Abstract
Breakthroughs in genetics over the last decade have radically advanced our understanding of the etiological basis of Parkinson's disease (PD). Although much research remains to be done, the main genetic causes of this neurodegenerative disorder are now partially unraveled, allowing us to feel more confident that our knowledge about the genetic architecture of PD will continue to increase exponentially. How and when these discoveries will be introduced into general clinical practice, however, remains uncertain. In this review, we provide a general summary of the progress in the genetics of PD and discuss how this knowledge will contribute to the diagnosis and clinical management of patients with, or at risk of this disorder.
Keywords: Genetics, Parkinson's disease, Mendelian genes, Genetic testing, PARK, Clinical genetics, Genetic risk factor.
INTRODUCTION
Dissection of the genetic architecture of any complex disorder like Parkinson's disease (PD) is paramount to understanding its biological basis, assess the individual predisposition of disease, and evaluate the capacity of novel therapeutic interventions in early, even pre-symptomatic stages. A striking progress in genetics of PD during the last decade has led to the identification of a substantial number of Mendelian genes and loci, as well as a large list of genes with significant effects on disease risk. In order to make an apprehensible description of what has been discovered during the last 15 years, we have performed a summary divided into three main topics: Mendelian genes, well established genes related to sporadic forms of disease, and genes with low risk effects resulting from genome-wide association analyses. We also discuss the usefulness and limitations of genetic testing.
1. MENDELIAN GENES
PARK1 (PARK4): α-synuclein; Chromosome 4q21-23
Three missense mutations (p.A30P, p.E46K, and p.A53T) in the gene encoding for α-synuclein (SNCA), the main protein that aggregates in Lewy bodies, have been reported in families with autosomal dominant forms of PD [1]. Broadly, these point mutations range from a relatively typical levodopa-responsive PD to a disorder reminiscent of diffuse Lewy body dementia, with a relatively early age at onset (mean age of 45 years). In 2003, a genomic triplication of 1.5 million base-pairs that includes the entire SNCA gene was found to segregate with disease in a large family called the "Iowa kindred" [2, 3]. Additional families with eitherSNCA triplication or duplication mutations were later discovered [4, 5]. In these cases, the α-synuclein gene is normal in sequence but abnormal in dose. Interestingly, duplication of the SNCA locus more closely resembles idiopathic PD, whereas patients with four copies of the gene tend to present an earlier onset and a more aggressive clinical course, including cognitive decline and shorter survival.
PARK2: Parkin; Chromosome 6q5.5-q27
Mutations in PARK2 are the leading cause of autosomal recessive early-onset PD. They account for up to 10% of patients with PD onset before 50 years [6, 7] and as high as 77% of patients with onset at 20 years or younger [8]. Since PARK2 was identified in 1998, a wide variety of mutations, including exon rearrangements, single base pair substitutions, and small deletions or insertions of one or several base pairs, have been identified in nearly all populations studied, regardless of ethnic origin. Patients with mutations present a clinical syndrome that is indistinguishable from that of idiopathic PD, with a good response to levodopa. Brain autopsy studies in PARK2 cases have shown pathological heterogeneity. Although the majority of reported cases present substantia nigra degeneration without neuronal inclusions of alpha-synuclein [9-11], other patients have been described with Lewy bodies (LB) in the nigra and locus coreuleus [12], basophilic LB-like inclusions in the pedunculopontine nucleus [13], or neurofibrillary tangle pathology in the cerebral cortex and brainstem nuclei [14].
Interestingly, carriers of heterozygous PARK2 mutations are at risk to develop PD [15-17], and some coding polymorphisms appear to be risk factors for sporadic and familial PD [18]. The role of heterozygous PARK2 mutations as a PD risk factor has been supported by positron emission tomography (PET) scanning studies showing preclinical changes in striatal structures in asymptomatic heterozygous mutation carriers [19, 20].
PARK6: PINK1; Chromosome 1p36
PTEN-induced putative kinase 1 gene (PINK1) is the second most frequent causative gene in early onset autosomal recessive PD. Homozygous and compound heterozygous mutations comprising nonsense, missense and small deletions, have been described in patients with a slowly progressive levodopa-responsive phenotype. Intriguingly, several alterations in imaging biomarkers have been reported in patients carrying a single PINK1 mutation. For example, a low uptake of the tracer iodine-123 metaiodobenzylguanidine in myocardial muscle [21], and a 20 to 30% reduction in caudate and putamen F-dopa have been shown in PINK1 mutation carriers [20]. Nonetheless, there is no clear evidence that PINK1 heterozygosity increases susceptibility to idiopathic PD [22].
PARK7: DJ1; Chromosome 1p36
Homozygous and compound heterozygous mutations in DJ1 are rare causes of early onset PD with a recessive inheritance [23]. Although patients with DJ1 mutations present a clinical picture of idiopathic PD with L-dopa responsiveness, three cases from the same family have been reported to have a phenotype comprising early-onset parkinsonism, dementia, and amyotrophic lateral sclerosis [24].
