting upstream modules regulate Hippo-YAP signaling
Several inter-related mechanisms signal through the Hippo pathway and sense the integrity and organization of cells in tissues; together they control contact and density-dependent regulation of growth. This can be thought of as several distinct upstream modules that control Hippo-YAP signaling (Fig. 1), but they interact in various ways. For the purpose of the present discussion, these can be divided in five categories: (1) adherens junction or cadherin–catenin complexes as adhesive elements, (2) epithelial polarity and tight junction proteins, (3) the FAT-Dachsous planar cell polarity (PCP) pathway, (4) cell shape or the actin cytoskeletal mechanotransduction pathway and (5) regulation by soluble extracellular growth factors.
Adherens junctions and the cadherin-catenin complex have been found to activate the Hippo signaling pathway and inhibit cell growth (Nishioka et al., 2009; Kim et al., 2011; Hirate et al., 2013). Cadherin-mediated stimulation of the Hippo signaling pathway requires cadherin ligation and the formation of a homophilic bond – consistent with a role in cell-cell contact – and works owing to phosphorylation of YAP by Lats and nuclear exclusion of YAP. α- and β-catenin are required for cadherin-mediated stimulation of the Hippo signaling pathway, although a cadherin-independent role of YAP regulation by α-catenin has been reported as well (Schlegelmilch et al., 2011; Silvis et al., 2011). Merlin and Kibra appear to link cadherin ligation to the Hippo cascade, although Merlin itself might be dispensable in some cell types because cadherins can stimulate the pathway in the Merlin-deficient MDA-MB-231 cell line (Kim et al., 2011). In Drosophila, the adherens junction protein Echinoid activates Hippo signaling through its direct interaction with Salvador (Yue et al., 2012). There is no obvious homologous adherens junction protein in mammals, but it is important to note that Echinoid also interacts with E-cadherin to control the formation of adherens junctions in flies (Wei et al., 2005).
Proteins that control apical-basolateral polarity in epithelia have also been strongly implicated in Hippo-YAP signaling (Grusche et al., 2010; Boggiano and Fehon, 2012; Tepass, 2012). The apical polarity regulators, including the crumbs complex and the aPKC–Par6–Par3 complex, interact with components of the Hippo pathway and stimulate signaling in both Drosophila and mammalian cells (Chen et al., 2010; Grzeschik et al., 2010; Robinson et al., 2010; Varelas et al., 2010a; Hirate et al., 2013). Mutations in the basolateral polarity genes scribble, Lgl and Dlg cause substantial tissue overgrowth in Drosophila, which is due at least in part to loss of Hippo pathway signaling (Grzeschik et al., 2010; Chen et al., 2012). In mammals, tight junctions are thought to be analogous to the apical determining polarity complex in Drosophila (Tepass, 2012) and, indeed, tight-junction-associated proteins, especially angiomotin, activate Hippo pathway signaling (Zhao et al., 2011; Hirate et al., 2013); similarly, Scribble has been found to antagonize YAP transcriptional activity (Skouloudaki et al., 2009).
Regulation of Hippo pathway activity by adherens junctions or cadherins and polarity proteins could operate through distinct molecular mechanisms, and many of the findings published so far are consistent with this notion. However, it is important to remember that tight junctions or associated polarity complexes and adherens junction or cadherin complexes are functionally highly interdependent. On one hand, cadherin-mediated adhesion is a basic early cell-cell interaction that helps to promote the formation of tight junctions and the development of polarized membrane domains (Gumbiner et al., 1988; Nejsum and Nelson, 2009). On the other hand, polarity proteins facilitate the formation and near apical positioning of the adherens junctions (Harris and Peifer, 2004; Tepass, 2012). Moreover, cadherin–catenin or adherens junction proteins have been found to interact with some tight junction and polarity proteins and, therefore, it is possible that they regulate the Hippo pathway through some common mechanisms.
Stimulation of Hippo pathway signaling, both by adherens junctions or cadherins and by polarity proteins, together suggests that they function to sense the integrity of the epithelium. Disruptions or discontinuities in the epithelium, reflected by the state of adhesion and polarity complexes, should lead to nuclear activity of YAP and/or TAZ, and activation of growth-promoting genes. The importance of this idea for contact inhibition is clear; cells at the edge of growing or damaged regions of an epithelium or at the edge of an epithelial tumor would be allowed to grow faster than cells well inside the epithelium.
Regulation of Hippo-YAP signaling by cell contact or cell density also appears to occur in non-epithelial cells (Zhao et al., 2007; Zhao et al., 2010; Zhao et al., 2011; Zhao et al., 2012), in which epithelial polarity and epithelial integrity are not thought to be especially important factors for growth control. In these cases, the cadherin–catenin-mediated mechanism and/or the cell shape or mechanotransduction mechanism (see below) may account for Hippo pathway activity. Moreover, several tight-junction-associated and polarity proteins are still expressed in non-epithelial cells, and they might have a role in Hippo-YAP signaling independent of their function in establishing apical and basolateral membrane domains.
