Background: Opn5 is considered to regulate nonvisual photoreception in the retina and brain of animals.
Results: Mouse and primate UV-sensitive Opn5 along with retinoid isomerase are localized in the preoptic hypothalamus.
Conclusion: Mammalian Opn5 can function as a high sensitivity photosensor in the deep brain with the assistance of 11-cis-retinal supplying system.
Significance: Mammals, including humans, may detect short wavelength light within the brain via Opn5.
Keywords: G Proteins, Molecular Evolution, Photoreceptors, Rhodopsin, Signal Transduction, Nonvisual Photoreception
Abstract
Opn5 is one of the recently identified opsin groups that is responsible for nonvisual photoreception in animals. We previously showed that a chicken homolog of mammalian Opn5 (Opn5m) is a Gi-coupled UV sensor having molecular properties typical of bistable pigments. Here we demonstrated that mammalian Opn5m evolved to be a more specialized photosensor by losing one of the characteristics of bistable pigments, direct binding of all-trans-retinal. We first confirmed that Opn5m proteins in zebrafish, Xenopus tropicalis, mouse, and human are also UV-sensitive pigments. Then we found that only mammalian Opn5m proteins lack the ability to directly bind all-trans-retinal. Mutational analysis showed that these characteristics were acquired by a single amino acid replacement at position 168. By comparing the expression patterns of Opn5m between mammals and chicken, we found that, like chicken Opn5m, mammalian Opn5m was localized in the ganglion cell layer and inner nuclear layer of the retina. However, the mouse and primate (common marmoset) opsins were distributed not in the posterior hypothalamus (including the region along the third ventricle) where chicken Opn5m is localized, but in the preoptic hypothalamus. Interestingly, RPE65, an essential enzyme for forming 11-cis-retinal in the visual cycle is expressed near the preoptic hypothalamus of the mouse and common marmoset brain but not near the region of the chicken brain where chicken Opn5m is expressed. Therefore, mammalian Opn5m may work exclusively as a short wavelength sensor in the brain as well as in the retina with the assistance of an 11-cis-retinal-supplying system.
Introduction
Opsins are the universal photosensitive proteins responsible for visual and nonvisual photoreception in animals and are G protein-coupled receptors having a seven-transmembrane α-helical structure. They contain vitamin A-derivative retinal as their light-absorbing chromophore, and the retinal binds to a conserved lysine residue located at the center of helix VII through a Schiff base linkage. A variety of opsins have been identified so far and are classified into at least seven groups according to their sequence similarity (1, 2). Opn5 is the most recently identified opsin in the human and mouse genomes (3) and is categorized in an independent group on the basis of its low sequence homology to the other opsin groups. Opn5 genes have been identified in various vertebrates from fishes to primates and are classified into several subgroups (4, 5). Most mammals have only one Opn5 gene (Opn5m), whereas nonmammalian vertebrates have additional Opn5 genes. This is probably because of the nocturnal period in the early evolution of mammals, during which mammals lost several nonvisual opsins as well as color opsin genes (6).
In our previous reports, we succeeded in the purification of recombinant Opn5 proteins obtained by the expression of chicken Opn5 genes and reported that these proteins are UV-sensitive bistable pigments that activate the Gi type of G protein (4, 7). We also speculated from the complete conservation of the amino acid residues surrounding the chromophore between chicken and mammalian Opn5m proteins that mammalian Opn5m proteins should be UV-sensitive pigments, which was recently confirmed in mouse and human Opn5m proteins (8). In the present study, we compared the molecular properties of Opn5m proteins from zebrafish (fish), Xenopus tropicalis (amphibian), chicken (bird), mouse (mammal), and human (primate), paying particular attention to the differences between mammals and nonmammalian vertebrates. In addition, we also analyzed the expression patterns of mouse and primate (common marmoset) Opn5m and RPE65, an enzyme essential for generating 11-cis-retinal in the visual cycle (9, 10), to compare with those of chicken Opn5m and RPE65.
EXPERIMENTAL PROCEDURES
Animals and Ethics Statement
Fertilized chicken eggs (Gallus gallus domesticus) were purchased from a commercial farm (Goto-furanjyo, Inc., Gifu, Japan) and incubated at 37.5 °C in a humidified incubator until hatching. Posthatch chicks were housed under a 12:12 light-dark cycle with food and water ad libitum. Postnatal and adult mice were purchased from Shimizu Laboratory Animal Center (ICR and C57BL/6 strains; Shizuoka, Japan). Animals were anesthetized and euthanized at Zeitgeber time 6–10. The eyes and brains of common marmosets were obtained through the Cooperation Research Program of Primate Research Institute of Kyoto University. The use of animals in these experiments was in accordance with the guidelines established by the Ministry of Education, Culture, Sports, Science, and Technology of Japan and University of Tokushima and Kyoto Prefectural University of Medicine (chick; mouse, Mus musculus domesticus) and Kyoto University (common marmoset, Callithrix jacchus). The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Tokushima (permits 08089, 11091, and 11120), Kyoto Prefectural University of Medicine (permit M22-197), and Kyoto University (permits 2011-142 and 2012-B-35). All surgery was performed under deep anesthesia (pentobarbital, 100 mg/kg of body weight), and all efforts were made to minimize suffering.
Preparation of Opn5 Proteins
The cDNAs of human (GenBankTM accession number AY377391), mouse (AY318865), X. tropicalis (XM_002935990), and zebrafish (Danio rerio) (AY493740) Opn5m were tagged by the epitope sequence of the anti-bovine rhodopsin monoclonal antibody Rho1D4 (ETSQVAPA) at the C terminus and were inserted into the mammalian expression vector pCAGGS (11). Site-directed mutations were introduced by the QuikChange kit (Agilent Technologies) according to the manufacturer's method. The plasmid DNA was transfected into HEK293 cells using the calcium phosphate method. After 1 day of incubation, 11-cis- or all-trans-retinal was added to the medium (final retinal concentration, 5 μm) (7). After additional incubation for 1 day in the dark, the cells were collected. The pigments were extracted with 1% digitonin (for human and mouse Opn5m proteins) or 1% n-dodecyl-β-d-maltoside (DM)3 (for Xenopus and zebrafish Opn5m proteins) in buffer A (50 mm HEPES, pH 6.5, and 140 mm NaCl) and were purified using Rho1D4-conjugated agarose. The purified pigments were eluted with 1% digitonin (for human and mouse Opn5m proteins) or 0.02% DM (for Xenopus and zebrafish Opn5m proteins) in buffer A containing the synthetic peptide that corresponds to the C terminus of bovine rhodopsin. The expression levels of human and Xenopus Opn5m proteins were very low, and thus to obtain sufficient amounts of recombinant proteins, we truncated 37 and 21 amino acid residues from the C terminus of human and Xenopus Opn5m proteins, respectively.
