Background: Insulin-like growth factor receptor-1 (IGF-1R) survival signal maintains caspase-3 at a low level of activity required for its non-apoptotic role in initiating lens cell differentiation.
Results: α6 integrin transactivates IGF-1R through a Src signal to regulate the caspase-3 differentiation pathway.
Conclusion: α6 integrin is necessary for non-apoptotic caspase-3 function in differentiation.
Significance: This is the first mechanistic evidence showing α6 integrin signals differentiation through affecting a cell survival pathway.
Keywords: Cell Differentiation, Development, Integrins, Lens, Signal Transduction
Abstract
The canonical mitochondrial death pathway was first discovered for its role in signaling apoptosis. It has since been found to have a requisite function in differentiation initiation in many cell types including the lens through low level activation of the caspase-3 protease. The ability of this pathway to function as a molecular switch in lens differentiation depends on the concurrent induction of survival molecules in the Bcl-2 and IAP families, induced downstream of an IGF-1R/NFκB coordinate survival signal, to regulate caspase-3 activity. Here we investigated whether α6 integrin signals upstream to this IGF-1R-mediated survival-linked differentiation signal. Our findings show that IGF-1R is recruited to and activated specifically in α6 integrin receptor signaling complexes in the lens equatorial region, where lens epithelial cells initiate their differentiation program. In studies with both α6 integrin knock-out mice lenses and primary lens cell cultures following α6 integrin siRNA knockdown, we show that IGF-1R activation is dependent on α6 integrin and that this transactivation requires Src kinase activity. In addition, without α6 integrin, activation and expression of NFκB was diminished, and expression of Bcl-2 and IAP family members were down-regulated, resulting in high levels of caspase-3 activation. As a result, a number of hallmarks of lens differentiation failed to be induced; including nuclear translocation of Prox1 in the differentiation initiation zone and apoptosis was promoted. We conclude that α6 integrin is an essential upstream regulator of the IGF-1R survival pathway that regulates the activity level of caspase-3 for it to signal differentiation initiation of lens epithelial cells.
Introduction
Precise regulation of caspase-3 activation (low level caspase-3 activity), induced through the canonical mitochondrial death pathway, allows caspase-3 to play a non-apoptotic role in a variety of cellular functions (1–4). This mechanism is in contrast to the high levels of caspase-3 activity, which induces apoptosis under many conditions including the cellular response to various apoptogens (5–7), the proper elimination of cells during development (8–11), and following the loss of cell survival-signaling pathways (12). High levels of caspase-3 activity resulting from the loss of an insulin-like growth factor receptor-1 (IGF-1R)2 survival-signaling pathway abrogates the non-apoptotic role of caspase-3 in promoting cell differentiation and pushes cell fate toward death (13). Growth factor receptor signaling required for cell survival, differentiation, and migration has often been linked to coordinated signaling with integrin receptors (14–18). Although direct inhibition of either integrin receptors or growth factor receptors can induce high levels of caspase-3 activity to cause cell death by apoptosis (13, 19), it was still unknown if an integrin-mediated growth factor receptor survival signal acts to regulate the canonical “mitochondrial death pathway” for its non-apoptotic functions in normal cellular processes such as cell differentiation.
Our studies have shown that lens epithelial cell differentiation initiation is dependent on low levels of caspase-3 activity (2). This signaling mechanism is now known to be required for promoting cell fate toward differentiation in many other cell types including skeletal muscle cells (3), osteoblasts (20), and keratinocytes (21). In skeletal muscle cells the caspase-3-dependent differentiation initiation pathway has been linked to a genetic reprogramming event leading to the expression of the cell cycle inhibitor p21 (4). The discovery of non-apoptotic functions for low levels of caspase-3 activity illustrated the need to understand the pathways responsible for the tight regulation of the caspase-3 activation state. IGF-1R is a classical cell survival molecule that has been linked to signaling of differentiation and development (22–24). In studies with lens differentiation in our model we reveal that the regulation of caspase-3 activity for its role in signaling differentiation initiation depended on an IGF-1R/nuclear factor κB (NFκB) survival-signaling pathway (13). Interestingly, previous studies from our laboratory demonstrate that there is a coordinated role for α6 integrin and IGF-1R in lens development. These studies include the finding that IGF-1R becomes linked, in vivo, to α6 integrin signaling complexes in cells located in the equatorial epithelium, the region where lens epithelial cells initiate their differentiation (25). In addition, we find that there is a requisite role for α6 integrin in the induction of the lens fiber cell-differentiated phenotype (25). In this study we examined α6 integrin as a potential upstream regulator of the IGF-1R/NFκB survival signal that mediates the caspase-3 differentiation signal.
Although previous studies have independently linked α6 integrin to IGF-1R signaling (25, 26) and IGF-1R to NFκB (13, 27), to date there is no direct evidence connecting α6 integrin to the IGF-1R/NFκB signaling axis, or to a role in modulating the caspase-3 differentiation initiation signal. Although IGF-1R can be activated in α6 integrin signaling complexes (25, 26), the signaling effector that could mediate a transactivation event within this receptor complex has remained unknown. Src family kinases stand out as likely candidates, as this kinase family has been shown to be involved in integrin/growth factor receptor association and, for other integrins such as αVβ3, transactivation of the associated growth factor receptor (15, 28–30).
α6 integrin, a laminin receptor, has been linked to roles in cell survival, cell differentiation, and tissue development (31–36). Specific examples include roles for α6 integrin in oligodendrocyte survival (34), lamination of the retina and development of the central nervous system (37, 38), branching morphogenesis of the submandibular gland (39), as well as differentiation of lens epithelial cells (25). In cultured myoblasts, knockdown of α6 integrin blocks differentiation and promotes sustained proliferation (40). Of particular relevance to our finding that IGF-1R is recruited to α6 integrin signaling complexes as lens epithelial cells begin their differentiation (25) is the discovery that insulin-like growth factor-1 (IGF-1), the ligand for IGF-1R, can associate directly with α6 integrin (26). Together, these results support the possibility that a coordinate signaling mechanism involving both α6 integrin and IGF-1R is required for differentiation initiation in the developing lens.
IGF-1R activates transcription factor NFκB to induce expression of survival proteins in both the Bcl-2 and inhibitor of apoptosis (IAP) families (41), and α6 integrin also is linked to cell survival through its induction of NFκB (33). In the differentiating lens the IGF-1R/NFκB signaling axis induces expression of survival proteins Bcl-2, Bcl-XL, XIAP, and ch-IAP-1, maintaining caspase-3 protease activation at the low levels required for its role in signaling the differentiation initiation process in this tissue (13). However, the function of α6 integrin in regulating the caspase-3 differentiation signal has not yet been investigated. Here we examined this possibility in studies with both embryonic α6 integrin knock-out lenses and in a primary differentiating lens culture system that mimics differentiation in vivo following siRNA knockdown of α6 integrin. Lenses are able to form in the absence of α6 integrin, likely due to compensation by α3 integrin as the double α6/α3 integrin knock-out mouse fails to form normal lenses (43), but α6−/− lenses have not yet been examined for potential differentiation defects. These lenses proved ideal for identifying the dependence of the IGF-1R/NFκB differentiation-signaling pathway on α6 integrin function, mechanisms that were paralleled in studies of lens epithelial cells in primary culture. Our findings reported here show that α6 integrin is necessary for the expression and activation of both IGF-1R and NFκB, and their downstream effectors in the Bcl-2 and IAP families, to maintain caspase-3 at the low levels at which it induces lens epithelial cell differentiation initiation.
EXPERIMENTAL PROCEDURES
Generation of α6 Integrin Null Mice and Genotyping of Embryos
α6−/− mice were generated as described previously (38, 44) and genotypes of embryos were obtained by PCR (40). The official nomenclature of the α6 integrin mice is B6.129S-Itga6tmZP149 (Jackson Laboratories). The α6 mutant line was maintained by backcrossing α6 heterozygous mice on C57BL/6J background. The status of CP49, which is spontaneously mutated in several mouse strains, was analyzed by PCR as previously described, using genomic tail DNA from α6 animals (45, 46). Briefly, PCR was carried out in a final volume of 25 μl, in a reaction mixture containing 1× PCR buffer, 2.5 mm MgCl2, 0.1 mm dNTP mixture, 0.5 μm of each primer, 0.625 units of Taq DNA polymerase and 1 μl of tail DNA. Wild-type and mutant CP49 alleles were detected using primers e (5′-TTG GAA ACA ACC TCC AGA CCA GAG-3′)/c′ (5′-ACA TTC TAT TTC GAG GCA GGG TCC-3′) and c (5′-TGG GGT TGG GCT AGA AAT CTC AGA-3′)/e′ (5′-AGC CCC TAC GAC CTG ATT TTT GAG-3′), respectively. Tail DNA from strain 129 was used as positive control for the CP49 mutation. The following PCR program was used: 95 °C, 1 min; 35 cycles of 3 steps: 95, 68, and 72 °C for 30 s each; and a final elongation at 72 °C for 10 min.
Chick Embryo Lens Microdissection
Embryonic day 10 (E10) lenses were isolated from chicken embryos (B&E Eggs, York Springs, PA) and microdissected into four distinct differentiation state-specific regions as previously described (47): central anterior epithelium (EC), equatorial epithelium (EQ), cortical fiber (FP), and nuclear fiber (FC) zones (modeled in Fig. 1A). EC is comprised of undifferentiated lens epithelial cells; EQ, the zone of differentiation initiation; FP, the region of lens fiber cell morphogenesis; and FC, the zone of fiber cell maturation.