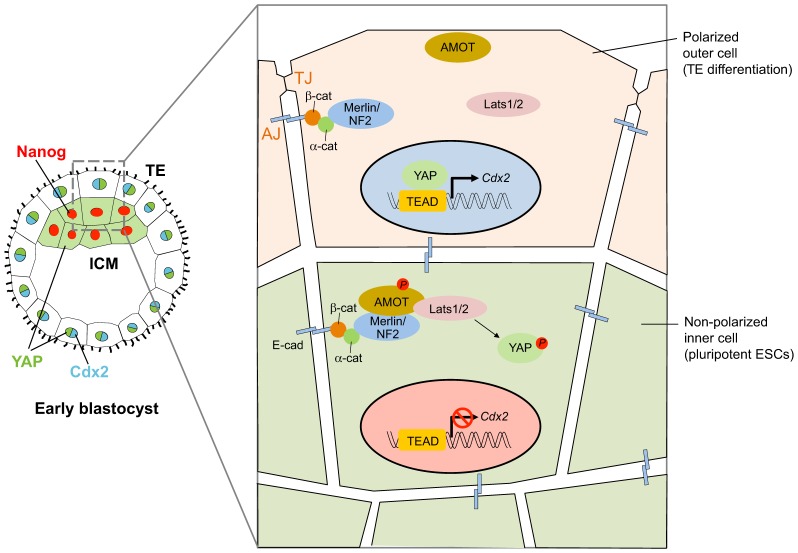
Indeed, changes in the functional interactions between the adherens junction or cadherin adhesion system and the epithelial polarity system provide important regulatory mechanisms and a way to diversify the roles of Hippo pathway signaling in response to tissue organization. The formation of the first polarized epithelium during compaction of the early mouse embryo, the trophectoderm, was found to switch off Hippo pathway activity and to lead to YAP nuclear accumulation and YAP- and TEAD-mediated gene expression (Nishioka et al., 2009; Hirate et al., 2013) (Fig. 2). In this case, the pathway regulates one of the first and most crucial cell fate decisions in mammalian embryos rather than cell proliferation. It determines the distinction between the inner cell mass, which leads to the formation of the embryo proper (the source of embryonic stem cells) and the trophectoderm – the outer epithelial cell layer that gives rise to extra-embryonic tissues and the placenta. Although the inner cells do not exhibit epithelial polarity, they do have cadherin-mediated junctions; and recruitment of the (normally) tight-junction-associated protein angiomotin to the cadherin-mediated junctions leads to activation of the Hippo signaling pathway and nuclear exclusion of YAP. When outer cells undergo epithelialization during compaction, polarity proteins act to segregate angiomotin into the apical domain, which leads to a loss of adherens-junction–angiomotin and/or cadherin–angiomotin stimulation of the Hippo pathway. Thus, establishment of cell polarity during compaction turns off Hippo pathway signaling, resulting in activation of YAP and TEAD target genes.
Fig. 2.
Hippo pathway-mediated regulation of cell lineage and pluripotency by adhesion and epithelial polarization in the early mouse embryo. Compaction of the mouse embryo at the pre-implantation stage leads to the development of two different cell lineages, trophectoderm (TE) and inner cell mass (ICM) (shown on the left). The TE is a polarized epithelium with E-cadherin (E-cad)-mediated adherens junctions (AJs), tight junctions (TJs) and apical–basolateral polarity (illustrated on the right); it differentiates into an extra-embryonic tissue, the placenta. The ICM contains adhesive cells that are non-polarized and form pluripotent embryonic stem cells (ESCs), which give rise to the embryo proper. Recruitment of angiomotin (AMOT) to the E-cad–catenin complexes of inner cells stimulates Hippo pathway signaling and restricts the transcriptional activity of YAP through its exclusion from the nucleus, thereby allowing expression of the pluripotency gene Nanog. AMOT becomes restricted to the apical domain of the outer cells by the action of polarity protein complexes during epithelialization, which leads to a loss of Hippo pathway signaling, nuclear accumulation of YAP and transcriptional activation of TE-specific transcription factor Cdx2. β-cat, β-catenin; α-cat, α-catenin.
Hippo-YAP signaling during mouse embryo compaction is regulated in a way that is completely opposite to what would be expected from previous studies, in several important ways. First, although both inner cell mass and trophectoderm express E-cadherin, activation of strong E-cadherin-mediated adhesion and formation of a complete zonular adherens type adherens junction occurs during compaction to make the trophectoderm (Vestweber et al., 1987; Fleming and Johnson, 1988). Thus, a loss of Hippo pathway signaling in the trophectoderm occurs despite a greater extent of cadherin adhesion and formation of adherens junctions. Second, epithelial polarization as mediated by polarity protein complexes leads to loss of Hippo pathway activity instead of stimulating it as has been observed for Drosophila and many studies of epithelial cells in culture. Nonetheless, the Hippo pathway still reads an aspect of tissue structure – inside versus outside cell layers, and still utilizes the same set of epithelial adhesion and polarity proteins, albeit in a different way. It is also worth noting that, in the early mouse embryo, YAP is excluded from the nucleus in the pluripotent cells of the inner cell mass, while its nuclear activity has been found to be associated with stem cell properties in somatic stem cells (Tremblay and Camargo, 2012). Therefore, there is a diversity of molecular regulatory mechanisms to control Hippo-YAP signaling activity as well as diverse outcomes, which we will have to elucidate to truly understand the role of Hippo-YAP signaling in tissue organization- and contact- regulation of growth and differentiation.