Spectrophotometry and HPLC Analysis
Absorption spectra were recorded at 10 °C (for human and mouse Opn5m proteins) or 0 °C (for Xenopus and zebrafish Opn5m proteins) with a Shimadzu UV-2400 spectrophotometer. The sample was irradiated with UV light through a UV-D35 glass filter (Asahi Technoglass), with yellow light through a Y-52 cutoff filter (Toshiba) or with orange light through an O-57 cutoff filter (Toshiba) from a 1 kW halogen lamp (Master HILUX-HR; Rikagaku). The absorption spectra of visible and UV light-absorbing forms of Opn5m proteins were calculated using the methods previously described (7). Briefly, the spectral region at wavelengths longer than the maximum of the main peak of the spectrum difference between the visible and UV light-absorbing forms of the pigment was best fitted with a template spectrum previously described (12, 13). The best fitting spectrum was considered to be the visible light-absorbing form of the pigment. The absorption spectrum of the UV light-absorbing form was then calculated by adding the visible light-absorbing form to the difference spectrum. The chromophore configurations of the sample were analyzed by HPLC (LC-10AT VP; Shimadzu) equipped with a silica column (150 × 6 mm, A-012–3; YMC) according to the previous report (14).
G Protein Activation Assay
A radionucleotide filter binding assay, which measures GDP/GTPγS exchange by G protein, was performed as described previously (4, 7). All procedures were carried out at 0 °C. The assay mixture consisted of 50 mm HEPES (pH 7.0), 140 mm NaCl, 5 mm MgCl2, 1 mm DTT, 0.01% DM, 1 μm [35S]GTPγS, and 2 μm GDP. Mouse Opn5m pigments after reconstitution with 11-cis-retinal were purified in 0.02% DM in buffer A for the assay. Purified mouse Opn5m pigments (final concentration, 300 nm) were mixed with G protein solution (final concentration, 600 nm) and were kept in the dark or irradiated with UV light for 1 min or with subsequent yellow light (>500 nm) for 1 min. After irradiation, the GDP/GTPγS exchange reaction was initiated by the addition of [35S]GTPγS solution to the mixture of the pigment and G protein. After incubation for the indicated time in the dark, an aliquot (20 μl) was removed from the sample into 200 μl of stop solution (20 mm Tris/Cl, pH 7.4, 100 mm NaCl, 25 mm MgCl2, 1 μm GTPγS, and 2 μm GDP), and it was immediately filtered through a nitrocellulose membrane to trap [35S]GTPγS bound to G proteins. The amount of bound [35S]GTPγS was quantitated by assaying the membrane with a liquid scintillation counter (Tri-Carb 2910 TR; PerkinElmer Life Sciences).
Antibodies
Specific antibodies were raised to the N terminus of mouse Opn5m and the C terminus of human Opn5m using guinea pigs. Each polyclonal antibody was commercially produced against a 15-amino acid synthetic peptide conjugated to keyhole limpet hemocyanin by Protein Purify Ltd. (Gunma, Japan), according to their standard procedures (mouse Opn5m, MALNHTALPQDERLPC; human Opn5m, CVRKSSAVLEIHEEWE). We confirmed that the sequence of this portion of human Opn5m (the C terminus) is identical to that of common marmoset Opn5m deposited in the public database. We also confirmed that this isoform of human Opn5m cDNA was isolated from human retina cDNA (Marathon-Ready cDNA; Clontech) with 3′ rapid amplification of cDNA ends. These antibodies were affinity-purified prior to use by Protein Purify Ltd. The primary antibodies used in this study included guinea pig anti-chick Opn5m antibody (7) and rabbit anti-RPE65 antibody (a gift from Dr. Rosalie K. Crouch, Medical University of South Carolina) (15). We performed Western blotting analysis using the antibodies and confirmed that the anti-mouse and human/monkey Opn5m antibodies recognized the recombinant proteins of full-length mouse and human Opn5m expressed in HEK293 cells, respectively (Fig. 1). The secondary antibody used for immunostaining in this study was Alexa Fluor 488 anti-guinea pig IgG (A-11073; Invitrogen).
FIGURE 1.
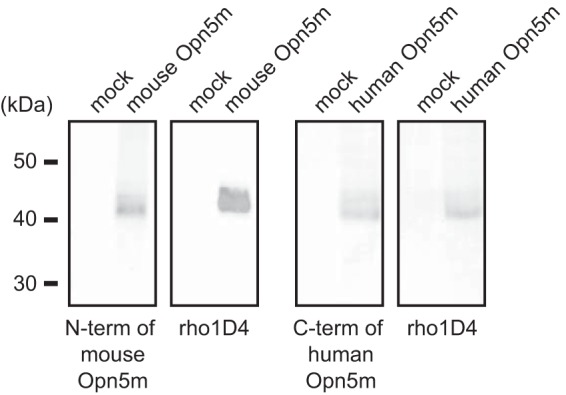
Characterization of the anti-mammalian Opn5m antibodies by Western blotting. Signals were detected in Opn5m-transfected cells but not in mock-transfected cells. Recombinant mammalian Opn5m proteins exhibited two bands, probably because of the heterogeneity of the post-translational modification within the cultured cells, such as the glycosylation in the N terminus of the protein, as shown for recombinant bovine rhodopsin (41).
Fixation and Sectioning
The animals were perfusion-fixed with 4% paraformaldehyde in PBS under deep pentobarbital anesthesia, and the eye and brain were quickly dissected, postfixed for 2–3 h (for eyes) or 16 h (for brain) in the same fixative at 4 °C and then transferred to 20% sucrose until they sank. After embedding the samples in optimal cutting temperature compound (Sakura, Japan), the tissues were sectioned with a cryostat (Leica) at 20-μm thickness. Sections were thaw-mounted onto Matsunami adhesive silane-coated slides (Matsunami, Japan), dried in air for a few hours, and stored at −30 °C until use. The anatomy of the chick, mouse, and common marmoset brain was determined according to Refs. 16–18, respectively, and the nomenclature adopted in this study was based on these atlases and previous studies.
Immunohistochemistry
Fluorescent immunolabeling was performed using standard techniques. Briefly, all slides were blocked for 30 min at room temperature in PBS Triton X-100 (0.25%) (PBST) with 5% serum from the same species as the corresponding secondary antibodies. Primary antibodies were diluted in PBST with 5% serum and secondary antibodies in PBS. All wash steps were performed three times with PBST for 5 min each. Primary antibodies (anti-N terminus of mouse Opn5m (diluted 1:2000), anti-C terminus of human Opn5m (diluted 1:2000), and anti-RPE65 (diluted 1:140) were incubated for 5 h at room temperature or for 16 h at 4 °C. Secondary antibodies were incubated for 1.5 h at room temperature and diluted 1:750. The cell nuclei were stained with DAPI (Vector Laboratories). The slides were mounted with anti-fade mountant, Vectashield (Vector, H-1500). Fluorescent images were collected using a Leica TCS-SP5 confocal laser-scanning microscope with excitation at 405, 488, and 543 nm and emission wavelengths of 424–489, 505–539, and 551–618 nm for DAPI, green, and Cy3, respectively.