PARK8: LRRK2; Chromosome 12p11.2-q13.1
The most frequently mutated gene in PD is the gene encoding for the Leucine-rich repeat kinase 2 (LRRK2) [25, 26]. Mutations in this gene lead to an autosomal dominant PD with onset in the sixth decade of life. Although more than 250 aminoacid substitutions have been reported in this gene [27], genetic evidence for pathogenicity by cosegregation with disease within families has only been proven for six variants: p.R1441C, p.R1441G, p.R1441H, p.Y1699C, p.G2019S, and p.I2020T. Among these mutations, p.G2019S is the most common, with a worldwide frequency ranging from 1% of patients with sporadic PD to 4% of patients with familial PD. These frequencies vary in different populations, however. It is present in ~40% of North African Berber Arabs with PD, in 28% of Ashkenazi Jews with hereditary disease (and in 10% with sporadic disease), and in less than 0.1% of Asians [28]. The age-related penetrance of the p.G2019S mutation has been estimated to be 28% at 59 years, 51% at 69 years, and 74% at 79 years. Patients harboring the p.G2019S mutation are clinically indistinguishable from those with idiopathic PD. Nonetheless, mutation carriers can present a more benign progression and less prevalence of dementia during the course of PD course than noncarriers.
PARK9: ATP13A2; Chromosome 1p36
A syndrome appearing at very young ages, between 11 and 16 years, was first described in 1994 in a consanguineous Jordanian family originating from a small community named Kufor-Rakeb [29]. This form of the disease consists of akinetic-rigid parkinsonism with concomitant bradykinesia, progressive spasticity, supranuclear upgaze paresis and dementia. It has a good response to L-dopa. The gene was mapped to the short arm of chromosome 1 (1p36) in 2001 [30], and the responsible gene (ATPase type 13A2, ATP 13A2) was cloned soon afterwards [31]. Since then, few families have been described worldwide and mutations in ATP13A2 seem to be a very rare cause of Parkinsonism.
PARK14: PLA2G6; Chromosome 22q13.1
Mutations in the PLA2G6 gene, encoding for the phospholipase A2, group VI (cytosolic, calcium-independent) gene, typically lead to a recessive degenerative disorder characterized by motor and cognitive regression starting at the first or second year of life [32]. Cerebellar cortical atrophy and basal ganglia iron accumulation are prominent radiologic features in these patients, and spasticity, dystonia and cerebellar features are also evident in mutation carriers [33]. It is important to note that patients with homozygous PLA2G6 mutations can also present with adult-onset levodopa-responsive complicated parkinsonism without brain iron accumulation on MRI. However, a clinical phenotype comprising slowly progressive gait problems, cognitive decline, clumsiness, hand tremor, bradykinesia, dysarthria and dystonia has also been described [34].
PARK15: F-box protein 7; Chromosome 22q12-q13
Pyramidal signs with an onset within the third decade of life, followed by L-dopa responsive extrapyramidal symptoms, were first described in a large Iranian family with an autosomal recessive pattern of inheritance [35]. The authors cloned the FBXO7 gene as the genetic cause of this disorder. A handful of families with early-onset, progressive parkinsonism with associated pyramidal tract signs due to FBXO7 homozygous and compound heterozygous mutations have since been reported in Italy, Turkey, the Netherlands and Pakistan [36, 37]. Clinical features such as bulbar signs, supranuclear gaze palsy and cognitive deterioration have been reported in some of these families, expanding the heterogeneity related to FBXO7 mutations.
PARK17: VPS35; Chromosome 16q12
The recent finding of VPS35 is a good example of how targeted enrichment of genomic DNA and next-generation sequencing are powerful tools for the discovery of Mendelian genes. This gene encodes for the vacuolar protein sorting 35 homolog (S. cerevisiae), and is the causal gene for some forms of late-onset, autosomal dominant PD. The gene was found in 2011, when two independent groups performed a caption of the ~180,000 exons that represent 22,000 genes of the human genome and sequenced all the resulting fragments by means of next generation sequencing systems in families with autosomal dominant PD from Switzerland and Australia [38, 39]. A missense mutation (p.D620N) in the VPS35 gene was found in both families. Subsequent analyses disclosed families from Tunisia, Israel and Austria carrying the same mutation. Although other missense mutations were found by both groups, the pathogenicity of these additional mutations remains unknown.
2. SNCA, GBA AND MAPT AS WELL ESTABLISHED GENES RELATED TO PD
SNCA Genetic Variation and PD Risk
In 1999, certain alleles of the polymorphic complex repeat site NACP-Rep1 (D4S3481), which is composed of a mixed length of contiguous dinucleotides, and located ~10Kilobases upstream of the transcriptional start site of the SNCA gene, were associated with an increased risk of sporadic PD [40]. Subsequent analyses trying to replicate this finding were not always successful. In 2006 however, a large study that included 2,692 PD cases and 2,652 controls and incorporated previous analyses but also added novel data revealed compelling evidence that NACP-Rep1 was associated to PD risk [41]. The biological link between this microsatellite and PD has been supported to some degree by functional data, which suggest that α-synuclein levels could be influenced by NACP-Rep1 alleles through a regulation of SNCA gene transcription [42]. The role of SNCA in sporadic forms of PD has been fueled by different genome-wide association studies. These analyses indicate that common polymorphisms located downstream of the gene (more than 125 Kb away from the NACP-Rep1 microsatellite) might also contribute to PD risk [43-45]. Whether the NACP-Rep1 variant, located upstream of SNCA, or biallelic polymorphisms located downstream of the gene are independent association signals is still a matter of controversy [46, 47].