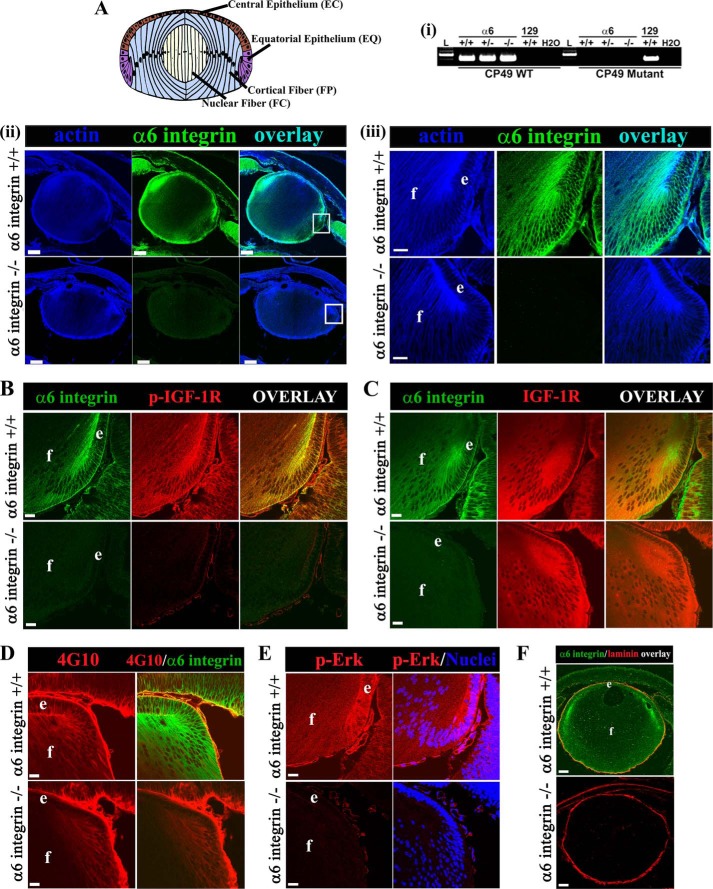
FIGURE 1.
Absence of α6 integrin in E18.5 mice lenses blocked by activation of IGF-1R, and suppressed IGF-1R signaling. A, lens model (top panel) showing the four distinct regions of lens differentiation including EC, undifferentiated lens epithelial cells; EQ cells, in the zone of differentiation initiation; FP, the region of lens fiber cell morphogenesis; and FC, the zone of fiber cell maturation and organelle loss. i, PCR analysis of CP49 WT and mutant alleles detected in genomic tail DNA isolated from representative WT (+/+), heterozygous (+/−), and mutant (−/−) α6 integrin embryos. Genomic tail DNA from a WT animal of the 129 line (which carries a spontaneous mutation of CP49) was used as positive control for detection of the CP49 mutant allele. L, DNA ladder. Results show that the C57BL/6 line used to prepare the α6 integrin knock-out does not carry the CP49 mutation. ii and iii and A–F, mid-sagittal lens cryosections from α6+/+ (wild type mice, upper panel) and α6−/− (knock-out mice, lower panel) at E18.5 (e, lens epithelium; f, lens fiber cells). A (ii and iii), sections were immunostained for α6 integrin (green) and co-labeled for F-actin (blue). A (ii), scale bar = 100 μm. Boxed areas shown at higher magnification in panel iii, scale bar = 20 μm. B–D and F, representative cryosections from E18.5 mice lenses were co-immunostained for α6 integrin (green) and p-IGF-1R (red) (B), IGF-1R (red) (C), phosphorylated-tyrosine residues (4G10, red) (D), or laminin-111 (red) (F). E, mid-sagittal lens cryosections from E18.5 mice were immunostained for phospho (activated) ERK (p-ERK, red) and co-labeled for nuclei (blue). Note that nuclear localization of active ERK observed in the α6+/+ mouse lens at E18.5 is distinct from that which occurs at E12.5, when it localized to the nuclei of differentiating primary lens fiber cells (42). All images were obtained by confocal microscopy. Images in A (ii and iii)–F, are single optical slices of 1-μm thickness selected from an acquired z-stack; B–E, scale bar = 20 μm. F, scale bar = 100 μm. Results show that in the absence of α6 integrin there was diminished activation of IGF-1R, moderate reduction in phosphotyrosine, and inhibition of ERK activation, a downstream effector of IGF-1R, whereas the lens capsule remained intact. Labeling of the lens capsule with the 4G10 antibody is nonspecific. Data are representative of three independent studies where lenses from 3 different α6+/+ or α6−/− mice embryos were examined.
Preparation of Primary Quail Lens Cell Cultures
Primary lens cell cultures that mimic differentiation as it occurs in vivo were prepared as described previously (48). Briefly, E9 quail lens cells were isolated by trypsinization followed by agitation, plated on laminin (Invitrogen), and cultured in Complete Medium (Medium 199 containing 10% fetal bovine serum, 1% penicillin and 1% streptomycin). For blocking activation of Src family kinases, cells were exposed to the Src family kinase-specific inhibitor PP1 (10 μm, Enzo Life Sciences, Farmingdale, NY) for 4 h. Controls were treated with the vehicle dimethyl sulfoxide. Cells were extracted in OG/T buffer for co-immunoprecipitation and immunoblot analysis.
siRNA Transfection
Lens epithelial cells in primary culture were transfected prior to differentiation initiation with either an avian-specific custom-made α6 integrin siRNA pool, or with the control ON-TARGET plus non-targeting siRNA pool (both from Dharmacon RNAi Technologies, Thermo Scientific). Before transfection, complete medium was replaced with Medium 199 devoid of antibiotics and serum. Cells were exposed overnight at 37 °C to either the α6 integrin-specific silencing siRNA (100 nm) or the non-silencing pool pre-mixed with Lipofectamine® 2000 transfection Reagent (Invitrogen), according to the manufacturer's protocol. The medium was changed to complete medium for the remainder of the study.
Antibodies
Antibodies to IGF-1R (sc-713), p-IGF-1R (sc-101704), for immunostaining, survivin (sc-10811), Bcl-2 (sc-492), NFκB p65 (sc-372), GAPDH (sc-25778), phospho-NFκB p65 Ser-276 (sc-101749), Bcl-XL/Xs (sc-1041), PARP-1 (sc-7150), c-IAP1/2 (equivalent to ch-IAP in chick), total Src (sc-18) and α6 integrin (sc-6596) were purchased from Santa Cruz Biotechnology, Inc. CD49f/GOH3 (number 555734) antibody was purchased from BD PharmingenTM. Antibody to phospho-IGF-1R ((pYpYpY1158/1162/1163) number 44-806G for immunoblotting) and phospho-Src number 44-660G were purchased from Invitrogen. β-Actin (A5441) and laminin-111 (L9393) were purchased from Sigma. Prox1 (ab37128) was purchased from Abcam. Antibodies to filensin and CP49 were received as generous gifts from Dr. Paul Fitzgerald (University of California, Davis, CA). Antibody to cleaved caspase-3 (number 9664), which recognizes the 17/19-kDa subunit produced by cleavage at Asp-175, was purchased from Cell Signaling. Antibody to X-IAP (number 610716) and E-cadherin (number 61082) was purchased from BD Transduction Laboratories. Antibody to phospho-ERK was purchased from Promega (V8031). Anti-Aquaporin-0 (AB3071) and 4G10 antibody (05-1050) were purchased from EMD Millipore. P2C62C4 mouse monoclonal antibody to chicken α6 integrin was obtained from the Developmental Studies Hybridoma Bank.
Immunostaining
Whole eyes isolated from α6+/+ and α6−/− mice were fixed in 3.7% formaldehyde solution for 18–24 h at 4 °C and then incubated in 30% sucrose solution prior to cryofreezing and cryosectioning. 20-μm thick cryosections were cut serially in the anterior to posterior direction. Sections were permeabilized with 0.25% Triton X-100 buffer for 10 min, blocked for 1 h in blocking buffer (5% goat serum, 1% BSA in PBS), and incubated sequentially in primary antibody at 37 °C followed by secondary antibody at 37 °C (1 h each). F-actin was labeled with Alexa 448-conjugated phalloidin (Invitrogen, number A12379) or Alexa 663-conjugated phalloidin (Invitrogen, number A22284). Nuclei were counterstained with TO-PRO-3 (Invitrogen, number T3605).
Immunoblotting
Microdissected lens tissue was extracted in Triton/octyl glucoside (OG/T) buffer (44.4 mm n-octyl β-d-glucopyranoside, 1% Triton X-100, 100 mm NaCl, 1 mm MgCl2, 5 mm EDTA, and 10 mm imidazole) containing 1 mm sodium vanadate, 0.2 mm H2O2, and protease inhibitor mixture (Sigma). Cultured lens cells were preincubated for 3 min in media with 1 mm sodium vanadate and 0.2 mm H2O2 to inhibit tyrosine phosphatase activity prior to extraction with the OG/T buffer. Protein concentration was determined using the BCA assay (Thermo Scientific). For direct immunoblots of whole cell lysates 30 μg of protein from each sample was subjected to SDS-PAGE on precast 8–16% Tris glycine gels (Novex, San Diego, CA). Proteins were electrophoretically transferred onto Immobilon-P (PVDF) membranes (Millipore Corp.) and membranes were blocked in 5% skim milk for 1 h. Membranes were probed for primary antibody at 4 °C overnight followed by secondary antibody-conjugated to horseradish peroxidase (BIO-RAD). Protein bands were detected using ECL reagent or ECL Plus reagent (Thermo Fisher Scientific). Images of immunoblots were acquired using the FluorChem E & M imager from Protein Simple (number FM0418), a digital darkroom technology. Densitometric analysis of immunoblots was performed using Alpha View® software (Protein Simple).
Immunoprecipitation Analysis
For each immunoprecipitation conducted with lens tissue study, 100 E10 lenses were microdissected into four regions of differentiation as described above and extracted with OG/T buffer. To determine the association of p-IGF-1R with α6 integrin, the entire EC, EQ, FP, and FC fractions were incubated with antibody to α6 integrin (4 °C overnight), followed by incubation with TrueBlot immunoprecipitation beads (eBiosciences) for 1 h as described previously (2). The immunoprecipitate was subjected to SDS-PAGE, transferred to Immobilon-P (PVDF) membranes, and association of p-IGF-1R with α6 integrin was determined using immunoblot techniques as described above. For the primary culture PP1 inhibitor studies, cells were extracted in OG/T buffer and 500 μg of protein was immunoprecipitated with α6 integrin following which the immunoprecipitates were subjected to SDS-PAGE and immunoblotted with antibodies to α6 integrin, p-Src, Src, p-IGF-1R, and IGF-1R.