In Situ Hybridization of Mouse Brain and Retinal Sections
Frozen sections (20-μm thickness) of mouse (ICR, postnatal days 0, 4, and 10 and 8 weeks, female; C57BL/6, 8 weeks, male and female) and adult common marmoset brain and retina were used. In situ hybridization was performed according to standard procedures. Briefly, digoxigenin-labeled RNA probes were synthesized from full-length cDNA of mouse Opn5m (purchased from IMAGE cDNA Collection), common marmoset Opn5m (GenBankTM accession number XM_002746623, synthesized by Medical & Biological Laboratories, Japan), mouse Rpe65 (GenBankTM accession number AF410461, cloned by PCR from mouse eye), and human Rpe65 (GenBankTM accession number BC075035, purchased from IMAGE cDNA Collection) using T7 T3 or Sp6 RNA polymerase. Sections were pretreated with methanol for 2 h and with proteinase K (20 μg/ml) for 40 min at room temperature. After prehybridization, sections were incubated with digoxigenin-labeled RNA antisense or sense probes (15∼30 ng/ml in hybridization buffer) at 65 °C overnight. They were washed with 1× SSC buffer containing 50% formamide three times at 65 °C and maleic acid buffer containing 0.1% Tween 20 and subsequently incubated with anti-digoxigenin antibody conjugated to alkaline phosphatase (1:2000 in PBS containing 0.1% Triton X-100; Roche Applied Science) overnight at 4 °C. Color was developed with BCIP/NBP. Sections were coverslipped and observed under a microscope.
Western Blotting
Extract from full-length human or mouse Opn5m-transfected HEK293 cells was subjected to SDS-PAGE, transferred onto a polyvinylidene difluoride membrane, and probed with anti-human or mouse Opn5m antibody (1:1000) (Fig. 1). Protein extracts from mouse tissues were prepared as follows (8). Eight-week-old male mice were anesthetized and perfused with ice-cold PBS. The tissues were dissected and solubilized in 1% DM in buffer B (50 mm HEPES, pH 6.5, 140 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, 4 mg/ml aprotinin, 4 mg/ml leupeptin, 0.1 mm phenylmethylsulfonyl fluoride). After centrifugation, the supernatant was collected for SDS-PAGE. Mouse Opn5m or RPE65 in the extracts from mouse neural retina, retinal pigment epithelium (RPE), hypothalamus, other brain area, lung, and liver was detected by anti-mouse Opn5m (1:1500) or RPE65 antibody (1:1000) (see Fig. 7, O and P).
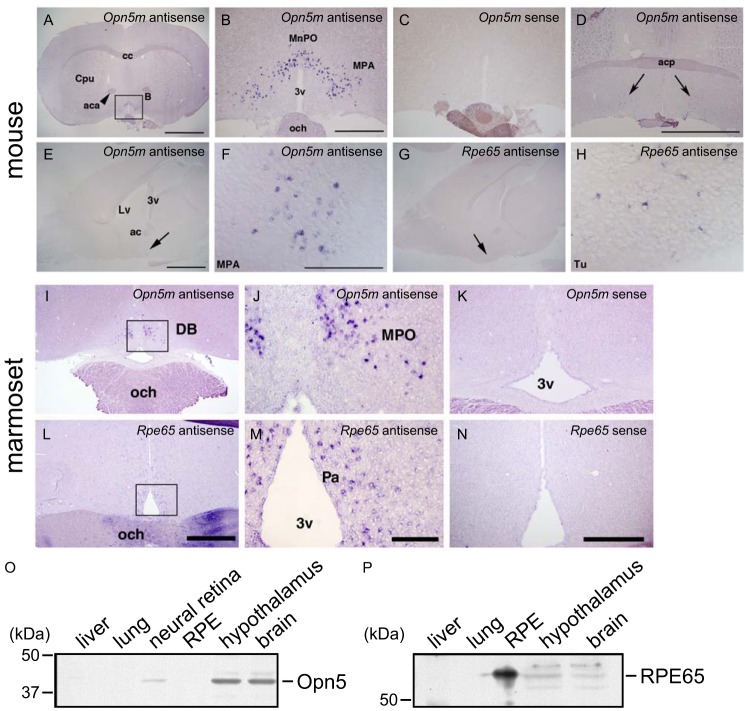
FIGURE 7.
Expression of Opn5m and Rpe65 in the mammalian brain. A–D, detection of Opn5m mRNA in the preoptic area. Coronal sections of mouse brain (ICR strain, female, 12 weeks) at the hypothalamus level. Approximate Bregma levels are 0.50 mm (A–C) and 0.14 mm (D). Large magnifications of A are shown in B as indicated. Opn5m mRNA was exclusively detected in the median preoptic nucleus and medial preoptic area at the anterior hypothalamus as shown in A, B, and D. C, sense probe did not give rise to any staining. D, a small number of Opn5m-expressing cells were detected in the medial preoptic area (arrows) at the level of the posterior part of the anterior commissure. Scale bars, 2 mm in A, 500 μm in B and C, and 2 mm in D. cc, corpus callosum; Cpu, caudate putamen (striatum); aca, anterior commissure, anterior part; MnPO, median preoptic nucleus; MPA, medial preoptic area; 3v, third ventricle; och, optic chiasm; acp, anterior commissure. E–H, detection of Rpe65 mRNA near the Opn5m expression domain in the mouse brain (approximate lateral level, 0.36 mm). The arrows show the localization of Opn5m mRNA in the medial optic area (E and F) and Rpe65 mRNA (G and H) in the olfactory tubercle. Scale bars, 2 mm in E and G and 0.2 mm in F and H. Lv, lateral ventricle; 3v, third ventricle; ac, anterior commissure; MPA, medial preoptic area; Tu, olfactory tubercle. I–N, detection of Opn5m and Rpe65 mRNA in the common marmoset anterior hypothalamus. Opn5m mRNA was expressed in the anterior part of the medial preoptic area (MPO; I and J) medially to the diagonal band (DB). The boxed area in I is magnified in J. Opn5m-positive cells were observed only in the anterior-most part of the MPO. Rpe65 mRNA was detected in the paraventricular nucleus (Pa) of the anterior hypothalamus, slightly caudal to the Opn5m-positive cells (L and M). The boxed area in L is magnified in M. Signals were not detected with sense probes (K and N). Scale bars, 1 mm in I and L, 200 μm in J and M, and 500 μm in K and N. O and P, expression of Opn5m and RPE65 proteins in the mouse brain analyzed by Western blotting. O, Opn5m protein was detected in the neural retina, hypothalamus, and other brain areas, not in the RPE, lung, or liver. P, REP65 protein was detected in the RPE, hypothalamus, and other brain areas, not in the lung or liver.