The Fat-Dachsous signaling pathway is a cell-contact-dependent mechanism that regulates the Hippo pathway in a very different context (Irvine, 2012). In Drosophila, it is a very strong regulator of the Hippo pathway; Fat acts upstream of Warts and Yorkie, and loss of Fat function results in very strong Hippo pathway phenotypes. Dachsous and Fat are both very large cell surface proteins containing a number of cadherin repeat domains, although they do not seem to mediate traditional physical cell adhesion. Neither do they appear to have a role in the local integrity of cells in the epithelial sheet, in contrast to E-cadherin and polarity proteins. Instead, they mediate a cell-contact-dependent morphogen gradient that patterns the overall tissue; in this case the whole imaginal disk, the primordium that gives rise to adult tissues. In fact the Fat-Dachsous system is not specific to Hippo signaling, because it is also a main mediator of planar PCP signaling, again modulating PCP across the whole imaginal disk rather than locally the Frizzled-mediated regulation of the PCP pathway. However, the PCP and Hippo signaling activities of Fat are separable and mechanistically distinct (Irvine, 2012; Bossuyt et al., 2013). Nonetheless, both pathways can detect a morphogen gradient set up by gradients of Dachsous and the Golgi kinase Four-jointed (Fj) that regulates Fat activity. Thus, Fat regulates cell proliferation across the imaginal disk as a way to pattern growth over a distance and to help shape the growth of the disk.
Homologues of Fat and other components of the Fat-Hippo pathway are expressed in mammals; of those, Fat4 is the one most similar to Drosophila Fat. It was found to have a role in PCP signaling in mammals (Saburi et al., 2012), but so far there has been minimal evidence that it functions in Hippo-YAP signaling in mammals or other vertebrates. One possibility is that none of the previous studies have looked in the right places or contexts for Fat-Hippo pathway signaling, e.g. over small regions of tissue where patterning by a local morphogen gradient might be important. Nonetheless, a recent study argued that Fat regulation of the Hippo pathway is specific to the arthropod lineage (Bossuyt et al., 2013). A molecular dissection of the Fat cytoplasmic tail reveals regions that function differentially in Hippo pathway signaling and PCP signaling; whereas the PCP signaling portions are conserved across a wide range of species, the Hippo pathway signaling-specific regions are not. Other molecules and molecular interactions also appear to diverge between Drosophila and vertebrates (Bossuyt et al., 2013). Notably, the vertebrate junction-associated adaptor protein angiomotin (Amot), which was found to have key roles in mammalian Hippo-YAP signaling (Zhao et al., 2011; Adler et al., 2013; Hirate et al., 2013), has no homologue in Drosophila. Nonetheless, most of the downstream core components of the Hippo-YAP signaling pathway, as well as some upstream regulators such as Merlin/NF2, function similarly in Drosophila and vertebrates.
Cell shape and mechanotransduction also have been found to regulate the Hippo-YAP signaling (Dupont et al., 2011; Wada et al., 2011; Halder et al., 2012) and might be involved in contact inhibition and/or cell-density-dependent growth (Aragona et al., 2013). As cells grow into dense colonies, they change shape and mechanical properties together with changes in cell-cell adhesions and junctions, and it can be difficult to separate out these different factors. Nonetheless, cell shape or tension has been found to regulate the nuclear accumulation of YAP and TAZ independently of the formation of cell-cell contacts (Dupont et al., 2011; Aragona et al., 2013). Highly spread individual cells that are grown on a hard substrate are under greater tension, contain numerous stress fibers and focal adhesions, and exhibit strong nuclear accumulation of YAP/TAZ. By contrast, rounded individual cells grown on soft substrates strongly exclude YAP/TAZ from the nucleus. There is evidence that this mechanical or shape regulation of the nuclear localization of YAP occurs independently of the Hippo pathway, i.e. that it does not involve the Hippo or Lats kinases, or the phosphorylation of YAP/TAZ on Lats target sites (Dupont et al., 2011; Aragona et al., 2013). This is consistent with the idea that the mechano- or shape-regulation of YAP/TAZ is independent of cell-cell contact per se, which does work through the Hippo pathway. Of course, cell-cell contact can also regulate cell shape and tension. The molecular mechanisms by which shape and tension regulate YAP/TAZ is unclear, but they appear to require an intact Rho-Rock pathway, as expected for a mechanical component.
The state of the actin cytoskeleton is very important in mediating the effects of shape and tension of nuclear YAP/TAZ. Perturbations of the actin cytoskeleton have strong effects on YAP localization (Wada et al., 2011; Zhao et al., 2012; Kim et al., 2013). Remarkably, genetic deficiencies in actin-capping proteins both in Drosophila and mammalian cells strongly activate nuclear Yorkie and YAP activity, respectively (Sansores-Garcia et al., 2011; Aragona et al., 2013). These findings are consistent with the idea that actomyosin-dependent tension regulates YAP/TAZ signaling, i.e. that such mutations reflect the cell shape or the mechanotransduction-mediated pathway described above. However, numerous studies have found that actin perturbations do, indeed, act through the Hippo pathway or, at least through YAP phosphorylation by Lats (Sansores-Garcia et al., 2011; Wada et al., 2011; Zhao et al., 2012; Kim et al., 2013) – in contrast to the studies on the role of the cell shape (Dupont et al., 2011; Aragona et al., 2013). To explain this apparent discrepancy, it has been proposed that mechanical cues can initiate both Lats-dependent and Lats-independent regulation of YAP/TAZ (Halder et al., 2012). These studies have not yet discerned which part of the actin cytoskeleton is involved in the regulation of Hippo-YAP signaling; for example, whether actin associated with focal-adhesion-associated stress fibers, cell junctions, other parts of the cell cortex, or the global state of actin effect cell-contact-dependent Hippo-YAP signaling.