TUNEL Assay and Quantification
TUNEL assay was performed to detect apoptotic cells in primary lens cell cultures and lens cryosections using the cell death detection kit (In Situ Cell Death Detection, TMR Red; Roche Molecular Biochemicals) according to the manufacturer's instructions. Nuclei were counterstained with TO-PRO-3 (Invitrogen). TUNEL and TO-PRO-3 staining was imaged by confocal microscopy. Quantification for the TUNEL assay in lens cell cultures was performed by analyzing four randomly selected fields from each sample over three independent studies, represented as the percent of total nuclei (stained with TO-PRO-3) that were TUNEL-positive.
Image Analysis
The Nikon Eclipse TE2000-U microscope (Optical Apparatus) was used to obtain phase images that were acquired using the Cool Snap HQ2 camera (Photometrics) with Image-Pro Plus software (Phase 3 Imaging Systems). Confocal microscopy was performed using the Zeiss LSM510 META confocal microscope. Single optical planes were selected from z-stacks, each 1 μm thick, unless otherwise indicated, using the LSM5 Image Browser.
Statistical Analysis
Statistical data analysis was performed with the t test on three or more independent experiments using SPSS statistics software. Error bars represent S.E. Differences were considered significant when (*) p ≤ 0.05.
RESULTS
IGF-1R Activation and Low Level Caspase-3 Activity in the Developing Lens Depends on α6 Integrin Function
To investigate whether α6 integrin is necessary for activation of IGF-1R in the zone of lens differentiation initiation we examined α6 integrin-null mouse lenses at E18.5, and WT age-matched controls. Mid-sagittal cryosections of whole eyes were immunolabeled with α6 integrin antibody to validate the absence of α6 integrin in the knock-out (KO) lenses, with an antibody to phospho-(p) IGF-1R to examine the activation state of IGF-1R, and co-labeled for F-actin to reveal the cytoarchitecture of the lens. In normal embryonic lenses α6 integrin was highly localized to lateral cell-cell borders of lens cells in the equatorial epithelial and cortical fiber zones (regions of lens cell differentiation of the embryonic lens (modeled in Fig. 1A)), in addition to the more classical localization of α6 integrin to basal aspects of lens cells where they contact the basement membrane (Fig. 1A, ii and iii), as we have described previously (47). No α6 integrin was detected in the KO lenses. Note that the mice used in this study are on a C57BL/6 background and do not carry a mutation for the lens-specific intermediate filament protein CP49 (phakinin) (Fig. 1A, i). Using a p-IGF-1R antibody that detects the major tyrosine phosphorylation site in the IGF-1R kinase domain required for the full activation of IGF-1R (49), we found that although IGF-1R activation was high in the equatorial zone of the WT E18.5 mouse lens where it co-localized with α6 integrin along lateral cell-cell borders, the IGF-1R activation state was significantly diminished in the embryonic α6−/− lens (Fig. 1B) without any detectable effect on IGF-1R expression (Fig. 1C). Immunolabeling with 4G10, which labels phosphotyrosine sites, revealed that the loss of IGF-1R activation did not reflect a general inhibition of tyrosine kinase activity in the absence of α6 integrin (Fig. 1D). However, activation of ERK, a well characterized downstream effector of IGF1R (25, 50, 51), was inhibited in the α6−/− mouse lenses (Fig. 1E). Immunofluorescence analysis for laminin-111, a major component of the lens basement membrane capsule (47), showed no gross effect of the loss of α6 integrin on distribution of this matrix protein in the lens capsule, to which the lens cells remained adherent (Fig. 1F).
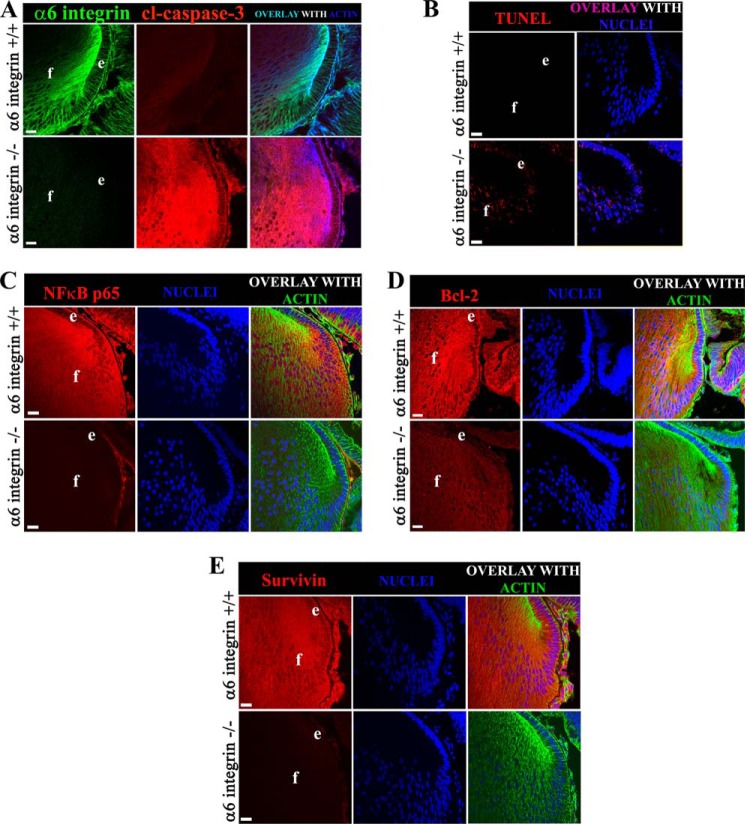
To examine whether α6 integrin is necessary to maintain the low levels of caspase-3 activation required for the role of this protease in signaling lens epithelial cell differentiation, cryosections of α6+/+ and α6−/− lenses were immunolabeled with an antibody to cleaved caspase-3 that recognizes the 17/19-kDa subunit produced by cleavage at Asp-175. Although in the α6+/+ lenses only low levels of activated caspase-3 were detected, the loss of α6 integrin resulted in high level caspase-3 activity (Fig. 2A). TUNEL assay (Fig. 2B) showed that this dysregulation of caspase-3 activity in the α6 KO lens leads to increased cell death by apoptosis in both lens epithelial and fiber cell zones. The TUNEL labeling of nuclei in the embryonic lenses of α6−/− mice lens was detected in the same population of cells that displayed high levels of cleaved caspase-3, suggesting a link between dysregulation of caspase-3 activity and induction of apoptosis. These findings show that α6 integrin is an upstream regulator of IGF-1R activation and that its function is required for maintaining the low level of caspase-3 activity in the developing lens.
FIGURE 2.
The absence of α6 integrin in the developing lens leads to low expression of NFκB and its target survival proteins resulting in high levels of caspase-3 activity and cell death by apoptosis. A–E, E18.5 lens cryosections from α6+/+ (upper panels) and α6−/− (lower panels) mice were co-labeled for: A, cleaved caspase-3 (red), F-actin (blue), α6 integrin (green); B, TUNEL (red), nuclei (blue); C, NFκB p65 (red), nuclei (blue), F-actin (green); D, Bcl-2 (red), nuclei (blue), F-actin (green); and E, survivin (red), nuclei (blue), and F-actin (green). All images were obtained by confocal microscopy. A–E are single optical slices of 1 μm thickness, selected from an acquired z-stack. D is a projection image (5 μm thickness), acquired from a z-stack of 1-μm thick optical slices. Scale bar = 20 μm in all images. Results show that in the absence of α6 integrin, expression of the transcription factor NFκB (C, red), and its downstream effectors Bcl-2 (D, red) and survivin (E, red), were significantly diminished, inducing a high level of caspase-3 activity and cell death by apoptosis. Whole eye cryosections were examined from a minimum of three different embryos taken from each of the α6+/+ and α6−/− mice groups and are thus representative of three independent studies.
α6 Integrin-dependent Expression of NFκB, Bcl-2, and Survivin during Lens Development
In previous studies we show that IGF-1R signaling is responsible for activation of NFκB and in turn for induction of survival proteins associated with maintaining caspase-3 at the low level of activity required for it to function as a differentiation signal (13). Because we have now demonstrated that α6 integrin is required for both activation of IGF-1R and the regulation of caspase-3 in developing lens tissue, we examined whether other elements of the IGF-1R survival-signaling pathway that we previously linked to the IGF-1R differentiation-initiation signal were dependent on α6 integrin function. Cryosections of E18.5 α6+/+ and α6−/− lenses were immunostained for NFκB (Fig. 2C), Bcl-2 (Fig. 2D), and survivin (Fig. 2E). The results showed that the expression of NFκB and survivin were significantly down-regulated in the absence of α6 integrin, and that only low levels of Bcl-2 expression could be detected. These findings suggest that α6/IGF-1R coordinate signaling regulates the activation state of caspase-3 for its role in lens epithelial cell differentiation initiation through induction of survival proteins by NFκB.