RESULTS AND DISCUSSION
Vertebrate Opn5m Proteins Reconstituted with 11-cis- or All-trans-retinal
We first expressed recombinant proteins of Opn5m from four vertebrates, zebrafish, X. tropicalis, mouse, and human, in cultured cells and examined whether or not these pigments have molecular properties similar to those of chicken Opn5m. As already reported, chicken Opn5m is a UV-sensitive bistable pigment that forms 11-cis- and all-trans-retinal bound states, the latter of which activates the Gi type of G protein (7). We also found that, although the apoprotein of Opn5m protein is extremely unstable, we could successfully obtain adequate amounts of chicken Opn5m pigment by adding 11-cis- or all-trans-retinal to the culture medium during the protein expression. Fig. 2C shows the photoreactions of chicken Opn5m that had been purified after incubation with 11-cis-retinal. The absorption spectrum of purified pigment exhibited UV and visible absorbances, indicating that the chicken Opn5m protein binds 11-cis-retinal to produce a UV light-absorbing form and also binds all-trans-retinal, which is formed by thermal isomerization of 11-cis-retinal in culture medium, to produce a visible light-absorbing form (Fig. 2H) (7). UV light irradiation of the pigment caused the conversion of the UV light-absorbing form to the visible light-absorbing form, and subsequent yellow light irradiation resulted in the complete conversion of the visible light-absorbing form to the UV light-absorbing form. The light-induced interconversion between the UV light- and visible light-absorbing forms could be observed repeatedly (Fig. 2C).
FIGURE 2.
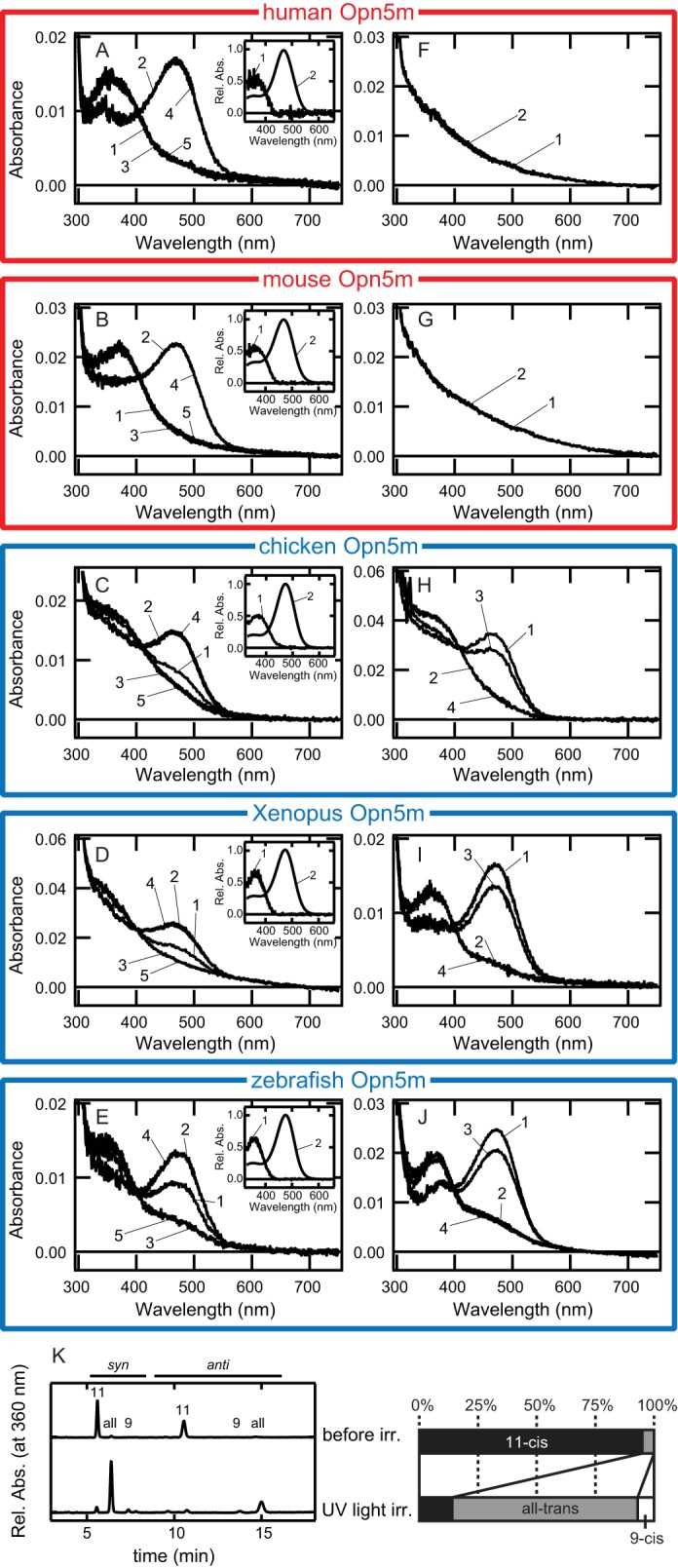
Comparison of the ability to directly bind 11-cis- or all-trans-retinal in vertebrate Opn5m proteins. A–E, absorption spectra of human (A), mouse (B), chicken (C), X. tropicalis (D), and zebrafish (E) Opn5m proteins after incubation with 11-cis-retinal. Spectra were measured in the dark (curve 1) and after UV light irradiation (curve 2), subsequent yellow light (>500 nm) irradiation (curve 3), UV light reirradiation (curve 4), or yellow light reirradiation (curve 5). Insets, the calculated absorption spectra of each Opn5m protein in the dark (curve 1) and after UV light irradiation (curve 2). F–J, absorption spectra of human (F), mouse (G), chicken (H), Xenopus (I), and zebrafish (J) Opn5m proteins after incubation with all-trans-retinal. Spectra were measured in the dark (curve 1) and after yellow light (>500 nm) irradiation (curve 2), subsequent UV light irradiation (curve 3), or yellow light reirradiation (curve 4). K, retinal configuration changes by UV light irradiation of human Opn5m. Left-hand panel, the retinal configurations were analyzed with HPLC after extraction of the chromophore as retinal oximes (syn and anti forms of 9-cis-, 11-cis-, and all-trans-retinal oximes). Right-hand panel, isomeric compositions of retinal before and after light irradiation of human Opn5m.