Soluble growth factors
Several recent studies have discovered that soluble hormones or growth factor can regulate Hippo-YAP in addition to its regulation by cell contact, polarity, and mechanical properties that are important for tissue organization. In most cases, these soluble growth factors inhibit Hippo-YAP signaling and counteract the effects of cell contact (Straßburger et al., 2012; Yu et al., 2012; Fan et al., 2013; Reddy and Irvine, 2013), suggesting that the Hippo pathway mediates a reciprocal relationship between contact inhibition and mitogenic signaling (Fig. 3). Several growth factors, including EGF, IGF, serum and LPA, inhibit Hippo-pathway signaling and lead to nuclear accumulation of Yorkie/YAP, which then activates growth-related genes. Significantly, Yorkie/YAP is found to be required for growth-factor-induced proliferation, suggesting that inhibition of Hippo pathway signaling is a crucial component of mitogenic signaling. Using genetics studies, this was shown to be true in vivo in Drosophila, but how important it is for mitogenic growth factor signaling in vivo in mammalian tissues or cancer remains to be determined.
Fig. 3.

The Hippo pathway mediates the reciprocal regulation of cell proliferation by contact inhibition and mitogenic signaling. Cell-cell contact inhibits cell proliferation through stimulation of the Hippo pathway. Mitogenic growth factors stimulate proliferation by well-known signaling mechanisms, but also counteract the growth inhibitory effects of the Hippo signaling pathway. Thus, the Hippo pathway can coordinate classic mitogenic signaling with the physical state of the cells in a tissue. TJ, tight junction; RTK, receptor tyrosine kinase; GPCR, G-protein-coupled receptor.
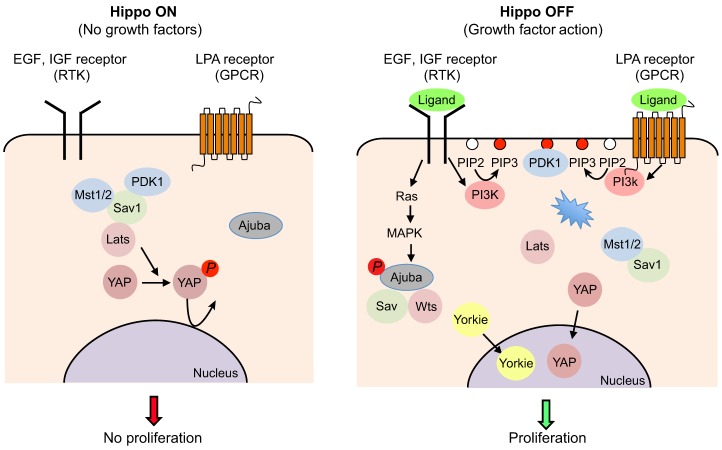
A few different mechanisms have been found to mediate the regulation of Hippo-YAP signaling by soluble factors. In mammalian cells, EGF, serum and LPA were found to rapidly stimulate the nuclear accumulation of YAP by a novel downstream effect of PI 3-kinase (PI3K) and PDPK1 (PDK1) on the Hippo pathway, largely independent of Akt signaling. PDK1 was found to interact with the core Hippo kinase complex, and stimulation of PI3K by EGF causes the complex to dissociate, which presumably impairs the ability of Lats to phosphorylate YAP (Fan et al., 2013) (Fig. 4). The PI3K-PDK pathway was similarly found to mediate the inhibition of the Hippo pathway through IGF signaling in Drosophila (Straßburger et al., 2012). EGF signaling was also found to inhibit Hippo pathway signaling in Drosophila, but in this case it was found to be mediated by MAP-kinase and the regulation of Warts by Ajuba (Reddy and Irvine, 2013). In some cases, hormones can stimulate, rather than inhibit, the Hippo pathway; for instance through G-protein-coupled receptor-mediated stimulation of adenyl-cyclase–PKA signaling to stimulate Lats activity (Yu et al., 2012; Yu et al., 2013). It will be interesting to see how widespread the regulation of the Hippo pathway by soluble factors is.
Fig. 4.