GBA Mutations and PD Risk
Homozygous mutations in the glucocerbrosidase (GBA) gene lead to Gaucher disease, the most common lysosomal storage disorder. The presence of progressive parkinsonian features in some patients with Gaucher disease was a key element in identifying mutations in GBA as an important risk factor for PD. Since the first discovery that resulted from the analysis of Ashkenazi Jewish patients, [48] many studies worldwide have replicated this finding [49-54]. In 2009, a multicentric international analysis that included 5,691 patients and 4,898 controls indicated that GBA mutations may be present in 3%-10% of PD patients from a non-Ashkenazi Jewish origin, and carriers of a GBA mutations have a fivefold increased risk to develop PD compared to noncarriers [55]. The clinical phenotype associated to a GBA mutation is indistinguishable from idiopathic PD. However, bradykinesia, resting tremor, rigidity and symmetric onset have been reported to be more frequent features in patients carrying a GBA mutant allele. A remarkable characteristic in patients harboring a GBA risk allele is the greater prevalence of cognitive decline and dementia during PD course [52, 56, 57], and carriers might have a six-fold increased risk to dementia compared to noncarriers [58].
MAPT H1 Haplotype and PD Risk
In 2002, the first genetic association between MAPT H1 halpotype (an extended haplotype that results from a common genomic inversion of approximately 800 kb in the large arm of chromosome 17 containing the MAPT gene) and PD risk was reported through a limited number of cases and controls [59]. With more than 20 studies performed to date, MAPT seems to be undoubtedly associated with PD risk in populations with European ancestry but not in Asians (http://www.pdgene.org) [60]. The MAPT H1 haplotype is present in all human populations, whereas the inverted haplotype (named H2) is mainly found in southwest Asian and southern European populations, with frequencies ranging from 21% to 32% [61, 62]. Interestingly, a longitudinal study performed in 2003 revealed that PD patients with the H1 variant followed for 3.5 years had a greater risk of cognitive decline than noncarriers [63]. A subsequent comprehensive analysis from same authors concluded that the MAPT H1 variant was the strongest independent predictor of dementia among PD patients, with an odds ratio of 12 over 5 years of follow-up [64]. Data originated in our and other's centers have provided compelling evidence that the MAPT H1 variant leads to an increased risk of cognitive decline and dementia in PD patients [65-67].
3. GENES WITH LOW RISK EFFECTS: RESULTS FROM GENOME WIDE ASSOCIATION STUDIES (GWAS)
In 2005 the first genome-wide association study of PD was performed in 443 sibling pairs discordant for PD, and a second tier of 332 patients and controls [68]. In 2006 a subsequent study that included 537 samples was not able to replicate previous findings and proposed that there are no common genetic variants with high risk effects on PD [69]. Three years later, two studies from Japan and Europe used more powerful sample sizes that evidenced the association with SNCA, MAPT, LRRK2, and disclosed novel loci in chromosome 1 (PARK16) and chromosome 4p15 (close to the BST1 gene) [70, 71]. In that same year, a similar study comprising 857 PD patients with a family history of PD and a similar number of controls disclosed a 112 kb region on the short arm of chromosome 4 that contained the genes GAK and DGKQ [72]. Additional analyses with similar sample sizes combined with data released from previous studies supported these genetic associations, [73-76] and yielded novel loci, like the human leukocyte antigen (HLA) region in choromosme 6p21.3 (which was designated PARK18) [74, 75]. In 2011 a meta-analysis of datasets from five PD GWAS was conducted by the International Parkinson's Disease Genomics Consortium (IPDGC). This study, which included a discovery phase with 5,333 cases and 12,019 controls, and a replication phase consisting of 7,053 cases and 9,007 controls, revealed five novel loci (ACMSD, STK39, MCC1/LAMP3, SYT11, and CCDC62/H1P1R) with subtle but significant risk effects [45]. A follow-up analysis performed by the same consortium revealed five additional PD risk loci that comprised the previously detected region in chromosome 1q32 (PARK16) and variants located close to the genes STBD1, GPNMB, FGF20, and STX1B [77]. Together with this study, an analysis performed by the Personal genetics company 23andMe, that incorporated over 3,400 cases and 29,000 controls, confirmed the outcomes from the IPDGC and yielded significant signals near SREBF1/RAI1 and SCARB2 genes [43]. Most recently, a meta-analysis was performed from data at the freely available online database "PDGene" (http://www.pdgene.org), which contains a comprehensive collection of all public genetic association studies performed on PD. It confirmed the existence of eleven loci previously shown to increase or decrease PD risk and revealed a novel association with an intronic polymorphism in ITGA8 gene on chromosome 10p13 [44].
4. MOLECULAR GENETIC TESTING
Up to 40% of PD patients with age at onset of less than 30 years and 17% of those with age at onset of less than 50 yeas will probably have a mutation in one of the known Mendelian genes linked to PD [78]. Therefore, genetic testing for diagnostic purposes in families with a Mendelian aggregation of disease is a very powerful tool and may be appropriate in many instances. Due to the increasing number of genes related to PD, genetic diagnostic process can be long, expensive and complex using classic Sanger-based sequencing approaches. The advent of next-generation sequencing (NGS) tools will allow a rapid, efficient and cost-effective process to test for genetic alterations in genetic forms of PD. Implementing NGS in diagnostic services, however, can be challenging. First, the large amount of data resulting from NGS can complicate the interpretation of pathogenic variants. Second, large gene dosage alterations (such as deletions and insertions) can be missed with NGS technologies. Finally, genomic regions with enriched G-C stretches are typically poorly captured and therefore mutations within these regions could be omitted. In any case, although genetic testing can be conducted in a successful and widely available manner, they should be performed in a multidisciplinary setting supported by personnel with expertise in this area. The reason for this multifaceted approach in genetic counseling is the possible ethical, social, psychological and legal consequences of a potential positive result from the test.