We performed similar experiments using zebrafish, Xenopus, mouse and human Opn5m proteins (Fig. 2, A, B, D, and E). The absorption spectra and photoreactions of zebrafish and Xenopus Opn5m were quite similar to those of chicken Opn5m. That is, the addition of 11-cis-retinal to the culture medium caused the production of UV light- and visible light-absorbing forms that were interconvertible by light (Fig. 2, D and E). In contrast, incubation of mouse and human Opn5m proteins with 11-cis-retinal in the culture medium caused the production of only the UV light-absorbing form. In fact, UV light irradiation of mouse and human pigments caused the production of the visible-light absorbing form, and subsequent yellow light irradiation resulted in the production of the UV-light absorbing form, whose absorption spectrum overlapped with that of the dark state (Fig. 2, A and B). The analysis of the retinal configurations of human Opn5m also showed that human Opn5m was exclusively reconstituted with 11-cis-retinal, and UV light irradiation induced the retinal isomerization from 11-cis to all-trans form (Fig. 2K). Therefore, mouse and human Opn5m showed photoreversibility similar to that of chicken Opn5m but had no ability to directly bind all-trans-retinal.
The absorption maxima of the UV light-absorbing forms of these Opn5m proteins are all located at 360 nm (Fig. 2, insets). In contrast, whereas the absorption maxima of visible light-absorbing forms of zebrafish, Xenopus, and chicken Opn5m proteins are located at 474 nm, those of mouse and human Opn5m proteins are located at 469 nm (Fig. 2, insets). These differences would reflect the differences of the ability to bind all-trans-retinal among these Opn5m proteins.
To confirm that mammalian Opn5m has no ability to directly bind all-trans-retinal, we performed similar expression experiments in the presence of all-trans-retinal instead of 11-cis-retinal in the culture medium (Fig. 2, F–J). In zebrafish and Xenopus Opn5m proteins, we observed the production of visible light-absorbing forms that exhibited photoreactions identical to that of chicken Opn5m (Fig. 2, H–J). On the other hand, we observed no pigments of mouse or human Opn5m under the present conditions (Fig. 2, F and G). These results clearly showed that mammalian Opn5m proteins have no ability to directly bind all-trans-retinal.
We also confirmed that the visible light-absorbing form of mammalian Opn5m is the active state that couples with Gi (Fig. 3). Purified mouse Opn5m reconstituted with 11-cis-retinal activated Gi in a UV light-dependent manner and lost the activity after subsequent yellow light irradiation. These alterations of the activity are consistent with the spectral changes shown in Fig. 2B, indicating that the visible light-absorbing form is the state-activating G protein.
FIGURE 3.
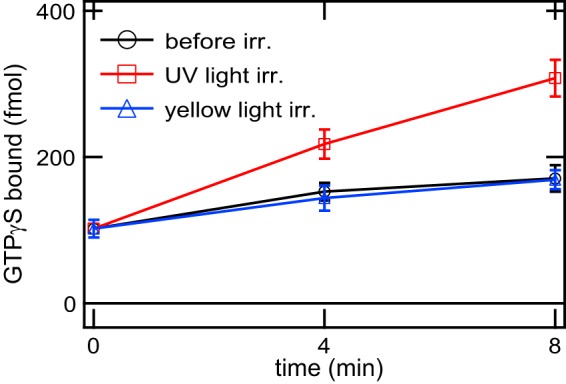
Time courses of G protein activation ability of mouse Opn5m. Gi activation ability of mouse Opn5m reconstituted with 11-cis-retinal was measured in the dark (circles), after UV light irradiation (squares), and after subsequent yellow light (>500 nm) irradiation (triangles). The data are presented as the means ± S.E. of three independent experiments. irr., irradiation.
Determination of Amino Acid Residue That Controls the Ability of Direct Binding of All-trans-retinal
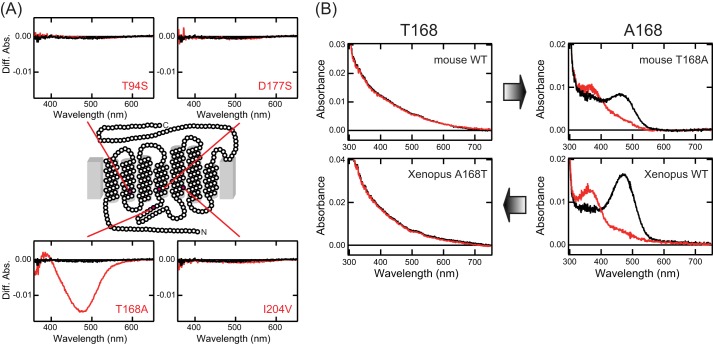
Next, we attempted to identify the amino acid residue(s) whose substitution(s) resulted in a loss of the ability to directly bind all-trans-retinal in mammalian Opn5m proteins. Comparison of the amino acid residues within 4.5 Å from the retinal, which are inferred from the three-dimensional structures of bovine and squid rhodopsins (19, 20), indicates that all the residues are completely conserved in mammalian and chicken Opn5m proteins. Thus, we compared the residues within 8 Å from the retinal among vertebrate Opn5m proteins. There are four residues that are conserved in human and mouse Opn5m proteins but not in nonmammalian Opn5m proteins (Table 1). We introduced each corresponding mutation (T94S, T168A, D177S, or I204V in the bovine rhodopsin numbering system) into mouse Opn5m and analyzed the alteration of the ability of direct incorporation of all-trans-retinal. Orange light irradiation of mouse Opn5m mutants incubated with all-trans-retinal led to a significant spectral change in T168A mutant, but not in wild type or other mutants (Fig. 4A). The purified mouse Opn5m T168A had the peak of its absorption spectrum in the visible region and was photoconverted into a pigment whose absorption maximum was in the UV region (Fig. 4B). This spectral change is the same as those observed in nonmammalian Opn5m proteins reconstituted with all-trans-retinal. It should be noted that T168A mutation of mouse Opn5m resulted in red shift of absorption maximum, close to those of nonmammalian Opn5m proteins. These results indicated that T168A mutation alters the all-trans-retinal binding ability as well as the electrostatic environment of the all-trans-retinal chromophore. In addition, we prepared A168T mutant of Xenopus Opn5m reconstituted with all-trans-retinal. The purified mutant exhibited no detectable peak of the absorption spectrum in the visible region and no spectral change after yellow light irradiation (Fig. 4B). Thus, this mutant lost the ability to directly bind all-trans-retinal. These data clearly showed that a single amino acid residue can modulate the ability of direct incorporation of all-trans-retinal as well as the electrostatic environment of the all-trans-retinal chromophore.
TABLE 1.