Two mechanisms for growth-factor-mediated regulation of the Hippo pathway: activation of Ras-MAPK signaling and activation of PI3K-PDK1 signaling. (Left panel, Hippo pathway on) In confluent cells in the absence of growth factors, PDK1 forms a complex with Hippo pathway components (Lats, Mst and Sav1) and the Hippo pathway is active. Mst phosphorylates Mob and Lats, which then phosphorylate YAP. YAP is then excluded from the nucleus and cell growth is arrested. (Right panel, Hippo pathway off) Activation of the Ras-MAPK pathway by EGF signaling phosphorylates Ajuba, which binds to and inhibits the activity of the Sav�–Wts complex, leading to dephosphorylation of Yorkie, its accumulation in the nucleus and increased cell proliferation. Growth factors (EGF, LPA or serum) can also activate PI3K and recruit PDK1 to the membrane, resulting in the dissociation of the PDK1–Hippo complex. As a result, the regulation of Lats by Mst is prevented, which eventually leads to nuclear accumulation of YAP and cell proliferation. Figure was modified with permission (Fan et al., 2013). PIP2, PtdIns(4,5)P2; PIP3, PtdIns(3,4,5)P3.
Soluble growth-factor-mediated regulation of the Hippo pathway may explain some of the cell nonautonomous features of Hippo-YAP signaling. Two of the well-established target genes for YAP and TEAD are connective tissue growth factor (CTGF) and amphiregulin (AREG), which can mediate effects of Hippo-YAP signaling on neighboring cells (Zhao et al., 2008; Zhang et al., 2009). Increased production of amphiregulin occurs at low-cell density due to loss of contact inhibition, decreased Hippo pathway signaling and increased concentration of nuclear YAP, which allows cells to proliferate rapidly (Zhang et al., 2009). Because amphiregulin is an EGF receptor (EGFR) ligand it can, in turn, inhibit Hippo pathway signaling and increase the nuclear accumulation of YAP (Fan et al., 2013), creating a positive autocrine or paracrine feedback loop to stimulate and spread the transcriptional activity of YAP. Several other examples of cell nonautonomy in Hippo pathway signaling have been observed, including Src kinase activation of Yorkie in neighboring cells (Enomoto and Igaki, 2013) and the effects of cell-cell competition on Hippo pathway signaling (Chen et al., 2012) (see below). In these cases the mediators that act between cells are not yet known, but soluble growth factors are likely to be candidates.
The findings that inhibition of Hippo pathway signaling can be a component of mitogenic signaling has important general implications and raises questions with regard to many different biological processes. How common is it for regulation of Hippo-YAP signaling to be coordinated with mitogenic signaling pathways during embryonic and/or tissue development? Is this coordination important for cancer growth? For example, mutational activation of the PI3K pathway, due to constitutively active mutations in PI3K or inactivation of PTEN, or activation of the EGFR pathway are known to drive the growth of many tumors (Engelman et al., 2006). Stimulation of PI3K signaling, including the presence of mutations that render PI3K constitutively active, cause nuclear accumulation of YAP (Fan et al., 2013), which suggests that YAP regulation by PI3K signaling is an important general mechanism in cancer. Understanding the role of Hippo-YAP signaling inhibition in mitogenic signaling in vivo is likely to be important for future research.
Broader implications – what is contact inhibition for?
The recent observations that regulation of Hippo-YAP signaling is intimately coordinated with signaling through traditional growth factor signaling pathways have additional implications for control of tissue growth; in particular, for activities at the edge of tissues or boundaries between tissue domains. How this might work was nicely demonstrated in a study describing the relationship between contact inhibition and mitogenic signaling (Kim et al., 2009). Contact inhibition is not an absolute switch that shuts off cell proliferation. Rather, increasing cell contact appears to shift the dose-dependence of cell proliferation in response to a mitogenic growth factor such as EGF to a higher threshold response (Fig. 5). As a result, a cell can differentially respond to a steady level of a growth factor depending on its location relative to other cells in the culture or – presumably – in a tissue. Although there are many potential mechanisms to explain this phenomenon, the Hippo pathway is ideally suited to mediate this effect, with the nuclear localization of YAP more likely to occur in cells at the edge of the cluster of cells (Fig. 5). In this way, cells at the edge of a colony or a wound in a tissue, or at the edge of a tumor would be more sensitive to ambient levels of growth factors and more likely to proliferate, migrate or differentiate in a YAP/TAZ-dependent process.
Fig. 5.