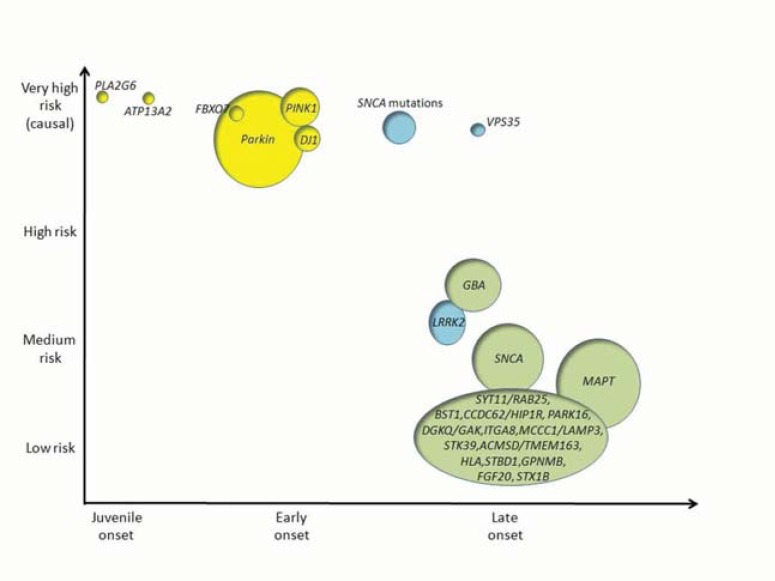
Genetic counseling and risk evaluation in sporadic, late onset PD, however, is likely of limited clinical utility at the present time. Predictive accuracy is poor due to the small effect sizes of genetic variants that have been associated with PD risk. As most of these genetic variants only explain a small proportion of the disease (Fig. 1), caution should be taken when interpreting this kind of genetic data for counseling purposes.
Fig. (1).
Schematic representation of the genetic architecture of PD. The Y axis shows the strength of genetic effects and the X axis indicates the age at onset of disease. Autosomal recessive genes are presented in yellow; autosomal dominant genes in blue and risk loci in green. The size of each circle is an approximation of the population attributable risk (PAR), that is, the proportion of PD that is ascribable to a mutation or a genetic risk variant at any gene. Percentages of PAR in genetic risk loci are based on references 56, 59 and 79. For genes related to monogenic forms of disease (PLA2G6, ATP13A2, FBOX7, PARK2, PINK1, DJ1, SNCA and VPS35) % of PAR have limited value due to the rarity of the deleterious alleles in the population and therefore the circle size represents an approximation of the % of cases due to known genetic causes. Relative risks for each loci are based on evidence from the PD gene database (www.pdgene.org).
The determination of genetic causes contributing to the modification of disease (modifier genes) is of extraordinary importance in terms of clinical follow up and management. GBA mutation or MAPT H1 allele status, for example, might be an independent risk factor for cognitive impairment in patients with PD and could therefore have strong implications in the disease course and therapeutic treatments [52, 56, 58, 64, 65].
Further insights into the genetic causes of PD are warranted, and an overwhelming amount of new data will probably come to light in coming years. How these data are handled in terms of genetic counseling and therapeutic interventions is a major responsibility that governments, scientists, the biotechnology industry, and civil society must approach with sensitivity, objectivity and rigor.
ACKNOWLEDGEMENTS
We would like to thank Carolyn Newey for editorial help.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Polymeropoulos MH, Hurko O, Hsu F, Rubenstein J, Basnet S, Lane K, Dietz H, Spetzler RF, Rigamonti D. Linkage of the locus for cerebral cavernous hemangiomas to human chromosome 7q in four families of Mexican-American descent. Neurology. 1997;48(3): 752–757. doi: 10.1212/wnl.48.3.752. [DOI] [PubMed] [Google Scholar]
- 2.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat. Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 3.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 4.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 5.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 6.Kann M, Jacobs H, Mohrmann K, Schumacher K, Hedrich K, Garrels J, Wiegers K, Schwinger E, Pram-staller PP, Breakefield XO, Ozelius LJ, Vieregge P, Klein C. Role of parkin mutations in 111 community-based patients with early-onset parkinsonism. Ann. Neurol. 2002;51(5): 621–625. doi: 10.1002/ana.10179. [DOI] [PubMed] [Google Scholar]
- 7.Mellick GD, Siebert GA, Funayama M, Buchanan DD, Li Y, Imamichi Y, Yoshino H, Silburn PA, Hattori N. Screening PARK genes for mutations in early-onset Parkinson's disease patients from Queensland, Australia. Parkinsonism Relat. Disord. 2009;15(2): 105–109. doi: 10.1016/j.parkreldis.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denefle P, Wood NW, Agid Y, Brice A. Association between early-onset Parkinson's disease and mutations in the parkin gene. N. Engl. J. Med. 2000;342(21): 1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 9.Gouider-Khouja N, Larnaout A, Amouri R, Sfar S, Belal S, Ben Hamida C, Ben Hamida M, Hattori N, Mizuno Y, Hentati F. Autosomal recessive parkinsonism linked to parkin gene in a Tunisian family.Cliical genetic and pathological study. Parkinsonism Relat. Disord. 2003; 9(5): 247–251. doi: 10.1016/s1353-8020(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 10.Pramstaller PP, Schlossmacher MG, Jacques TS, Scaravilli F, Eskelson C, Pepivani I, Hedrich K, Adel S, Gonzales-McNeal M, Hilker R, Kramer PL, Klein C. Lewy body Parkinson's disease in a large pedigree with 77 Parkin mutation carriers. Ann. Neurol. 2005;58(3): 411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi H, Ohama E, Suzuki S, Horikawa Y, Ishikawa A, Morita T, Tsuji S, Ikuta F. Familial juvenile parkinsonism: clinical and pathologic study in a family. Neurology. 1994;44(3 Pt 1):437–441. doi: 10.1212/wnl.44.3_part_1.437. [DOI] [PubMed] [Google Scholar]