Comparison of the amino acid residues among vertebrate Opn5m proteins
| Residue no. | Bovine rhodopsina | Squid rhodopsin | Zebrafish Opn5m | Xenopus Opn5m | Chicken Opn5m | Platypus/opossum Opn5m | Mouse/human Opn5m |
|---|---|---|---|---|---|---|---|
| 94b | T | M | F | A | S | T | T |
| 168 | A | A | A | A | A | A | T |
| 177 | R | A | N | N | S | N | D |
| 204 | V | I | V | V | V | I | I |
a NCBI accession numbers of opsin sequences are as follows: bovine rhodopsin, K00506; squid rhodopsin, X70498; platypus Opn5m, XM_001511941; and opossum Opn5m, XM_001369165.
b Amino acid residue numbers are designated using the bovine rhodopsin numbering system.
FIGURE 4.
Alteration of the ability to directly bind all-trans-retinal in mouse and Xenopus Opn5m mutants. A, spectral changes of mouse Opn5m proteins caused by orange light (>550 nm) irradiation. Extracts containing wild type, T94S, T168A, D177S, and I204V of mouse Opn5m with 1% DM after reconstitution with all-trans-retinal were irradiated, and then the difference spectra before and after irradiation were calculated. Each panel contains the spectra of wild type (black curves) and mutant (red curves). B, modulation of the ability to directly bind all-trans-retinal by a single mutation at position 168. Absorption spectra of wild type and mutant of mouse and Xenopus Opn5m reconstituted with all-trans-retinal were measured in the dark (black curves) and after yellow light (>500 nm) irradiation (red curves).
It has been reported that bovine rhodopsin cannot be reconstituted with all-trans-retinal to form the active state (21), because in neutral pH the equilibrium of inactive and active conformations of apoprotein of bovine rhodopsin is mostly biased toward the inactive conformation (22). Bovine rhodopsin has an alanine residue at position 168, the same as nonmammalian vertebrate Opn5m (Table 1). Therefore, the lack of ability of all-trans-retinal binding in bovine rhodopsin cannot be explained by the same molecular mechanism. Thus, the loss of all-trans-retinal binding ability in vertebrate visual opsin and mammalian Opn5m is the result of convergent evolution.
The alanine residue at position 168, which is well conserved in nonmammalian Opn5m proteins, is replaced by a threonine residue in most mammalian Opn5m proteins. The exceptions are the platypus (Ornithorhynchus anatinus) and the opossum (Monodelphis domestica) Opn5m proteins, which have an alanine residue in this position (Table 1). We speculate that A168T mutation occurred after the divergence of eutherian and metatherian mammals.
Localization of Mammalian Opn5m in the Retina and the Brain
Mammals have several nonvisual opsins, including Opn5m. The mRNA transcript of mammalian Opn4 (melanopsin) is exclusively expressed in the retina (23), whereas the mRNA of mammalian Opn3 (encephalopsin) is detected in the brain, but not in the retina (24). On the other hand, the mRNA of mammalian Opn5m is present in both the retina and brain (3, 8, 25), suggesting the multiple physiological roles of Opn5m. Therefore, it is of interest to investigate the detailed expression patterns of mammalian Opn5m.
For this, first we performed in situ hybridization and immunostaining on mouse retinal sections. Although our standard in situ hybridization technique could not detect Opn5m mRNA in the adult mouse retina, we observed the expression of Opn5m in the immature retina at postnatal day 1 (P1) and 11 (P11) (Fig. 5). Furthermore, we found Opn5m immunoreactive cells in the ganglion cell layer of the adult retina (Fig. 6A). These signals were not seen when immunostaining was performed using antigen-adsorbed antibodies (Fig. 6B). We also performed immunohistochemical studies using the common marmoset retina and detected signals in the ganglion cell layer and the inner nuclear cell layer (Fig. 6, C, C′, and D). The expression pattern of Opn5m in the retina was similar between mouse and common marmoset and consistent with those of mouse and rat seen in the previous studies (8, 25). We previously observed immunostaining signals of Opn5m in the ganglion cell layer and the inner nuclear cell layer of chick retina (7). Thus, the localization of Opn5m in the retina is conserved between mammals and chicken.
FIGURE 5.
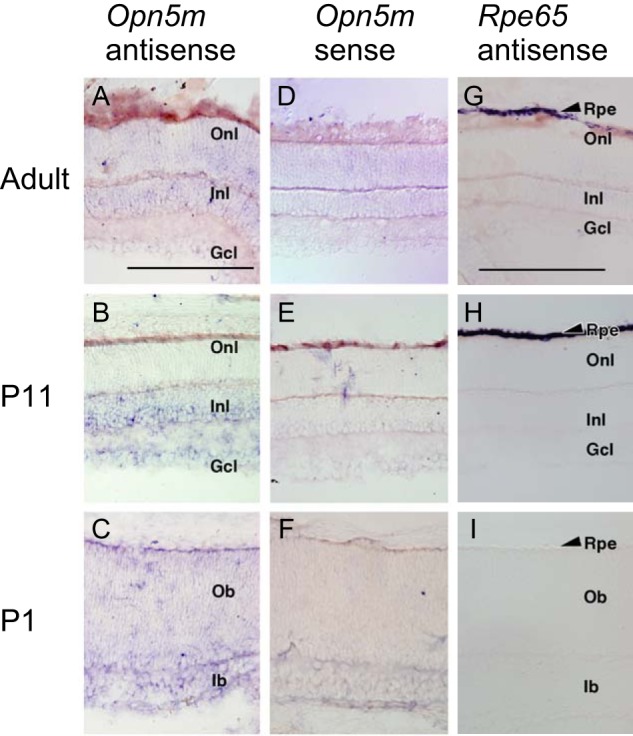
Expression of Opn5m and Rpe65 mRNA in the mouse retina. A–F, detection of Opn5m mRNA in the retina of adult (ICR strain, 8 weeks, male), P11, and P1 mice. D–F, sense probes gave essentially no signals. G–I, detection of Rpe65 mRNA in the RPE of adult, P11, and P1 mouse. I, Rpe65 mRNA was undetectable in P1 retina. Rpe, retinal pigment epithelium; Onl, outer nuclear layer; Inl, inner nuclear layer; Gcl, ganglion cell layer; Ob, outer neuroblastic layer; Ib, inner neuroblastic layer. Scale bars, 0.2 mm in A–F and 0.2 mm in G–I.
FIGURE 6.