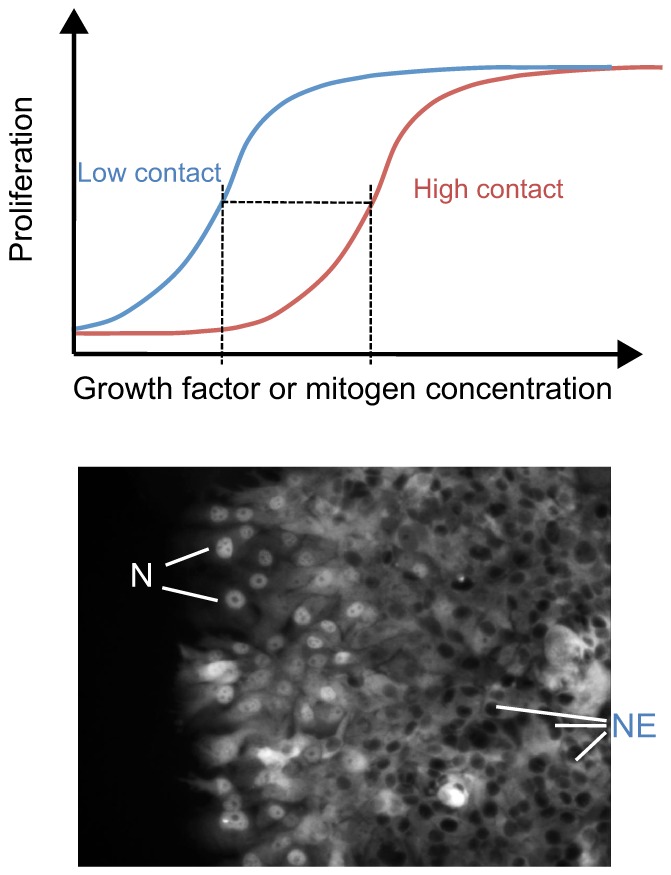
Role of contact inhibition and Hippo-YAP signaling in the spatial control of mitogenic signaling by growth factors. (Top) The graph shows the dose-response curves for growth factor-stimulated proliferation. A high degree of cell-cell contact shifts the curve on the x-axis, such that a higher concentration of growth factors is required to elicit the same response. This graph is an interpretation of the findings of Kim et al., 2009. (Bottom) Illustration how cell-cell contact regulates transcription factor activity through the Hippo pathway. At high cell density in the middle of the cell monolayer, the Hippo pathway is active, leading to nuclear exclusion (NE) of YAP and/or TEAD (TEAD is labeled by immunofluorescence staining in this example). At the edge of the culture where cells have lost contact inhibition, YAP and/or TEAD (as shown here) accumulate in the nuclei (N) and stimulate proliferation. Thus, proliferation is spatially regulated despite uniform levels of growth factors acting on these cells.
The Hippo pathway also plays a role in responding to boundaries between populations of cells that have different growth potential within the same tissue, even when they have continuous contact. Cell competition is a homeostatic mechanism that can eliminate abnormal or cancerous cells from a tissue during development (Johnston, 2009). Moreover, a recent study found that regulation of Hippo pathway signaling by normal cells prevents the overgrowth of tumor cells in Drosophila imaginal disks (Chen et al., 2012). Null-mutations in the scribble gene (scrib–) cause neoplastic tumor-like growths when all cells in the tissue harbor the mutation (Chen et al., 2012). However, in mosaic tissues, the growth of scrib– clones is completely suppressed by neighboring clones of wild-type cells. Wild-type clones prevent the overproliferation of scrib– clones by suppressing Yorkie/YAP activity in the mutant clones (Chen et al., 2012). Thus, cell competition through a regulation of Hippo pathway signaling acts as a tumor suppressor mechanism. It will be interesting to see whether a similar mechanism suppresses tumor growth in mammals.
Hippo-YAP signaling might be particularly important in sensing other three-dimensional features of tissues. This is beautifully illustrated by the process of mouse embryo compaction described above, in which cells in the outside layer of a tissue differentiate along a completely different path than the inner cells. In this case, the difference in Hippo-YAP signaling between the two layers is brought about by the acquisition of cell polarity and by switching off of the stimulation of the pathway that is dependent on cadherin-angiomotin coupling. Another potential way in which the position of outer versus inner cells of a tissue might be sensed is through the mechanotransduction or tension-mediated regulation of YAP, because outer cells may be more spread and, thus, might experience a higher surface tension.
Concluding remarks
One remarkable and particularly interesting aspect of the Hippo-YAP signaling pathway is that it senses and responds in so many ways to the physical organization of cells in tissues; through cell-cell adhesion, cell junctions and other contact-dependent signals, cell polarity mechanisms, cell shape, tension and, finally, actin organization. Thus, Hippo signaling provides an important explanation for many of the observed phenomena by which physical cues regulate tissue growth and differentiation. Future discoveries in this fast-moving field are likely to reveal even greater insights into the physical mechanisms that control cell growth and differentiation, as well as their coordination with classic growth-factor-mediated pathways.
Acknowledgments
We thank our colleagues Jing Yu and Xiaowei Lu for many valuable discussions about the ideas presented in this Commentary, and for reading and making comments on the manuscript.
Footnotes
Competing interests
The authors declare no conflict of interest.
Funding
This work was supported by a National Institutes of Health grant to B.M.G. Deposited in PMC for release after 12 months.