Lens Differentiation Is Altered in the Absence of α6 Integrin
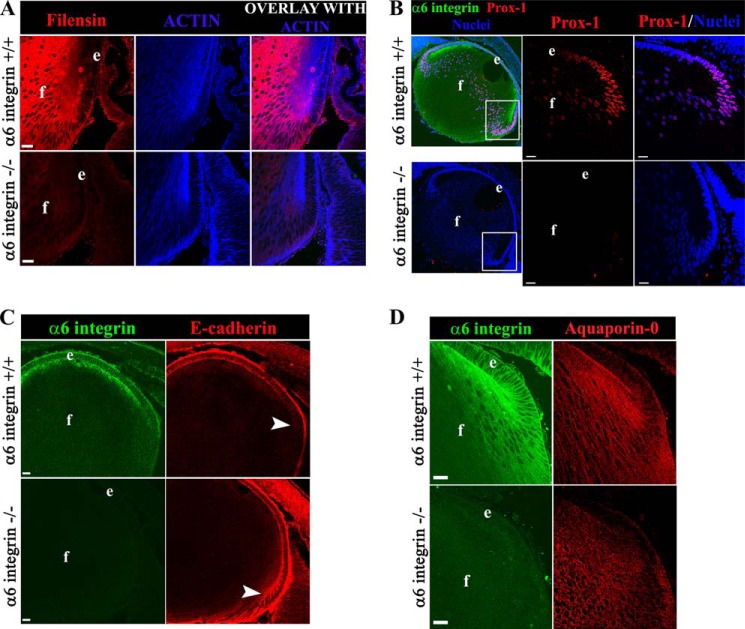
Although the formation of lenses in α6−/− mice shows that many aspects of development of this tissue are able to proceed in the absence of α6 integrin, likely due to compensatory signaling (38, 44), the significant effects of the absence of α6 integrin on IGF-1/NFκB signaling indicated that this knock-out model would reveal elements of lens differentiation that were guided by α6 integrin signaling. To examine this question we studied hallmarks of lens differentiation at E18.5 using an immunolocalization approach with antibodies to the lens intermediate filament protein filensin, the transcription factor Prox1, the cell-cell adhesion protein E-cadherin that is limited to lens epithelial cells (52, 53), and the lens-specific water channel protein Aquaporin-0 (Figs. 3, A–D). Filensin, highly expressed in fiber cells of the WT lens (54, 55), was only present at low levels in fiber cells of lenses developed in the absence of α6 integrin (Fig. 3A). Normally, as lens epithelial cells begin their transition to a fiber cell phenotype Prox1 translocates to the nuclei (56, 57), as seen here in the WT lenses at E18.5 (Fig. 3B). In age-matched α6−/− lenses there was significantly diminished nuclear localization of Prox1 in cells of the transition zone, where it typically first appears, and this inhibition is maintained into the lens fiber cell zone. Immunolocalization analysis for E-cadherin revealed that this adhesion protein persisted at cell-cell borders into the region of the young cortical fiber cells, consistent with a failure to properly shut off a molecular marker of lens epithelial cells (Fig. 3C). No significant effect on expression of Aquaporin-0 was detected in α6−/− lenses (Fig. 3D). These results show that there are distinct defects in a subset of molecules required for normal lens differentiation in the absence of α6 integrin.
FIGURE 3.
The absence of α6 integrin in the developing lens suppresses specific hallmarks of lens differentiation. Mid-sagittal lens cryosections from α6+/+ (upper panel) and α6−/− (lower panel) mice at E18.5 were co-labeled for filensin (red), F-actin (blue) (A); α6 integrin (green), Prox1 (red), nuclei (blue) (B); α6 integrin (green), E-cadherin (red) (C); and α6 integrin (green), Aquaporin-0 (red) (e, lens epithelium; f, lens fiber cells) (D). Results show diminished expression of the lens-specific intermediate filament protein filensin (A, red) and Prox1 (B, red) nuclear expression/translocation in the lens equatorial region was also prevented in the absence of α6 integrin. E-cadherin (C, red) was misexpressed in α6−/− lenses, persisting into the youngest differentiating cortical lens fiber cells (region noted by arrowheads). Little effect on the expression of the lens-specific differentiation protein Aquaporin-0/MIP-28 (D, red) was noted. All images were obtained by confocal microscopy. A and D and B and C, single optical slices of 1 μm thickness and 0.5 μm thickness, respectively, selected from an acquired z-stack. Scale bar = 20 μm in all images. Lens cryosections of whole eyes were examined from a minimum of three different embryos from each of the α6+/+ and α6−/− mice groups, thus these results are representative of three independent studies.
IGF-1R Is Activated Specifically in α6 Integrin Signaling Complexes Formed in the Zone of Lens Differentiation Initiation
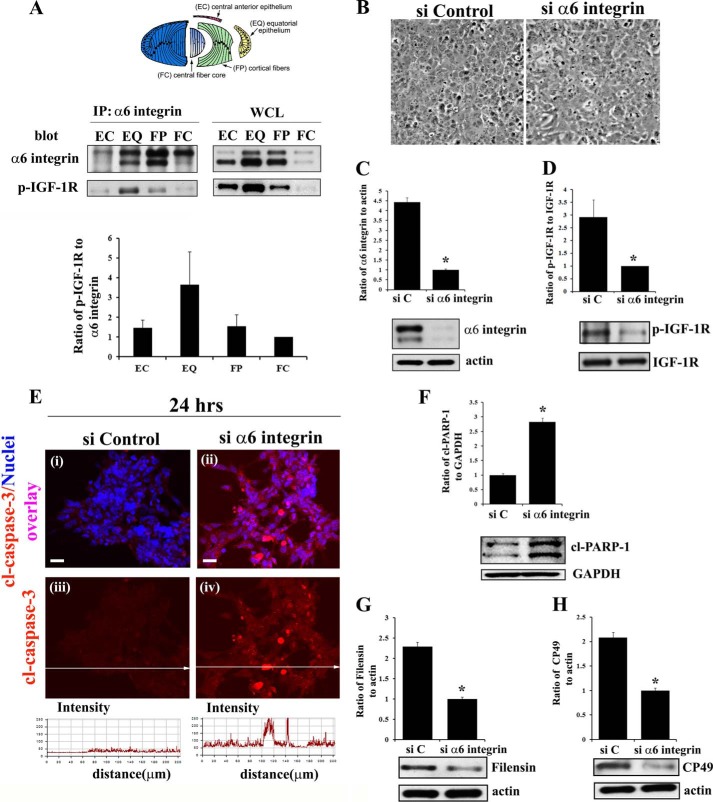
Our previous studies have shown a differentiation state-specific association between IGF-1R and α6 integrin in the chick embryo lens (25). Using a co-immunoprecipitation approach, we investigated the activation state of IGF-1R (p-IGF-1R) in α6 integrin signaling complexes as a factor of the lens cell differentiation state. Four distinct regions of lens differentiation were isolated from the E10 chick embryo lenses by microdissection. These stages of differentiation are all expressed concurrently in the embryonic lens, representing a continuum of the differentiation process, and include undifferentiated lens epithelial cells (EC); the zone of differentiation initiation (EQ); the region of lens fiber cell morphogenesis (FP); and the zone of fiber cell maturation (FC), (microdissection modeled in Fig. 4A). Each fraction was immunoprecipitated for α6 integrin, and immunoblotted for both α6 integrin and activated IGF-1R (p-IGF-1R). The results show that differentiation state-specific activation of IGF-1R in an α6 integrin signaling complex is greatest in the equatorial zone of the embryonic lens (Fig. 4A), the region of lens epithelial cell differentiation initiation. This finding is consistent with the role of α6 integrin/IGF-1R signaling in regulating caspase-3 to maintain it at the low level of activity required to execute differentiation initiation in this region of the developing lens.
FIGURE 4.
α6 integrin transactivation of IGF-1R maintains low level caspase-3 activity for its role in signaling differentiation initiation of lens epithelial cells. A, diagram of the microdissected lens areas (top panel); co-immunoprecipitation (IP, α6 integrin; blot, α6 integrin and activated (phosphorylated) IGF-1R (p-IGF-1R)) analysis in differentiation specific zones of E10 chick embryo lenses, obtained by microdissection. α6 integrin was immunoprecipitated with an α6A integrin-specific polyclonal antibody because this is the predominant isoform associated with lens differentiation (47). Immunoprecipitates and whole cell lysates (WCL) were immunoblotted with a monoclonal antibody to the α6 integrin extracellular domain that recognizes two bands, the cleaved (lower band) and uncleaved (upper band) form of α6 integrin as described previously (47, 62). Results of three independent experiments were quantified and represented graphically as a ratio of p-IGF1R/α6 integrin (both cleaved and uncleaved bands), showing that IGF-1R is activated specifically in the α6 integrin signaling complexes in the EQ zone. B–H, α6 integrin was knocked down using a siRNA approach (si α6 integrin) in primary lens cells in culture. Control cultures were treated with non-targeting siRNA (siControl/siC). B, phase images of lens epithelial cells following siRNA knockdown of α6 integrin compared with siControl. C, immunoblot for α6 integrin showing siα6 integrin effectively knocked down α6 integrin expression, and D, activation of IGF-1R (p-IGF-1R), without affecting expression of total IGF-1R. E, immunostaining for cleaved (activated) caspase-3 (red, i–iv), co-stained with TO-PRO-3 to detect nuclei (blue, i–ii), showed increased caspase-3 activation following α6 integrin knockdown. Fluorescence intensity of cl-caspase-3 was quantified by creating line scans (panels iii and iv, quantified below) across the field of cells using LSM Image Examiner, confirming increased caspase-3 activation in response to α6 integrin knockdown. F, immunoblot for the caspase-3 target cl-PARP-1. Immunoblot analysis was also performed for the lens differentiation specific proteins filensin (G) and CP49 (H). The results show that α6 integrin is necessary to maintain low levels of caspase-3 activity and inducing lens epithelial cell differentiation initiation. Densitometric analyses of immunoblots for α6 integrin, filensin, and CP49 were plotted as a ratio to actin; cl-PARP-1 as a ratio to GAPDH; and p-IGF-1R against total IGF-1R. For confocal imaging, z-stacks were collected and analyzed, and the data are presented as a single optical plane of 0.5 μm. Results are representative of at least three or more independent studies. Error bars represent S.E. *, p < 0.05, t test. Scale bar, 20 μm.
Knockdown of α6 Integrin in Primary Lens Epithelial Cell Cultures Inhibited Activation of IGF-1R, Deregulated Caspase-3 Activity, and Suppressed Lens Epithelial Cell Differentiation
To further examine the requirement for α6 integrin in the activation of IGF-1R and investigate their synergistic role in lens epithelial cell differentiation we used a siRNA approach in primary quail lens epithelial cell cultures. These cultures mimic lens differentiation as it occurs in vivo, providing an ideal system to study the role of integrin-dependent signaling in lens differentiation (48). α6 integrin was knocked down prior to differentiation initiation with a custom-made siRNA pool specific for α6 integrin. This approach effectively knocked down α6 integrin expression (Fig. 4C), with efficiency close to 80%. Knockdown of α6 integrin in these lens epithelial cell cultures suppressed activation of IGF-1R without affecting IGF-1R expression, demonstrating that in these cells IGF-1R activation was dependent on α6 integrin (Fig. 4D). These results provided the first direct evidence of α6 integrin transactivation of IGF-1R. This coordinate loss of α6 integrin and IGF-1R activity removed the constraints on caspase-3 activation, shown both by the increased levels of activated, cleaved caspase-3 (Fig. 4E) and increased cleavage of the direct caspase-3 substrate PARP-1 (Fig. 4F). Knockdown of α6 integrin also resulted in significantly lower levels of the lens-specific intermediate filament proteins filensin (Fig. 4G) and CP49 (Fig. 4H). These results parallel our findings with the α6 integrin-null lenses and confirm that α6 integrin functions upstream to the IGF-1R signaling pathway to maintain the low levels of caspase-3 activity required to induce differentiation initiation.