- 12.Farrer M, Chan P, Chen R, Tan L, Lincoln S, Hernandez D, Forno L, Gwinn-Hardy K, Petrucelli L, Hussey J, Singleton A, Tanner C, Hardy J, Langston JW. Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 2001;50(3):293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki S, Shirata A, Yamane K, Iwata M. Parkin-positive autosomal recessive juvenile Parkinsonism with alpha-synuclein-positive inclusions. Neurology. 2004;63(4):678–682. doi: 10.1212/01.wnl.0000134657.25904.0b. [DOI] [PubMed] [Google Scholar]
- 14.Mori H, Kondo T, Yokochi M, Matsumine H, Nakagawa-Hattori Y, Miyake T, Suda K, Mizuno Y. Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology. 1998;51(3): 890–892. doi: 10.1212/wnl.51.3.890. [DOI] [PubMed] [Google Scholar]
- 15.Foroud T, Uniacke SK, Liu L, Pankratz N, Rudolph A, Halter C, Shults C, Marder K, Conneally PM, Nichols WC. Heterozygosity for a mutation in the parkin gene leads to later onset Parkinson disease. Neurology. 2003;60(5):796–801. doi: 10.1212/01.wnl.0000049470.00180.07. [DOI] [PubMed] [Google Scholar]
- 16.Hedrich K, Marder K, Harris J, Kann M, Lynch T, Meija-Santana H, Pramstaller PP, Schwinger E, Bressman SB, Fahn S, Klein C. Evaluation of 50 probands with early-onset Parkinson's disease for Parkin mutations. Neurology. 2002;58(8):1239–1246. doi: 10.1212/wnl.58.8.1239. [DOI] [PubMed] [Google Scholar]
- 17.Lohmann E, Periquet M, Bonifati V, Wood NW, De Michele G, Bonnet AM, Fraix V, Broussolle E, Horstink MW, Vidailhet M, Verpillat P, Gasser T, Nicholl D, Teive H, Raskin S, Rascol O, Destee A, Ruberg M, Gasparini F, Meco G, Agid Y, Durr A, Brice A. How much phenotypic variation can be attributed to parkin genotype?. Ann. Neurol. 2003;54(2): 176–185. doi: 10.1002/ana.10613. [DOI] [PubMed] [Google Scholar]
- 18.Lucking CB, Chesneau V, Lohmann E, Verpillat P, Dulac C, Bonnet AM, Gasparini F, Agid Y, Durr A, Brice A. Coding polymorphisms in the parkin gene and susceptibility to Parkinson disease. Arch. Neurol. 2003;60(9):1253–1256. doi: 10.1001/archneur.60.9.1253. [DOI] [PubMed] [Google Scholar]
- 19.Hilker R, Klein C, Ghaemi M, Kis B, Strotmann T, Ozelius LJ, Lenz O, Vieregge P, Herholz K, Heiss WD, Pramstaller PP. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial parkinsonism associated with mutations in the parkin gene. Ann. Neurol. 2001;49(3): 367–376. [PubMed] [Google Scholar]
- 20.Khan NL, Valente EM, Bentivoglio AR, Wood NW, Albanese A, Brooks DJ, Piccini P. Clinical and subclinical dopaminergic dysfunction in PARK6-linked parkinsonism: an 18F-dopa PET study. Ann. Neurol. 2002;52(6): 849–853. doi: 10.1002/ana.10417. [DOI] [PubMed] [Google Scholar]
- 21.Kumazawa R, Tomiyama H, Li Y, Imamichi Y, Funayama M, Yoshino H, Yokochi F, Fukusako T, Take-hisa Y, Kashihara K, Kondo T, Elibol B, Bostantjopoulou S, Toda T, Takahashi H, Yoshii F, Mizuno Y, Hattori N. Mutation analysis of the PINK1 gene in 391 patients with Parkinson disease. Arch. Neurol. 2008;65(6): 802–808. doi: 10.1001/archneur.65.6.802. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara-Paul L, Hulihan MM, Kachergus J, Upmanyu R, Warren L, Amouri R, Elango R, Prinjha RK, Soto A, Kefi M, Zouari M, Sassi SB, Yahmed SB, El Euch-Fayeche G, Matthews PM, Middleton LT, Gibson RA, Hentati F, Farrer MJ. PINK1 mutations and parkinsonism. Neurology. 2008;71(12): 896–902. doi: 10.1212/01.wnl.0000323812.40708.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifati V, Rohe CF, Breedveld GJ, Fabrizio E, De Mari M, Tassorelli C, Tavella A, Marconi R, Nicholl DJ, Chien HF, Fincati E, Abbruzzese G, Marini P, De Gaetano A, Horstink MW, Maat-Kievit JA, Sampaio C, Antonini A, Stocchi F, Montagna P, Toni V, Guidi M, Dalla Libera A, Tinazzi M, De Pandis F, Fabbrini G, Goldwurm S, de Klein A, Barbosa E, Lopiano L, Martignoni E, Lamberti P, Vanacore N, Meco G, Oostra BA. Early-onset parkinsonism associated with PINK1 mutations: frequency, genotypes, and phenotypes. Neurology. 2005;65(1): 87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- 24.Annesi G, Savettieri G, Pugliese P, D'Amelio M, Tarantino P, Ragonese P, La Bella V, Piccoli T, Civitelli D, Annesi F, Fierro B, Piccoli F, Arabia G, Caracciolo M, Ciro Candiano IC, Quattrone A. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann. Neurol. 2005;58(5): 803–807. doi: 10.1002/ana.20666. [DOI] [PubMed] [Google Scholar]