References
- Adler J. J., Johnson D. E., Heller B. L., Bringman L. R., Ranahan W. P., Conwell M. D., Sun Y., Hudmon A., Wells C. D. (2013). Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. USA 110, 17368–17373 10.1073/pnas.1308236110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- Basu S., Totty N. F., Irwin M. S., Sudol M., Downward J. (2003). Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 10.1016/S1097-2765(02)00776-1 [DOI] [PubMed] [Google Scholar]
- Boggiano J. C., Fehon R. G. (2012). Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev. Cell 22, 695–702 10.1016/j.devcel.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt W., Chen C. L., Chen Q., Sudol M., McNeill H., Pan D., Kopp A., Halder G. (2013). An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene 10.1038/onc.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Gajewski K. M., Hamaratoglu F., Bossuyt W., Sansores-Garcia L., Tao C., Halder G. (2010). The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. USA 107, 15810–15815 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Schroeder M. C., Kango-Singh M., Tao C., Halder G. (2012). Tumor suppression by cell competition through regulation of the Hippo pathway. Proc. Natl. Acad. Sci. USA 109, 484–489 10.1073/pnas.1113882109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Engelman J. A., Luo J., Cantley L. C. (2006). The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 7, 606–619 10.1038/nrg1879 [DOI] [PubMed] [Google Scholar]
- Enomoto M., Igaki T. (2013). Src controls tumorigenesis via JNK-dependent regulation of the Hippo pathway in Drosophila. EMBO Rep. 14, 65–72 10.1038/embor.2012.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F., Gumbiner B. M. (1996). Cell contact-dependent signaling. Dev. Biol. 180, 445–454 10.1006/dbio.1996.0318 [DOI] [PubMed] [Google Scholar]
- Fan R., Kim N. G., Gumbiner B. M. (2013). Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. USA 110, 2569–2574 10.1073/pnas.1216462110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust D., Dolado I., Cuadrado A., Oesch F., Weiss C., Nebreda A. R., Dietrich C. (2005). p38alpha MAPK is required for contact inhibition. Oncogene 24, 7941–7945 10.1038/sj.onc.1208948 [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Johnson M. H. (1988). From egg to epithelium. Annu. Rev. Cell Biol. 4, 459–485 10.1146/annurev.cb.04.110188.002331 [DOI] [PubMed] [Google Scholar]
- Grusche F. A., Richardson H. E., Harvey K. F. (2010). Upstream regulation of the hippo size control pathway. Curr. Biol. 20, R574–R582 10.1016/j.cub.2010.05.023 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., Richardson H. E. (2010). Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 10.1016/j.cub.2010.01.055 [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. (1988). The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107, 1575–1587 10.1083/jcb.107.4.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G., Johnson R. L. (2011). Hippo signaling: growth control and beyond. Development 138, 9–22 10.1242/dev.045500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G., Dupont S., Piccolo S. (2012). Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13, 591–600 10.1038/nrm3416 [DOI] [PubMed] [Google Scholar]
- Harris T. J., Peifer M. (2004). Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167, 135–147 10.1083/jcb.200406024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K. F., Zhang X., Thomas D. M. (2013). The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 10.1038/nrc3458 [DOI] [PubMed] [Google Scholar]
- Heit I., Wieser R. J., Herget T., Faust D., Borchert-Stuhlträger M., Oesch F., Dietrich C. (2001). Involvement of protein kinase Cdelta in contact-dependent inhibition of growth in human and murine fibroblasts. Oncogene 20, 5143–5154 10.1038/sj.onc.1204657 [DOI] [PubMed] [Google Scholar]
- Hirate Y., Hirahara S., Inoue K., Suzuki A., Alarcon V. B., Akimoto K., Hirai T., Hara T., Adachi M., Chida K. et al. (2013). Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 23, 1181–1194 10.1016/j.cub.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K. D. (2012). Integration of intercellular signaling through the Hippo pathway. Semin. Cell Dev. Biol. 23, 812–817 10.1016/j.semcdb.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. A. (2009). Competitive interactions between cells: death, growth, and geography. Science 324, 1679–1682 10.1126/science.1163862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D., Xie B., Rister J., Terrell D., Charlton-Perkins M., Pistillo D., Gebelein B., Desplan C., Cook T. (2013). Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science 342, 1238016 10.1126/science.1238016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Kushiro K., Graham N. A., Asthagiri A. R. (2009). Tunable interplay between epidermal growth factor and cell-cell contact governs the spatial dynamics of epithelial growth. Proc. Natl. Acad. Sci. USA 106, 11149–11153 10.1073/pnas.0812651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. G., Koh E., Chen X., Gumbiner B. M. (2011). E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA 108, 11930–11935 10.1073/pnas.1103345108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Kim M., Lee S., Kuninaka S., Saya H., Lee H., Lee S., Lim D. S. (2013). cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 32, 1543–1555 10.1038/emboj.2013.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro A., Nagai M., Navin N. E., Sudol M. (2003). WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J. Biol. Chem. 278, 33334–33341 10.1074/jbc.M305597200 [DOI] [PubMed] [Google Scholar]
- Li W., Cooper J., Karajannis M. A., Giancotti F. G. (2012). Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO Rep. 13, 204–215 10.1038/embor.2012.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Y., Zha Z. Y., Zhou X., Zhang H., Huang W., Zhao D., Li T., Chan S. W., Lim C. J., Hong W. et al. (2010). The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J. Biol. Chem. 285, 37159–37169 10.1074/jbc.M110.152942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., Yap A. S. (2012). Contact inhibition (of proliferation) redux. Curr. Opin. Cell Biol. 24, 685–694 10.1016/j.ceb.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Nejsum L. N., Nelson W. J. (2009). Epithelial cell surface polarity: the early steps. Front. Biosci. (Landmark Ed.) 14, 1088–1098 10.2741/3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N., Inoue K., Adachi K., Kiyonari H., Ota M., Ralston A., Yabuta N., Hirahara S., Stephenson R. O., Ogonuki N. et al. (2009). The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 10.1016/j.devcel.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Perrais M., Chen X., Perez-Moreno M., Gumbiner B. M. (2007). E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol. Biol. Cell 18, 2013–2025 10.1091/mbc.E06-04-0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. (1994). p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8, 9–22 10.1101/gad.8.1.9 [DOI] [PubMed] [Google Scholar]