Knockdown of α6 Integrin in Primary Lens Epithelial Cells Suppressed Expression of Survival Proteins and Led to Cell Death by Apoptosis
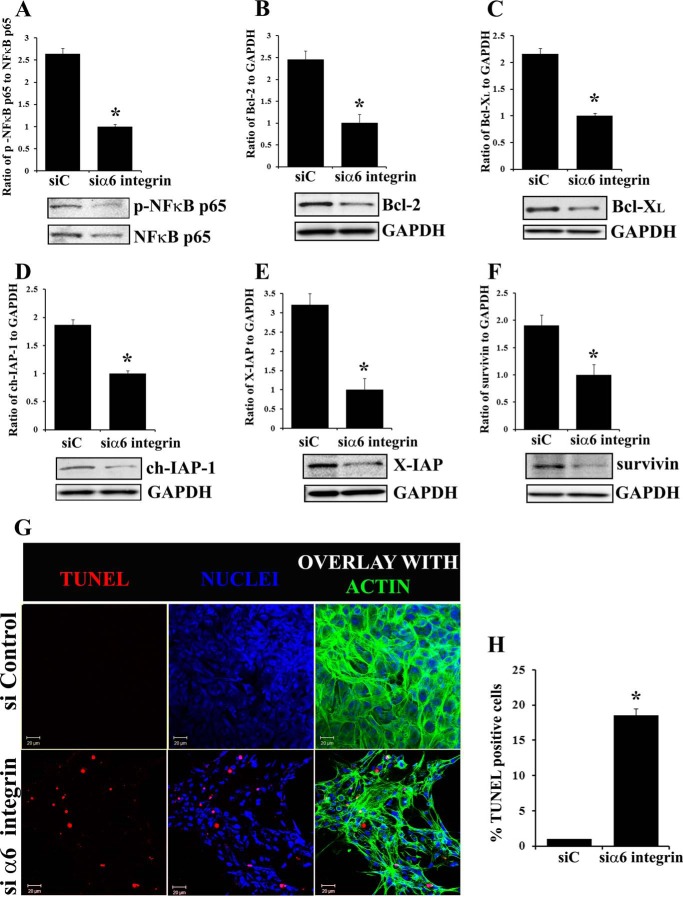
As caspase-3 activity was found to be deregulated in the absence of α6 integrin in primary lens cell cultures we examined if this result reflected a block in the expression/activation of transcription factor NFκB and expression of survival proteins regulated by NFκB (13). As above, α6 integrin was knocked down in primary lens epithelial cells with an siRNA approach and analyzed for expression of the p65 subunit of NFκB (Fig. 5A), and for survival proteins in the Bcl-2 and IAP families, including Bcl-2, Bcl-XL, ch-IAP-1, X-IAP, and survivin (Fig. 5, B–F). In the absence of α6 integrin, expression of the NFκB p65 subunit was suppressed significantly along with all Bcl-2 and IAP family members examined. TUNEL analysis revealed that the loss of these survival proteins in the absence of α6 integrin lead to increased cell death by apoptosis (Fig. 5, G and H). These results demonstrated that the α6 integrin/IGF-1R/NFκB signaling axis maintains caspase-3 activity at the low level required for its role in inducing lens differentiation initiation.
FIGURE 5.
siRNA knockdown of α6 integrin in primary lens cell cultures led to suppression of survival proteins in the Bcl-2 and IAP families and cell death by apoptosis. A–F, α6 integrin was knocked down using a siRNA approach (siα6 integrin) in primary lens cell cultures. Controls were treated with non-targeting siRNA (siC). A, following α6 integrin siRNA knockdown cultures were immunoblotted for activated p-NFκB (p65 Ser-276) and total NFκB p65; Bcl-2 (B), Bcl-XL (C), and IAP family proteins (D–F): ch-IAP-1 (D), X-IAP (E), and survivin (F). G, induction of apoptosis following α6 integrin knockdown was examined by TUNEL assay. Confocal images show that α6 integrin knockdown induced cell death by apoptosis, TUNEL (red), nuclei (blue), F-actin (green); H, quantification for TUNEL positive cells showed a significant increase in the number of TUNEL positive cells in the absence of α6 integrin. Results show that α6 integrin is an essential upstream signal of NFκB-mediated expression of survival proteins in the Bcl-2 and IAP families in lens epithelial cells. Densitometric analyses of immunoblots for Bcl-2, Bcl-XL, ch-IAP-1, X-IAP, and survivin were plotted as a ratio to GAPDH; and p-NFκB p65 against total NFκB p65. For confocal imaging, z-stacks were collected and analyzed, and the data are presented as a single optical plane of 0.5 μm. Results are representative of at least three studies. Error bars represent S.E. *, p < 0.05, t test. Scale bar, 20 μm.
Transactivation of IGF-1R by α6 Integrin Is Mediated by a Src Family Kinase
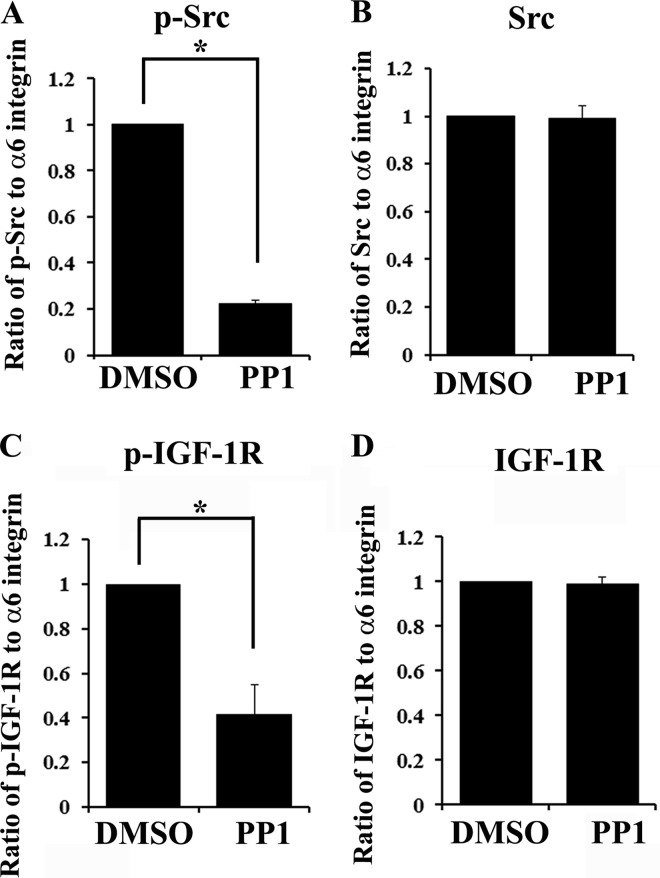
Having discovered that α6 integrin is upstream to IGF-1R and is required for its activation in this survival-linked differentiation pathway we next examined the mechanism by which α6 integrin regulates IGF-1R activity. There is substantial evidence in the literature implicating Src kinases as a principal downstream effector of integrins (58, 59) and in transactivation of growth factor receptors by integrins (15). Because Src family kinase members have requisite, regulatory functions in lens differentiation (60), we examined whether α6 integrin-mediated activation of Src kinase activity was responsible for transduction of the α6 integrin survival-linked differentiation signal to IGF-1R. For these studies lens cells in primary culture were exposed to PP1, a specific Src kinase inhibitor, or its vehicle dimethyl sulfoxide. Cells were extracted and subjected to co-immunoprecipitation analysis (IP, α6 integrin; blot, pIGF1R or IGF-1R). The activation status of Src in these complexes was determined by immunoblotting for p-Src. The results showed that PP1 exposure effectively blocked the activation of Src in α6 integrin signaling complexes (Fig. 6A), without affecting the association of Src with the α6 integrin receptor (Fig. 6B). Importantly, this loss of Src kinase activity blocked activation of IGF-1R in these α6 integrin-signaling complexes (p-IGF1R, Fig. 6C), whereas IGF-1R association with these complexes remained unchanged (Fig. 6D). These results demonstrate that transactivation of IGF-1R by α6 integrin in the developing lens is mediated by Src kinase activity.
FIGURE 6.
Activation of IGF-1R in α6 integrin signaling complexes is dependent on the kinase activity of Src family kinases. Primary lens cells in culture were exposed to the highly specific Src family kinase inhibitor, PP1, or to the vehicle dimethyl sulfoxide (DMSO) for 4 h. Following PP1 treatment, cells were immunoprecipitated for α6 integrin and the immunoprecipitates were immunoblotted for α6 integrin and phospho-Src (p-Src) (A), Src (B), phospho-IGF-1R (p-IGF-1R) (C), and IGF-1R (D). A–D, graphical representation of densitometric analysis of co-immunoprecipitation studies showing the ratio of activated, p-Src/α6 integrin (A), total Src/α6 integrin (B), activated p-IGF-1R/α6 integrin (C), and total IGF-1R/α6 integrin (D). Data shows that blocking activation of Src kinases prevented α6 integrin transactivation of IGF-1R. Results shown are quantified from more than three independent studies. Error bars represent S.E. *, p < 0.05, t test.
DISCUSSION
Previous findings from our laboratory had shown a requirement for the α6 integrin receptor in signaling lens differentiation, and the potential that this signal is related to the association of the growth factor receptor IGF-1R with α6 integrin in the lens equatorial zone, the region of the embryonic lens where differentiation is initiated (47). This finding took on additional significance when we discovered that low level caspase-3 activity, induced through the mitochondrial death pathway, was necessary for lens epithelial cell differentiation (2). Particularly intriguing was that concurrent with this low level activation of caspase-3 was the induction of anti-apoptotic proteins in both Bcl-2 (Bcl-2, p-Bad, and Bcl-XL) and IAP (ch-IAP, XIAP, and survivin) families of survival molecules (2). Further investigation revealed that the non-apoptotic function for caspase-3 in cell differentiation is dependent on an IGF-1R/NFκB survival signal that is responsible for inducing the expression of both Bcl-2 and IAP survival proteins (13). Blocking this survival signal results in high level caspase-3 activation, and apoptosis is induced instead of lens cell differentiation.