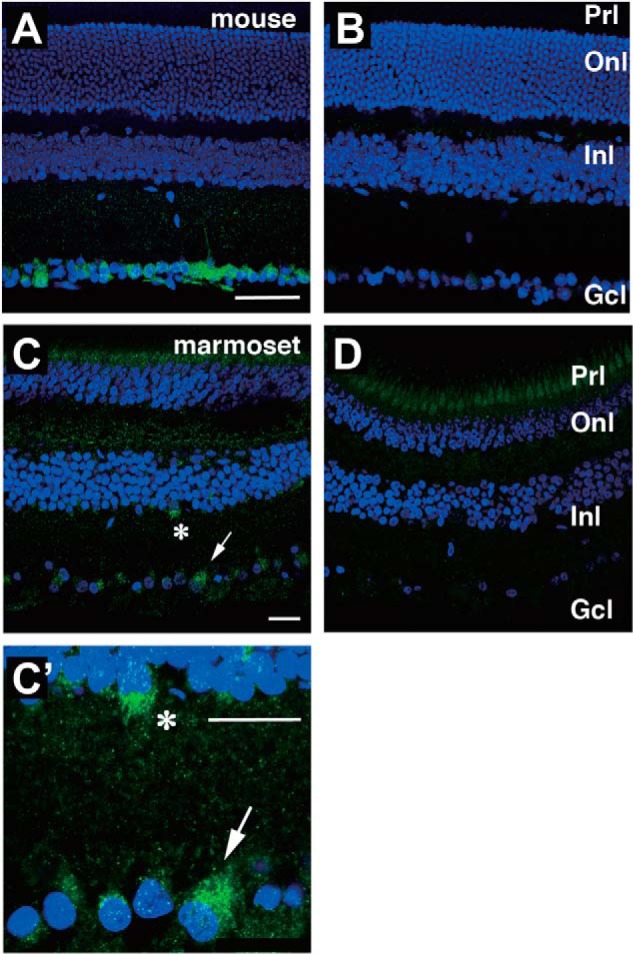
Localization of Opn5m-expressing cells in the mammalian retina. Immunostaining of adult mouse (A and B) and common marmoset (C, C′, and D) retina is shown. B and D, when using the antibody preadsorbed with the antigenic peptide, there were no signals except weak signals in the ganglion cell layer in B and photoreceptor cell layer in D. Asterisk and arrow in C show Opn5m-expressing cells enlarged in C′. Onl, outer nuclear layer; Inl, inner nuclear layer; Gcl, Ganglion cell layer; Prl, photoreceptor layer. Scale bars, 50 μm in A and B and 25 μm in C, C′, and D.
We next investigated the localization of Opn5m mRNA in the mouse brain. The results showed that Opn5m was expressed exclusively in the preoptic area, specifically the median preoptic nucleus and medial preoptic area of the anterior hypothalamus (Fig. 7, A–D). However, there was no Opn5m mRNA in the posterior hypothalamus or hippocampus (Fig. 8), whose counterparts in birds are known to contain photoreceptive cells (26). Moreover, our Western blotting analysis showed that Opn5m protein was detected in the hypothalamus as well as in the neural retina and other brain areas with the expected molecular weight (Fig. 7O).
FIGURE 8.
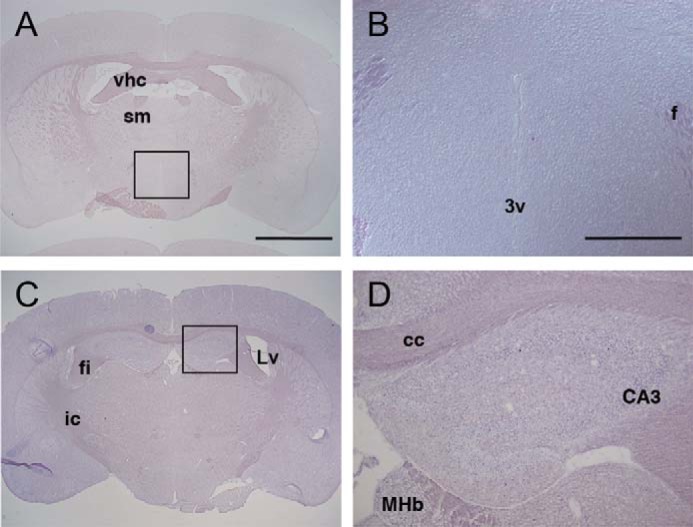
Expression of Opn5m mRNA in the mouse posterior hypothalamus and hippocampus. A–D, coronal sections of mouse brain (ICR strain, female, 12 weeks) at the posterior hypothalamus (A and B) and hippocampus (C and D) levels. Approximate Bregma levels are −0.58 mm (A and B) and −1.34 mm (C and D). Large magnifications of A and C are shown in B and D as indicated. A and B, at the posterior hypothalamus, Opn5m mRNA was not detected in the paraventricular nucleus along the third ventricle. C and D, Opn5m mRNA was not detectable in the hippocampus. vhc, ventral hippocampal commissure; sm, stria medullaris; 3v, third ventricle; f, fornix; fi, fimbria of the hippocampus; Lv, lateral ventricle; ic, internal capsule; cc, corpus callosum; CA3, field CA3 of the hippocampus; MHb, medial habenular nucleus. Scale bars, 2 mm in A and C and 500 μm in B and D.
Comparison of Localization of Opn5m and an 11-cis-Retinol-generating Enzyme, RPE65, in the Mammalian Brain
We were surprised to find that mouse Opn5m is expressed in the preoptic hypothalamus, a deep brain region, because, in addition to the fact that UV light would scarcely penetrate into such a deep brain region, our biochemical analysis showed that mouse Opn5m protein has lost the ability to directly bind all-trans-retinal. We previously speculated that chicken Opn5m in the paraventricular organ (PVO) of the hypothalamus can be regenerated by all-trans-retinal binding and work as a photoreceptive molecule by absorbing visible light even without an 11-cis-retinal supply, because visible light can more easily be transmitted into the deep brain than UV light (7). However, mouse Opn5m cannot be regenerated by all-trans-retinal, and therefore it appears essential to have an 11-cis-retinal-supplying system (as in the visual cycle mechanism) in the vicinity. One important enzyme constituting the visual cycle is the RPE-specific 65-kDa protein, RPE65. RPE65 in the RPE is involved in the conversion of all-trans-retinyl ester to 11-cis-retinol during the visual cycle (9, 10). We therefore examined the expression domain of Rpe65 in the mouse brain. We first confirmed that a high level of Rpe65 mRNA is observed in the RPE of the adult mouse retina (Fig. 5). Then, we performed in situ hybridization on mouse brain sections and showed that Rpe65 is expressed in the domains (Fig. 7, G and H) near the Opn5m-expressing cells (Fig. 7, E and F). In addition, by the Western blotting analysis we detected multiple bands of putative RPE65 proteins in the dissected hypothalamus and other brain areas including a band of the same molecular weight as the band observed in the RPE (Fig. 7P). These results clearly showed that an RPE65-dependent retinal isomerization system is present near the region where Opn5m is expressed. It has been shown that lecithin retinol acyltransferase, an enzyme that produces the substrate of RPE65 from all-trans-retinol in the visual cycle, is also expressed in the mouse brain, although its detailed expression pattern remains unknown (27). Thus, lecithin retinol acyltransferase may also contribute to Opn5m-RPE65 system in the brain.