- 25.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44(4): 595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4): 601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Rubio JP, Topp S, Warren L, StJean PL, Wegmann D, Kessner D, Novembre J, Shen J, Fraser D, Aponte J, Nangle K, Cardon LR, Ehm MG, Chissoe SL, Whittaker JC, Nelson MR, Mooser VE. Deep sequencing of the LRRK2 gene in 14 002 individuals reveals evidence of purifying selection and independent origin of the p.rg1628Pro mutation in. Europe. Hum. Mutat. 33(7):1087–1098. doi: 10.1002/humu.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7(7): 583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.al-Din SN, Anderson M, Eeg-Olofsson O, Trontelj JV. Neuro-ophthalmic manifestations of the syndrome of ophthalmoplegia, ataxia and areflexia: a review. Acta Neurol. Scand. 1994;89(3): 157–163. doi: 10.1111/j.1600-0404.1994.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 30.Hampshire DJ, Roberts E, Crow Y, Bond J, Mubaidin A, Wriekat AL, Al-Din A, Woods CG. Kufor-Rakeb syndrome, pallido-pyramidal degeneration with supranuclear upgaze paresis and dementia, maps to 1p36. J. Med. Genet. 2001;38(10): 680–682. doi: 10.1136/jmg.38.10.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 2006;38(10): 1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 32.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J, Canham N, Nardocci N, Zorzi G, Pasha S, Rodriguez D, Desguerre I, Mubaidin A, Bertini E, Trembath RC, Simonati A, Schanen C, Johnson CA, Levinson B, Woods CG, Wilmot B, Kramer P, Gitschier J, Maher ER, Hayflick SJ. PLA2G6 encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat. Genet. 2006;38(7): 752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, Philip SG, Hendriksz C, Morton JE, Kingston HM, Rosser EM, Wassmer E, Gissen P, Maher ER. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN). Neurology. 2008;70(18): 1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 34.Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H, Singleton A, Schneider SA. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann. Neurol. 2009;65(1):19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shojaee S, Sina F, Banihosseini SS, Kazemi MH, Kalhor R, Shahidi GA, Fakhrai-Rad H, Ronaghi M, Elahi E. Genome-wide linkage analysis of a Parkinsonian-pyramidal syndrome pedigree by 500 K SNP arrays. Am. J. Hum. Genet. 2008;82(6): 1375–1384. doi: 10.1016/j.ajhg.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Fonzo A, Dekker MC, Montagna P, Baruzzi A, Yonova EH, Correia Guedes L, Szczerbinska A, Zhao T, Dubbel-Hulsman LO, Wouters CH, de Graaff E, Oyen WJ, Simons EJ, Breedveld GJ, Oostra BA, Horstink MW, Bonifati V. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology. 2009;72(3): 240–245. doi: 10.1212/01.wnl.0000338144.10967.2b. [DOI] [PubMed] [Google Scholar]
- 37.Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, Schneider SA, Schwingenschuh P, Bajaj N, Emre M, Singleton AB, Hardy J, Bhatia KP, Brandner S, Lees AJ, Houlden H. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov. Disord. 2010;25(12): 1791–1800. doi: 10.1002/mds.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89(1): 162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brucke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 2011;89(1): 168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruger R, Vieira-Saecker AM, Kuhn W, Berg D, Muller T, Kuhnl N, Fuchs GA, Storch A, Hungs M, Woitalla D, Przuntek H, Epplen JT, Schols L, Riess O. Increased susceptibility to sporadic Parkinson's disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann. Neurol. 1999;45(5): 611–617. doi: 10.1002/1531-8249(199905)45:5<611::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 41.Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296(6): 661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 42.Chiba-Falek O, Nussbaum RL. Effect of allelic variation at the NACP-Rep1 repeat upstream of the alpha-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum. Mol. Genet. 2001;10(26): 3101–3109. doi: 10.1093/hmg/10.26.3101. [DOI] [PubMed] [Google Scholar]
- 43.Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston JW, Wojcicki A, Eriksson N. Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson's disease. PLoS Genet. 2011;7(6): e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM, Schjeide LM, Meissner E, Zauft U, Allen NC, Liu T, Schilling M, Anderson KJ, Beecham G, Berg D, Biernacka JM, Brice A, DeStefano AL, Do CB, Eriksson N, Factor SA, Farrer MJ, Foroud T, Gasser T, Hamza T, Hardy JA, Heutink P, Hill-Burns EM, Klein C, Latourelle JC, Maraganore DM, Martin ER, Martinez M, Myers RH, Nalls MA, Pankratz N, Payami H, Satake W, Scott WK, Sharma M, Singleton AB, Stefansson K, Toda T, Tung JY, Vance J, Wood NW, Zabetian CP, Young P, Tanzi RE, Khoury MJ, Zipp F, Lehrach H, Ioannidis JP, Bertram L. Comprehensive research synopsis and systematic meta-analyses in Parkinson's disease genetics: The PDGene database. PLoS Genet. 2012;8(3):e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377(9766): 641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mata IF, Shi M, Agarwal P, Chung KA, Edwards KL, Factor SA, Galasko DR, Ginghina C, Griffith A, Higgins DS, Kay DM, Kim H, Leverenz JB, Quinn JF, Roberts JW, Samii A, Snapinn KW, Tsuang DW, Yearout D, Zhang J, Payami H, Zabetian CP. SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch. Neurol. 2010;67(11): 1350–1356. doi: 10.1001/archneurol.