The current study revealed that IGF-1R activation in the developing lens and its function in the regulation of lens cell differentiation initiation were both dependent on the α6 integrin receptor. In the absence of α6 integrin (α6−/− lenses, siRNA knockdown in differentiating lens cell cultures) IGF-1R activation was blocked, NFκB signaling was compromised, and molecular regulators of caspase-3 in both the Bcl-2 and IAP families failed to be induced (modeled in Fig. 7). These findings paralleled the effects of directly blocking activation of IGF-1R or NFκB that we have published previously (13). The effects of α6 integrin loss led to a high level of caspase-3 activation that suppressed lens cell differentiation and instead induced cell death by apoptosis. Therefore, coordinate α6 integrin/IGF-1R survival signaling was essential to induce the expression of survival proteins that regulate caspase-3 activation levels so that it is able to signal cell differentiation and not death. Our studies with the α6 integrin−/− lens also revealed significant deficits in the lens differentiation process that validate it as an important model for identifying α6 integrin-dependent signaling pathways important to normal lens differentiation and development.
FIGURE 7.
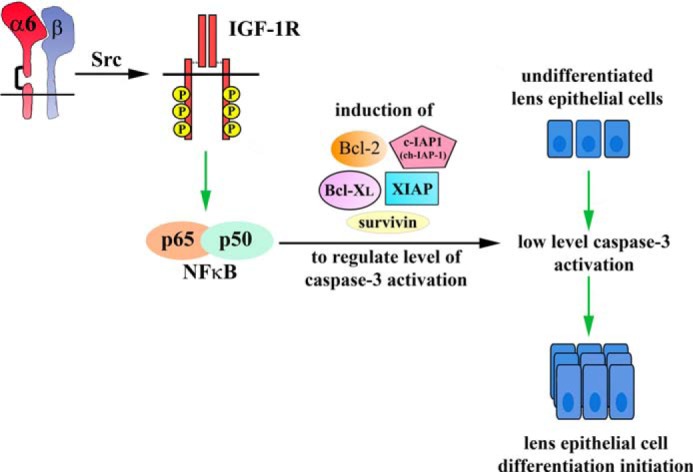
α6 integrin is the upstream regulator of the IGF-1R signaling pathway that enables low levels of caspase-3 activation to signal lens epithelial cell differentiation initiation. Differentiation initiation of lens epithelial cells require low levels of caspase-3 activity that are induced downstream of the canonical mitochondrial death pathway, which acts as a molecular switch in inducing lens epithelial cell differentiation initiation. Previous studies have shown that an IGF-1R/NFκB (p65/p50 complex)-mediated survival signal regulates the level of caspase-3 activity for its role in lens differentiation. Here we show that α6 integrin transactivates IGF-1R to regulate the level of caspase-3 activity through the induction of survival proteins in the Bcl-2 (Bcl-2 and Bcl XL) and IAP families (ch-IAP-1, X-IAP, and survivin). This pathway allows caspase-3 to play a non-apoptotic role in lens epithelial cell differentiation initiation. Our findings show a novel role for the α6 integrin/IGF-1R coordinated survival signal in lens differentiation by modulating the activation of caspase-3.
There is precedence for both the collaborative signaling between integrin and growth factor receptors (15, 26, 34) and a role for the α6 integrin receptor in cell survival (14, 38) and cell differentiation (25, 61, 62). In this context, and consistent with our findings, are studies from ffrench-Constant's laboratory (16, 63) that provide strong evidence for integrin-growth factor receptor-regulated signals in the survival and differentiation of oligodendrocytes. The authors show that prior to oligodendrocyte differentiation the PDGFα receptor associates in a complex with αvβ3 integrin to signal proliferation, and that the PDGFα receptor switches its association to α6β1 integrin as oligodendrocyte precursors initiate their differentiation (16, 63). Interesting to our studies, during their transition from progenitor to differentiating cell phenotype oligodendrocytes are particularly sensitive to programmed cell death (64). The switch of the PDGFα receptor from αvβ3 to α6β1 receptor complexes provides the essential survival signal for oligodendrocytes to survive and differentiate (34). In fact, among all the integrins that have been linked separately to either cell survival (19, 65–69) or cell differentiation (70–73), α6 integrin stands out as crucial for both (33, 34, 37, 38, 44). However, prior to our studies there was no evidence as to the mechanism of how α6 integrin can induce, concurrently, both survival and differentiation, or whether these functions were interconnected. Our investigations reveal the mechanism by which α6 integrin provides a survival-linked differentiation signal. These findings (modeled in Fig. 7) show that the α6 integrin/IGF-1R coordinate survival signal functions to induce pro-survival proteins that maintain caspase-3 at a low level of activity that initiates differentiation. Loss of this signal releases the cellular controls on caspase-3, resulting in high levels of caspase-3 activity that instead induce apoptotic cell death. These results provide a likely mechanism for the coordinated survival/differentiation function of α6 integrin observed in previous studies (33, 34, 37, 38, 44).
In our investigations with the α6 integrin−/− lenses we discovered that this receptor function was also required for the appropriate, temporal regulation of a number of molecules associated with the lens cell differentiation process, including that of the transcription factor Prox1. Previously, Duncan and co-workers (56) showed the importance of Prox1 nuclear localization during the transition of lens epithelial cells to fiber cells, including that Prox1 interacts with proliferating cell nuclear antigen to induce expression of lens differentiation-specific proteins (74). The absence of α6 integrin suppressed the translocation of Prox1 to the nucleus as lens epithelial cells begin their differentiation into lens fiber cells. Interestingly, nuclear translocation of Prox1 was not affected in the absence of β1 integrin (57). Because α6 integrin can heterodimerize with both β1 and β4 integrins, our results suggest that the α6β4 integrin heterodimer is responsible for the signal that induces translocation of Prox1 to the nucleus at the start of lens epithelial cell differentiation. Misexpression of E-cadherin was noted into the early differentiating fiber cells of the cortical zone and was not restricted to the lens epithelium. This finding is consistent with studies reported by Wigle et al. (75) where ablation of Prox1 resulted in misexpression of E-cadherin in the lens. Thus failure in Prox1 expression/translocation to the nucleus in the α6 integrin knock-out lenses was likely responsible for the misexpression of E-cadherin in these lenses. Other effects of the absence of α6 integrin on differentiating lens cells included the inability to induce/maintain high levels of the lens-specific intermediate filament protein filensin. The diminished immunolabeling for filensin in the α6−/− lenses may reflect the fact that filensin can be a caspase-3 substrate. The absence of α6 integrin did not significantly affect the expression of Aquaporin-0. This may be explained by the fact that Aquaporin-0 expression is regulated by the homeodomain transcription factor Pitx3 (76). Our studies suggest that α6 integrin is crucial in regulating a subset of molecules fundamental to lens epithelial cell differentiation. In addition, the effects of α6 integrin loss on Prox1, E-cadherin, and filensin showed that the α6 integrin receptor has roles in lens cell differentiation beyond that of mediating the non-apoptotic signaling function of caspase-3.
There are multiple ways by which the α6 integrin/IGF-1R survival linked-differentiation signal can be activated in the lens. Classically, integrins are activated through engagement of their extracellular matrix ligands (77, 78). Both α6 integrin ligands laminin-111 (47) and laminin-332 (79) are expressed in the embryonic lens where they could activate an α6 integrin-dependent cell-survival differentiation-initiation signal. Although in this mechanism IGF-1R would be transactivated by α6 integrin following its engagement of the matrix ligand, activation of the α6 integrin/IGF-1R survival/differentiation pathway could be induced in a matrix-independent manner, as described in a recent paradigm shifting study. There it was shown that IGF-1, the ligand of IGF-1R, can bind directly to α6β4 and induce the formation of an α6β4-IGF1-IGF1R ternary complex that activates the IGF-1R signaling pathway (26). Alternatively, activation of the α6 integrin/IGF-1R pathway essential to lens differentiation could occur independently of both laminin and IGF-1 in a mechanism where α6 integrin mediates the transactivation of IGF-1R in a ligand-independent manner (15). Our studies show that the mechanism by which α6 integrin transactivates IGF-1R depends on activation of a Src family tyrosine kinase. It remains to be determined which Src kinase member is responsible for this transactivation event. Together our results provide strong evidence that IGF-1R activation in the developing lens is dependent on α6 integrin.
Survival signaling is necessary to regulate caspase-3 activation and maintain it at low levels of activity at which it can perform its non-apoptotic functions in the cell. In addition to our findings, regulation of caspase-3 activity has also been linked to α-crystallins, molecular chaperones that can function as protectors against apoptosis in lens differentiation. Apoptotic cell death has been reported in developing lenses of αA/αB crystallin double knock-out mice, and associated with the induction of high levels of caspase-3 (80). Interestingly, we found that αA-crystallin associates with α6 integrin receptor complexes in regions of active lens cell differentiation (81). Furthermore, activation of α6 integrin following exposure to PMA induced recruitment of αA-crystallin to α6 integrin receptor complexes, providing evidence of a direct signaling connection between α6 integrin and αA-crystallin (81). These studies suggest that αA-crystallin association with α6 integrin in differentiating cells of the lens could play a role in regulating the α6 integrin-linked survival-signaling pathway responsible for maintaining caspase-3 at the low levels of activity required for its function as a differentiation-initiation signal.
It is still a mystery as to what factors or molecules are activated to induce lens cell differentiation downstream of the low level caspase-3 activity. Larsen et al. (4) have described a novel mechanism involving the caspase-activated DNase (CAD) and its inhibitor, the inhibitor of caspase-activated DNase (ICAD), in inducing the differentiation of skeletal muscle cells. This study showed that low levels of caspase-3 activity induce limited cleavage of ICAD to result in low level activation of CAD that translocates to the nucleus where it makes limited strand breaks at the p21 promoter site to initiate a genomewide reprogramming event necessary for skeletal muscle cell differentiation. It will be interesting to determine whether this mechanism can be extended to lens epithelial cell differentiation.