- Reddy B. V., Irvine K. D. (2013). Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev. Cell 24, 459–471 10.1016/j.devcel.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. S., Huang J., Hong Y., Moberg K. H. (2010). Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20, 582–590 10.1016/j.cub.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi S., Hester I., Goodrich L., McNeill H. (2012). Functional interactions between Fat family cadherins in tissue morphogenesis and planar polarity. Development 139, 1806–1820 10.1242/dev.077461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L., Bossuyt W., Wada K., Yonemura S., Tao C., Sasaki H., Halder G. (2011). Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 10.1038/emboj.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J. R., Zhou D., Kreger B. T., Vasioukhin V., Avruch J., Brummelkamp T. R. et al. (2011). Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 10.1016/j.cell.2011.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis M. R., Kreger B. T., Lien W. H., Klezovitch O., Rudakova G. M., Camargo F. D., Lantz D. M., Seykora J. T., Vasioukhin V. (2011). α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 4, ra33 10.1126/scisignal.2001823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouloudaki K., Puetz M., Simons M., Courbard J. R., Boehlke C., Hartleben B., Engel C., Moeller M. J., Englert C., Bollig F. et al. (2009). Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc. Natl. Acad. Sci. USA 106, 8579–8584 10.1073/pnas.0811691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straßburger K., Tiebe M., Pinna F., Breuhahn K., Teleman A. A. (2012). Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev. Biol. 367, 187–196 10.1016/j.ydbio.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Tepass U. (2012). The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 28, 655–685 10.1146/annurev-cellbio-092910-154033 [DOI] [PubMed] [Google Scholar]
- Tremblay A. M., Camargo F. D. (2012). Hippo signaling in mammalian stem cells. Semin. Cell Dev. Biol. 23, 818–826 10.1016/j.semcdb.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Tumaneng K., Russell R. C., Guan K. L. (2012). Organ size control by Hippo and TOR pathways. Curr. Biol. 22, R368–R379 10.1016/j.cub.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B. G., Rossant J., Wrana J. L. (2010a). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Varelas X., Miller B. W., Sopko R., Song S., Gregorieff A., Fellouse F. A., Sakuma R., Pawson T., Hunziker W., McNeill H. et al. (2010b). The Hippo pathway regulates Wnt/beta-catenin signaling. Dev. Cell 18, 579–591 10.1016/j.devcel.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Vestweber D., Gossler A., Boller K., Kemler R. (1987). Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Dev. Biol. 124, 451–456 10.1016/0012-1606(87)90498-2 [DOI] [PubMed] [Google Scholar]
- Wada K., Itoga K., Okano T., Yonemura S., Sasaki H. (2011). Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 10.1242/dev.070987 [DOI] [PubMed] [Google Scholar]
- Wei S. Y., Escudero L. M., Yu F., Chang L. H., Chen L. Y., Ho Y. H., Lin C. M., Chou C. S., Chia W., Modolell J. et al. (2005). Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev. Cell 8, 493–504 10.1016/j.devcel.2005.03.015 [DOI] [PubMed] [Google Scholar]
- Wieser R. J., Faust D., Dietrich C., Oesch F. (1999). p16INK4 mediates contact-inhibition of growth. Oncogene 18, 277–281 10.1038/sj.onc.1202270 [DOI] [PubMed] [Google Scholar]
- Yagi R., Chen L. F., Shigesada K., Murakami Y., Ito Y. (1999). A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 18, 2551–2562 10.1093/emboj/18.9.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F., Yu J., Zheng Y., Chen Q., Zhang N., Pan D. (2013). Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154, 1342–1355 10.1016/j.cell.2013.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. X., Guan K. L. (2013). The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 10.1101/gad.210773.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. X., Zhao B., Panupinthu N., Jewell J. L., Lian I., Wang L. H., Zhao J., Yuan H., Tumaneng K., Li H. et al. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 10.1016/j.cell.2012.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. X., Zhang Y., Park H. W., Jewell J. L., Chen Q., Deng Y., Pan D., Taylor S. S., Lai Z. C., Guan K. L. (2013). Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 27, 1223–1232 10.1101/gad.219402.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T., Tian A., Jiang J. (2012). The cell adhesion molecule echinoid functions as a tumor suppressor and upstream regulator of the Hippo signaling pathway. Dev. Cell 22, 255–267 10.1016/j.devcel.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ji J. Y., Yu M., Overholtzer M., Smolen G. A., Wang R., Brugge J. S., Dyson N. J., Haber D. A. (2009). YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat. Cell Biol. 11, 1444–1450 10.1038/ncb1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L. et al. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J. D., Wang C. Y., Chinnaiyan A. M. et al. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 10.1101/gad.1664408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Tumaneng K., Wang C. Y., Guan K. L. (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 24, 72–85 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lu Q., Wang L. H., Liu C. Y., Lei Q., Guan K. L. (2011). Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 25, 51–63 10.1101/gad.2000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Wang L., Wang C. Y., Yu J., Guan K. L. (2012). Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 10.1101/gad.173435.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Conrad C., Xia F., Park J. S., Payer B., Yin Y., Lauwers G. Y., Thasler W., Lee J. T., Avruch J. et al. (2009). Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 10.1016/j.ccr.2009.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]