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myhre R, Toft M, Kachergus J, Hulihan MM, Aasly JO, Klungland H, Farrer MJ. Multiple alpha-synuclein gene polymorphisms are associated with Parkinson's disease in a Norwegian population. Acta Neurol. Scand. 2008;118(5): 320–327. doi: 10.1111/j.1600-0404.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 48.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N. Engl. J. Med. 2004;351(19):1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 49.Bras J, Paisan-Ruiz C, Guerreiro R, Ribeiro MH, Morgadinho A, Januario C, Sidransky E, Oliveira C, Singleton A. Complete screening for glucocerebrosidase mutations in Parkinson disease patients from Portugal. Neurobiol. Aging. 2009;30(9): 1515–1517. doi: 10.1016/j.neurobiolaging.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L. Complete screening for glu-cocerebrosidase mutations in Parkinson disease patients from Greece. Neurosci. Lett. 2009;452(2): 87–89. doi: 10.1016/j.neulet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 51.Mao XY, Burgunder JM, Zhang ZJ, An XK, Zhang JH, Yang Y, Li T, Wang YC, Chang XL, Peng R. Association between GBA L444P mutation and sporadic Parkinson's disease from Mainland China. Neurosci. Lett. 2010;469(2):256–259. doi: 10.1016/j.neulet.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132 (Pt 7):1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spitz M, Rozenberg R, Pereira Lda V, Reis Barbosa E. Association between Parkinson's disease and gluco-cerebrosidase mutations in Brazil. Parkinsonism Relat. Disord. 2008;14(1): 58–62. doi: 10.1016/j.parkreldis.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Toft M, Pielsticker L, Ross OA, Aasly JO, Farrer MJ. Glucocerebrosidase gene mutations and Parkinson disease in the Norwegian population. Neurology. 2006;66(3): 415–417. doi: 10.1212/01.wnl.0000196492.80676.7c. [DOI] [PubMed] [Google Scholar]
- 55.Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N. Engl. J. Med. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe Reilly M, Ruiz D, Ross B, Verbitsky M, Kisselev S, Louis E, Comella C, Colcher A, Jennings D, Nance M, Bressman S, Scott WK, Tanner C, Mickel S, Andrews H, Waters C, Fahn S, Cote L, Frucht S, Ford B, Rezak M, Novak K, Friedman JH, Pfeiffer R, Marsh L, Hiner B, Siderowf A, Payami H, Molho E, Factor S, Ottman R, Clark LN, Marder K. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78(18): 1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, Duran R, Mencacci NE, Sawcer SJ, Barker RA. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community-based incident cohort. Brain. 2013;136(Pt 2): 392–399. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- 58.Seto-Salvia N, Pagonabarraga J, Houlden H, Pascual-Sedano B, Dols-Icardo O, Tucci A, Paisan-Ruiz C, Campolongo A, Anton-Aguirre S, Martin I, Munoz L, Bufill E, Vilageliu L, Grinberg D, Cozar M, Blesa R, Lleo A, Hardy J, Kulisevsky J, Clarimon J. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson's disease course. Mov. Disord. 2012;27(3): 393–399. doi: 10.1002/mds.24045. [DOI] [PubMed] [Google Scholar]
- 59.Farrer M, Skipper L, Berg M, Bisceglio G, Hanson M, Hardy J, Adam A, Gwinn-Hardy K, Aasly J. The tau H1 haplotype is associated with Parkinson's disease in the Norwegian population. Neurosci. Lett. 2002;322(2): 83–86. doi: 10.1016/s0304-3940(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 60.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y, Toda T. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 2009;41(12): 1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 61.Donnelly MP, Paschou P, Grigorenko E, Gurwitz D, Mehdi SQ, Kajuna SL, Barta C, Kungulilo S, Karoma NJ, Lu RB, Zhukova OV, Kim JJ, Comas D, Siniscalco M, New M, Li P, Li H, Manolopoulos VG, Speed WC, Rajeevan H, Pakstis AJ, Kidd JR, Kidd KK. The distribution and most recent common ancestor of the 17q21 inversion in humans. Am. J. Hum. Genet. 2010;86(2): 161–171. doi: 10.1016/j.ajhg.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Evans W, Fung HC, Steele J, Eerola J, Tienari P, Pittman A, Silva R, Myers A, Vrieze FW, Singleton A, Hardy J. The tau H2 haplotype is almost exclusively Caucasian in origin. Neurosci. Lett. 2004;369(3): 183–185. doi: 10.1016/j.neulet.2004.05.119. [DOI] [PubMed] [Google Scholar]
- 63.Goris A, Williams-Gray CH, Clark GR, Foltynie T, Lewis SJ, Brown J, Ban M, Spillantini MG, Compston A, Burn DJ, Chinnery PF, Barker RA, Sawcer SJ. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann. Neurol. 2007;62(2):145–153. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- 64.Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132 (Pt 11): 2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 65.Seto-Salvia N, Clarimon J, Pagonabarraga J, Pascual-Sedano B, Campolongo A, Combarros O, Mateo JI, Regana D, Martinez-Corral M, Marquie M, Alcolea D, Suarez-Calvet M, Molina-Porcel L, Dols O, Gomez-Isla T, Blesa R, Lleo A, Kulisevsky J. Dementia risk in Parkinson disease: disentangling the role of MAPT haplotypes. Arch. Neurol. 2011;68(3): 359–364. doi: 10.1001/archneurol.2011.17. [DOI] [PubMed] [Google Scholar]