In conclusion, our findings show a novel mechanism by which α6 integrin-coordinated signaling with the classic survival receptor IGF-1R regulates the level of caspase-3 activation for its role in inducing lens epithelial cell differentiation initiation. We show that α6 integrin transactivates IGF-1R through a Src family kinase to induce expression of Bcl-2 and IAP family survival proteins through transcription factor NFκB. These survival proteins modulate caspase-3 activity and keep it in check, allowing caspase-3 to play a non-apoptotic role in cell differentiation. Our study provides an explanation to the long existing question of how α6 integrin induces both cell survival and differentiation, and shows how these functions are linked. With both avian and mammalian models of lens development we reveal that low levels of caspase-3 activity initiate lens epithelial cell differentiation downstream of a α6 integrin/IGF-1R/NFκB coordinated cell-survival signal.
Acknowledgments
We appreciate the generous gifts of antibodies to filensin and CP49 from Dr. Paul Fitzgerald. We thank V. Pfister for help with the genotyping, PCR, and maintenance of the mice lines. We thank Dr. Janice L. Walker for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant EY10577 (to A. S. M.).
- IGF-1R
- insulin-like growth factor receptor-1
- IGF-1
- insulin-like growth factor-1
- NFκB
- nuclear factor κB
- E
- embryonic day
- TUNEL
- terminal deoxynucleotidyltransferase-mediated biotinylated UTP nick end labeling
- IAP
- inhibitor of apoptosis protein
- ch-IAP1/2
- chicken IAP1/2
- Bcl-2
- B-cell lymphoma-2
- ICAD
- inhibitor of caspase-activated DNase
- PMA
- phorbol 12-myristate 13-acetate
- PARP
- poly(ADP-ribose) polymerase
- EC
- undifferentiated lens epithelial cells
- EQ
- equatorial epithelium
- FP
- cortical fiber zone
- NC
- nuclear fiber zone.
REFERENCES
- 1. Hyman B. T., Yuan J. (2012) Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat. Rev. Neurosci. 13, 395–406 [DOI] [PubMed] [Google Scholar]
- 2. Weber G. F., Menko A. S. (2005) The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J. Biol. Chem. 280, 22135–22145 [DOI] [PubMed] [Google Scholar]
- 3. Fernando P., Kelly J. F., Balazsi K., Slack R. S., Megeney L. A. (2002) Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. U.S.A. 99, 11025–11030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larsen B. D., Rampalli S., Burns L. E., Brunette S., Dilworth F. J., Megeney L. A. (2010) Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc. Natl. Acad. Sci. U.S.A. 107, 4230–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keramaris E., Stefanis L., MacLaurin J., Harada N., Takaku K., Ishikawa T., Taketo M. M., Robertson G. S., Nicholson D. W., Slack R. S., Park D. S. (2000) Involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. Mol. Cell. Neurosci. 15, 368–379 [DOI] [PubMed] [Google Scholar]
- 6. Belmokhtar C. A., Hillion J., Ségal-Bendirdjian E. (2001) Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20, 3354–3362 [DOI] [PubMed] [Google Scholar]
- 7. Belmokhtar C. A., Torriglia A., Counis M. F., Courtois Y., Jacquemin-Sablon A., Ségal-Bendirdjian E. (2000) Nuclear translocation of a leukocyte elastase Inhibitor/Elastase complex during staurosporine-induced apoptosis. Role in the generation of nuclear L-DNase II activity. Exp. Cell Res. 254, 99–109 [DOI] [PubMed] [Google Scholar]
- 8. Kuida K., Zheng T. S., Na S., Kuan C., Yang D., Karasuyama H., Rakic P., Flavell R. A. (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384, 368–372 [DOI] [PubMed] [Google Scholar]
- 9. Haydar T. F., Kuan C. Y., Flavell R. A., Rakic P. (1999) The role of cell death in regulating the size and shape of the mammalian forebrain. Cerebral. Cortex 9, 621–626 [DOI] [PubMed] [Google Scholar]
- 10. Zeiss C. J., Neal J., Johnson E. A. (2004) Caspase-3 in postnatal retinal development and degeneration. Invest. Ophthalmol. Vis. Sci. 45, 964–970 [DOI] [PubMed] [Google Scholar]
- 11. Mori C., Nakamura N., Kimura S., Irie H., Takigawa T., Shiota K. (1995) Programmed cell death in the interdigital tissue of the fetal mouse limb is apoptosis with DNA fragmentation. Anat. Rec. 242, 103–110 [DOI] [PubMed] [Google Scholar]
- 12. Yang S. Y., Sales K. M., Fuller B. J., Seifalian A. M., Winslet M. C. (2008) Inducing apoptosis of human colon cancer cells by an IGF-I D domain analogue peptide. Mol. Cancer 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basu S., Rajakaruna S., Menko A. S. (2012) Insulin-like growth factor receptor-1 and nuclear factor κB are crucial survival signals that regulate caspase-3-mediated lens epithelial cell differentiation initiation. J. Biol. Chem. 287, 8384–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moro L., Venturino M., Bozzo C., Silengo L., Altruda F., Beguinot L., Tarone G., Defilippi P. (1998) Integrins induce activation of EGF receptor. Role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 17, 6622–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moro L., Dolce L., Cabodi S., Bergatto E., Boeri Erba E., Smeriglio M., Turco E., Retta S. F., Giuffrida M. G., Venturino M., Godovac-Zimmermann J., Conti A., Schaefer E., Beguinot L., Tacchetti C., Gaggini P., Silengo L., Tarone G., Defilippi P. (2002) Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 277, 9405–9414 [DOI] [PubMed] [Google Scholar]
- 16. Baron W., Colognato H., ffrench-Constant C. (2005) Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia 49, 467–479 [DOI] [PubMed] [Google Scholar]
- 17. Baron W., Decker L., Colognato H., ffrench-Constant C. (2003) Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13, 151–155 [DOI] [PubMed] [Google Scholar]
- 18. Zheng B., Clemmons D. R. (1998) Blocking ligand occupancy of the αVβ3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 95, 11217–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonfoco E., Chen W., Paul R., Cheresh D. A., Cooper N. R. (2000) β1 integrin antagonism on adherent, differentiated human neuroblastoma cells triggers an apoptotic signaling pathway. Neuroscience 101, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 20. Mogi M., Togari A. (2003) Activation of caspases is required for osteoblastic differentiation. J. Biol. Chem. 278, 47477–47482 [DOI] [PubMed] [Google Scholar]
- 21. Weil M., Raff M. C., Braga V. M. (1999) Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr. Biol. 9, 361–364 [DOI] [PubMed] [Google Scholar]
- 22. Epaud R., Aubey F., Xu J., Chaker Z., Clemessy M., Dautin A., Ahamed K., Bonora M., Hoyeau N., Fléjou J. F., Mailleux A., Clement A., Henrion-Caude A., Holzenberger M. (2012) Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PloS One 7, e48071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 [PubMed] [Google Scholar]
- 24. Kiely P., O'Gorman D., Lyons A., O'Connor R. (2005) The IGF-1 receptor in cell survival. Signalling and regulation. In Cell Engineering (Al-Rubeai M., Fussenegger M., eds) pp. 49–92, Springer, The Netherlands [Google Scholar]
- 25. Walker J. L., Zhang L., Zhou J., Woolkalis M. J., Menko A. S. (2002) Role for α6 integrin during lens development. Evidence for signaling through IGF-1R and ERK. Dev. Dyn. 223, 273–284 [DOI] [PubMed] [Google Scholar]
- 26. Fujita M., Ieguchi K., Davari P., Yamaji S., Taniguchi Y., Sekiguchi K., Takada Y. K., Takada Y. (2012) Cross-talk between integrin α6β4 and insulin-like growth factor-1 receptor (IGF1R) through direct α6β4 binding to IGF1 and subsequent α6β4-IGF1-IGF1R ternary complex formation in anchorage-independent conditions. J. Biol. Chem. 287, 12491–12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitsiades C. S., Mitsiades N., Poulaki V., Schlossman R., Akiyama M., Chauhan D., Hideshima T., Treon S. P., Munshi N. C., Richardson P. G., Anderson K. C. (2002) Activation of NF-κB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells. Therapeutic implications. Oncogene 21, 5673–5683 [DOI] [PubMed] [Google Scholar]
- 28. Alam N., Goel H. L., Zarif M. J., Butterfield J. E., Perkins H. M., Sansoucy B. G., Sawyer T. K., Languino L. R. (2007) The integrin-growth factor receptor duet. J. Cell. Physiol. 213, 649–653 [DOI] [PubMed] [Google Scholar]
- 29. Colognato H., Ramachandrappa S., Olsen I. M., ffrench-Constant C. (2004) Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J. Cell Biol. 167, 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng F., Lowell C. A. (1998) A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 17, 4391–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walker J., Menko A. S. (2009) Integrins in lens development and disease. Exp. Eye Res. 88, 216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmid R. S., Anton E. S. (2003) Role of integrins in the development of the cerebral cortex. Cerebral. Cortex 13, 219–224 [DOI] [PubMed] [Google Scholar]
- 33. Friedland J. C., Lakins J. N., Kazanietz M. G., Chernoff J., Boettiger D., Weaver V. M. (2007) α6β4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-κB-dependent resistance to apoptosis in 3D mammary acini. J. Cell Sci. 120, 3700–3712 [DOI] [PubMed] [Google Scholar]
- 34. Colognato H., Baron W., Avellana-Adalid V., Relvas J. B., Baron-Van Evercooren A., Georges-Labouesse E., ffrench-Constant C. (2002) CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4, 833–841 [DOI] [PubMed] [Google Scholar]
- 35. Hierck B. P., Poelmann R. E., van Iperen L., Brouwer A., Gittenberger-de Groot A. C. (1996) Differential expression of α-6 and other subunits of laminin binding integrins during development of the murine heart. Dev. Dyn. 206, 100–111 [DOI] [PubMed] [Google Scholar]