- 66.Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry. 2013;84(11): 1258–64. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 67.Morley JF, Xie SX, Hurtig HI, Stern MB, Colcher A, Horn S, Dahodwala N, Duda JE, Weintraub D, Chen-Plotkin AS, Van Deerlin V, Falcone D, Siderowf A. Genetic influences on cognitive decline in Parkinson's disease. Mov. Disord. 2012;27(4): 512–518. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, Pant PV, Frazer KA, Cox DR, Ballinger DG. High-resolution whole-genome association study of Parkinson disease. Am. J. Hum. Genet. 2005;77(5): 685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fung HC, Scholz S, Matarin M, Simon-Sanchez J, Hernandez D, Britton A, Gibbs JR, Langefeld C, Stiegert ML, Schymick J, Okun MS, Mandel RJ, Fernandez HH, Foote KD, Rodriguez RL, Peckham E, De Vrieze FW, Gwinn-Hardy K, Hardy JA, Singleton A. Genome-wide genotyping in Parkinson's disease and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 2006;5(11): 911–916. doi: 10.1016/S1474-4422(06)70578-6. [DOI] [PubMed] [Google Scholar]
- 70.Kruger R, Sharma M, Riess O, Gasser T, Van Broeckhoven C, Theuns J, Aasly J, Annesi G, Bentivoglio AR, Brice A, Djarmati A, Elbaz A, Farrer M, Ferrarese C, Gibson JM, Hadjigeorgiou GM, Hattori N, Ioannidis JP, Jasinska-Myga B, Klein C, Lambert JC, Lesage S, Lin JJ, Lynch T, Mellick GD, de Nigris F, Opala G, Prigione A, Quattrone A, Ross OA, Satake W, Silburn PA, Tan EK, Toda T, Tomiyama H, Wirdefeldt K, Wszolek Z, Xiromerisiou G, Maraganore DM. A large-scale genetic association study to evaluate the contribution of Omi/HtrA2 (PARK13) to Parkinson's disease. Neurobiol. Aging. 2011;32(3): 548 e549–518. doi: 10.1016/j.neurobiolaging.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, van der Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 2009;41(12): 1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC, Foroud T, Myers RH. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum. Genet. 2009;124(6): 593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW, Wang L, Zuchner S, Konidari I, Wang G, Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J, Vance JM, Martin ER. Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 2010;74(2): 97–109. doi: 10.1111/j.1469-1809.2009.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Mon-timurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42(9): 781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simon-Sanchez J, van Hilten JJ, van de Warrenburg B, Post B, Berendse HW, Arepalli S, Hernandez DG, de Bie RM, Velseboer D, Scheffer H, Bloem B, van Dijk KD, Rivadeneira F, Hofman A, Uitterlinden AG, Rizzu P, Bochdanovits Z, Singleton AB, Heutink P. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur. J. Hum. Genet. 2011;19(6): 655–661. doi: 10.1038/ejhg.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spencer CC, Plagnol V, Strange A, Gardner M, Paisan-Ruiz C, Band G, Barker RA, Bellenguez C, Bhatia K, Blackburn H, Blackwell JM, Bramon E, Brown MA, Burn D, Casas JP, Chinnery PF, Clarke CE, Corvin A, Craddock N, Deloukas P, Edkins S, Evans J, Freeman C, Gray E, Hardy J, Hudson G, Hunt S, Jankowski J, Langford C, Lees AJ, Markus HS, Mathew CG, McCarthy MI, Morrison KE, Palmer CN, Pearson JP, Peltonen L, Pirinen M, Plomin R, Potter S, Rautanen A, Sawcer SJ, Su Z, Trembath RC, Viswanathan AC, Williams NW, Morris HR, Donnelly P, Wood NW. Dissection of the genetics of Parkinson's disease identifies an additional association 5' of SNCA and multiple associated haplotypes at 17q21. Hum. Mol. Genet. 2011;20(2): 345–353. doi: 10.1093/hmg/ddq469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.A two-stage meta-analysis identifies several new loci for Parkinson's disease. PLoS Genet. 2011;7(6): e1002142. doi: 10.1371/journal.pgen.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Ross BM, Verbitsky M, Kisselev S, Louis ED, Comella C, Colcher A, Jennings D, Nance MA, Bressman SB, Scott WK, Tanner C, Mickel S, Andrews H, Waters C, Fahn S, Cote L, Frucht S, Ford B, Rezak M, Novak K, Friedman JH, Pfeiffer R, Marsh L, Hiner B, Siderowf A, Ottman R, Marder K, Clark LN. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch. Neurol. 2010;67(9): 1116–1122. doi: 10.1001/archneurol.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mata IF, Samii A, Schneer SH, Roberts JW, Griffith A, Leis BC, Schellenberg GD, Sidransky E, Bird TD, Leverenz JB, Tsuang D, Zabetian CP. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch. Neurol. 2008;65(3): 379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]