- 36. Wewer U. M., Shaw L. M., Albrechtsen R., Mercurio A. M. (1997) The integrin α6β1 promotes the survival of metastatic human breast carcinoma cells in mice. Am. J. Pathol. 151, 1191–1198 [PMC free article] [PubMed] [Google Scholar]
- 37. Lallier T. E., Whittaker C. A., DeSimone D. W. (1996) Integrin α6 expression is required for early nervous system development in Xenopus laevis. Development 122, 2539–2554 [DOI] [PubMed] [Google Scholar]
- 38. Georges-Labouesse E., Mark M., Messaddeq N., Gansmüller A. (1998) Essential role of α6 integrins in cortical and retinal lamination. Curr. Biol. 8, 983–986 [DOI] [PubMed] [Google Scholar]
- 39. Koyama N., Hayashi T., Gresik E. W., Kashimata M. (2009) Role of alpha 6 integrin subunit in branching morphogenesis of fetal mouse submandibular gland. Investigation by mesenchyme-free epithelial culture system. J. Med. Invest. 56, 247–249 [DOI] [PubMed] [Google Scholar]
- 40. Sastry S. K., Lakonishok M., Thomas D. A., Muschler J., Horwitz A. F. (1996) Integrin α subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J. Cell Biol. 133, 169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bernal-Mizrachi L., Lovly C. M., Ratner L. (2006) The role of NF-κB-1 and NF-κB-2-mediated resistance to apoptosis in lymphomas. Proc. Natl. Acad. Sci. U.S.A. 103, 9220–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C., Robinson M. L. (2008) Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Arcangelis A., Mark M., Kreidberg J., Sorokin L., Georges-Labouesse E. (1999) Synergistic activities of α3 and α6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development 126, 3957–3968 [DOI] [PubMed] [Google Scholar]
- 44. Georges-Labouesse E., Messaddeq N., Yehia G., Cadalbert L., Dierich A., Le Meur M. (1996) Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370–373 [DOI] [PubMed] [Google Scholar]
- 45. Alizadeh A., Clark J., Seeberger T., Hess J., Blankenship T., FitzGerald P. G. (2004) Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest. Ophthalmol. Vis. Sci. 45, 884–891 [DOI] [PubMed] [Google Scholar]
- 46. Son A. I., Cooper M. A., Sheleg M., Sun Y., Kleiman N. J., Zhou R. (2013) Further analysis of the lens of ephrin-A5−/− mice. Development of postnatal defects. Mol. Vis. 19, 254–266 [PMC free article] [PubMed] [Google Scholar]
- 47. Walker J. L., Menko A. S. (1999) α6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev. Biol. 210, 497–511 [DOI] [PubMed] [Google Scholar]
- 48. Menko A. S., Klukas K. A., Johnson R. G. (1984) Chicken embryo lens cultures mimic differentiation in the lens. Dev. Biol. 103, 129–141 [DOI] [PubMed] [Google Scholar]
- 49. Hernández-Sánchez C., Blakesley V., Kalebic T., Helman L., LeRoith D. (1995) The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J. Biol. Chem. 270, 29176–29181 [DOI] [PubMed] [Google Scholar]
- 50. Sasaoka T., Ishiki M., Wada T., Hori H., Hirai H., Haruta T., Ishihara H., Kobayashi M. (2001) Tyrosine phosphorylation-dependent and -independent role of Shc in the regulation of IGF-1-induced mitogenesis and glycogen synthesis. Endocrinology 142, 5226–5235 [DOI] [PubMed] [Google Scholar]
- 51. Jameson M. J., Beckler A. D., Taniguchi L. E., Allak A., Vanwagner L. B., Lee N. G., Thomsen W. C., Hubbard M. A., Thomas C. Y. (2011) Activation of the insulin-like growth factor-1 receptor induces resistance to epidermal growth factor receptor antagonism in head and neck squamous carcinoma cells. Mol. Cancer Ther. 10, 2124–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takeichi M. (1988) The cadherins. Cell-cell adhesion molecules controlling animal morphogenesis. Development 102, 639–655 [DOI] [PubMed] [Google Scholar]
- 53. Xu L., Overbeek P. A., Reneker L. W. (2002) Systematic analysis of E-, N-, and P-cadherin expression in mouse eye development. Exp. Eye Res. 74, 753–760 [DOI] [PubMed] [Google Scholar]
- 54. Alizadeh A., Clark J., Seeberger T., Hess J., Blankenship T., FitzGerald P. G. (2003) Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest. Ophthalmol. Vis. Sci. 44, 5252–5258 [DOI] [PubMed] [Google Scholar]
- 55. Merdes A., Brunkener M., Horstmann H., Georgatos S. D. (1991) Filensin. A new vimentin-binding, polymerization-competent, and membrane-associated protein of the lens fiber cell. J. Cell Biol. 115, 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duncan M. K., Cui W., Oh D. J., Tomarev S. I. (2002) Prox1 is differentially localized during lens development. Mech. Dev. 112, 195–198 [DOI] [PubMed] [Google Scholar]
- 57. Simirskii V. N., Wang Y., Duncan M. K. (2007) Conditional deletion of β1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev. Biol. 306, 658–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zou L., Cao S., Kang N., Huebert R. C., Shah V. H. (2012) Fibronectin induces endothelial cell migration through beta1 integrin and Src-dependent phosphorylation of fibroblast growth factor receptor-1 at tyrosines 653/654 and 766. J. Biol. Chem. 287, 7190–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dayyani F., Parikh N. U., Varkaris A. S., Song J. H., Moorthy S., Chatterji T., Maity S. N., Wolfe A. R., Carboni J. M., Gottardis M. M., Logothetis C. J., Gallick G. E. (2012) Combined Inhibition of IGF-1R/IR and Src family kinases enhances antitumor effects in prostate cancer by decreasing activated survival pathways. PloS One 7, e51189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Leonard M., Zhang L., Bleaken B. M., Menko A. S. (2013) Distinct roles for N-cadherin linked c-Src and fyn kinases in lens development. Dev. Dyn. 242, 469–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilschut K. J., van Tol H. T., Arkesteijn G. J., Haagsman H. P., Roelen B. A. (2011) α6 Integrin is important for myogenic stem cell differentiation. Stem Cell Res. 7, 112–123 [DOI] [PubMed] [Google Scholar]
- 62. Walker J. L., Zhang L., Menko A. S. (2002) A signaling role for the uncleaved form of α6 integrin in differentiating lens fiber cells. Dev. Biol. 251, 195–205 [DOI] [PubMed] [Google Scholar]
- 63. Baron W., Shattil S. J., ffrench-Constant C. (2002) The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of α(v) β3 integrins. EMBO J. 21, 1957–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70, 31–46 [DOI] [PubMed] [Google Scholar]
- 65. DiPersio C. M., Hodivala-Dilke K. M., Jaenisch R., Kreidberg J. A., Hynes R. O. (1997) α3β1 Integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 137, 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guo W., Pylayeva Y., Pepe A., Yoshioka T., Muller W. J., Inghirami G., Giancotti F. G. (2006) β4 Integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489–502 [DOI] [PubMed] [Google Scholar]
- 67. Kreidberg J. A., Donovan M. J., Goldstein S. L., Rennke H., Shepherd K., Jones R. C., Jaenisch R. (1996) α3β 1 Integrin has a crucial role in kidney and lung organogenesis. Development 122, 3537–3547 [DOI] [PubMed] [Google Scholar]
- 68. Mayer U., Saher G., Fässler R., Bornemann A., Echtermeyer F., von der Mark H., Miosge N., Pöschl E., von der Mark K. (1997) Absence of integrin α7 causes a novel form of muscular dystrophy. Nat. Genet. 17, 318–323 [DOI] [PubMed] [Google Scholar]
- 69. Dowling J., Yu Q. C., Fuchs E. (1996) β4 Integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134, 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levy L., Broad S., Diekmann D., Evans R. D., Watt F. M. (2000) β1 integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol. Biol. Cell 11, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Menko A. S., Kreidberg J. A., Ryan T. T., Van Bockstaele E., Kukuruzinska M. A. (2001) Loss of α3β1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev. Dyn. 220, 337–349 [DOI] [PubMed] [Google Scholar]
- 72. Schreiber T. D., Steinl C., Essl M., Abele H., Geiger K., Müller C. A., Aicher W. K., Klein G. (2009) The integrin α9β1 on hematopoietic stem and progenitor cells. Involvement in cell adhesion, proliferation and differentiation. Haematologica 94, 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lygoe K. A., Norman J. T., Marshall J. F., Lewis M. P. (2004) αV integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 12, 461–470 [DOI] [PubMed] [Google Scholar]
- 74. Chen X., Patel T. P., Simirskii V. I., Duncan M. K. (2008) PCNA interacts with Prox1 and represses its transcriptional activity. Mol. Vis. 14, 2076–2086 [PMC free article] [PubMed] [Google Scholar]
- 75. Wigle J. T., Chowdhury K., Gruss P., Oliver G. (1999) Prox1 function is crucial for mouse lens-fibre elongation. Nat. Genet. 21, 318–322 [DOI] [PubMed] [Google Scholar]
- 76. Sorokina E. A., Muheisen S., Mlodik N., Semina E. V. (2011) MIP/Aquaporin 0 represents a direct transcriptional target of PITX3 in the developing lens. PloS One 6, e21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hynes R. O. (1992) Integrins. Versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 [DOI] [PubMed] [Google Scholar]
- 78. Menko A. S., Boettiger D. (1987) Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell 51, 51–57 [DOI] [PubMed] [Google Scholar]
- 79. Walker J., Menko A. S. (2004) Role of matrix and cell adhesion molecules in lens differentiation (Lovicu F. J., Robinson M. L., eds), pp. 245–260, Cambridge University Press, Cambridge [Google Scholar]
- 80. Morozov V., Wawrousek E. F. (2006) Caspase-dependent secondary lens fiber cell disintegration in αA-/αB-crystallin double-knockout mice. Development 133, 813–821 [DOI] [PubMed] [Google Scholar]
- 81. Menko A. S., Andley U. P. (2010) αA-Crystallin associates with α6 integrin receptor complexes and regulates cellular signaling. Exp. Eye Res. 91, 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]