Opn5m mRNA has also been detected in the human brain by RT-PCR analysis (3). Thus, to analyze the detailed distributions of Opn5m and Rpe65 in the primate brain, we performed in situ hybridization on the common marmoset brain. We detected the expression of Opn5m mRNA in the anterior part of the medial preoptic area (Fig. 7, I–K), which is in the vicinity of Rpe65-expressing domains (Fig. 7, L–N). These results showed that Opn5m and RPE65 are expressed in the primate anterior hypothalamus.
We also examined the expression pattern of RPE65 in the chick brain by immunostaining. We found that anti-RPE65 antibody (Fig. 9, D and E) detected a subset of ependymal cells lining the third ventricle (Fig. 9, B and C). However, in the PVO region, there were no RPE65-immunoreactive cells in the ependymal layer, which abuts the Opn5m-immunoreactive neurons (Fig. 9A). These results support the notion that, in the chick brain, Opn5m-expressing cells of the PVO, which are thought to be responsible for the photoperiodism in birds (28, 29), do not require assistance by RPE65 in generating 11-cis-retinal, because chicken Opn5m is a bistable pigment that can generate the 11-cis-retinal-binding form from the all-trans-retinal-binding form (7).
FIGURE 9.
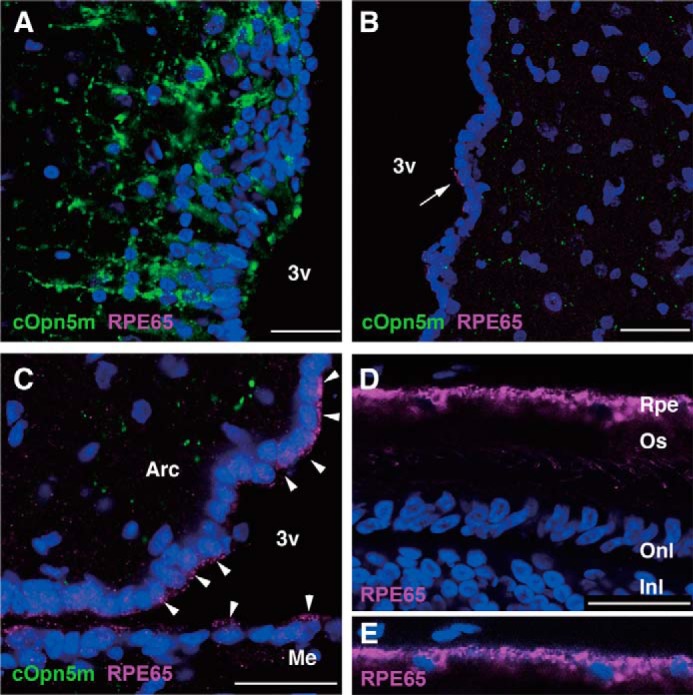
Localization of RPE65-immunoreactive cells in the chick brain. A, Opn5m-expressing neurons in the PVO. There were no RPE65-immunoreactive cells in and near the PVO. B, in the anterior hypothalamus, a subset of ependymal cells were immunoreactive for RPE65. The arrow shows RPE65 protein localized to the apical side of an ependymal cell. C, more intense expression of RPE65 protein was observed in the ependymal cells lining the ventral region of the third ventricle (arrowheads). D, the anti-RPE65 antibody recognized retinal pigment epithelium (Rpe) in the retina at P10. E, to better visualize nuclei of RPE cells, the blue channel is enhanced. Nuclei are stained with DAPI. 3v, third ventricle; Arc, arcuate nucleus; Me, median eminence; Os, outer segments of photoreceptors; Onl, outer nuclear layer; Inl, inner nuclear layer. Scale bar, 25 μm.
Photoreception within Mammalian Retina and Brain via Opn5m
In this study, we demonstrated that not only rodents but also primates have the UV-sensitive Opn5 in the retina. Like the expression of melanopsin in a subset of retinal ganglion cells in mammals, including primates (30, 31), the present findings suggest that retinal photoreception in mammals and primates is not restricted to classical rod and cone photoreceptor cells or intrinsically photosensitive retinal ganglion cells. More importantly, we demonstrated here the expression of Opn5m in the preoptic hypothalamus in the mouse and common marmoset deep brain. Mammalian Opn5m proteins exclusively bind 11-cis-retinal because of their loss of the ability to directly bind all-trans-retinal. Direct binding of all-trans-retinal causes the formation of the active state and elevates the G protein activation ability without light irradiation, which lowers the signal to noise ratio in light-dependent signaling. Light can penetrate the skull in several mammalian species, including humans (26), and mammalian cerebral cortical tissues were reported to respond to low intensity light to regulate neurotransmitter release (32). In birds, the measurement of the light transmittance into the deep brain revealed that the hypothalamus can receive light enough to trigger opsin-mediated signal transduction (33) because of the high quantum yield of opsins (34). Thus, although there is no direct evidence to show that mammals actually utilize Opn5m to sense short wavelength light, by the acquisition of exclusive 11-cis-retinal binding ability, Opn5m could function as a high sensitivity photosensor within the mammalian brain. It is known that the sexually dimorphic nucleus is located in a cluster of cells of the preoptic area in the hypothalamus of the brain, that it is related to sexual behavior in animals, and that its volume is usually larger in males than in females (35). Although we found no obvious differences in the amount of Opn5m-expressing cells between males and females (Fig. 10), Opn5m in this region might have a role in some sexual behaviors. In addition, the preoptic area of the hypothalamus is an important region to control the body temperature (36). Thus, Opn5m might contribute to light-dependent thermoregulation (37). In birds, Opn5m in the deep brain can regulate photoperiodic response (28). However, in mammals, visual opsins and Opn4 in the retina exclusively contribute to the control of melatonin release from the pineal gland (38, 39), and thus Opn5m in the deep brain is not involved in photoperiodism (40). Compared with chicken Opn5m, mammalian Opn5m has changed its molecular property (i.e., has lost direct binding to all-trans-retinal) and its expression pattern within the brain, although it is difficult to precisely compare the areas of the brain between mammals and birds. The change of the molecular property and the expression pattern of Opn5m may be related to the difference of the physiological role of Opn5m between mammals and birds. Therefore, it is intriguing to speculate that Opn5m might have obtained a new physiological role in the early evolution of mammals, which will be the subject of our future research.
FIGURE 10.
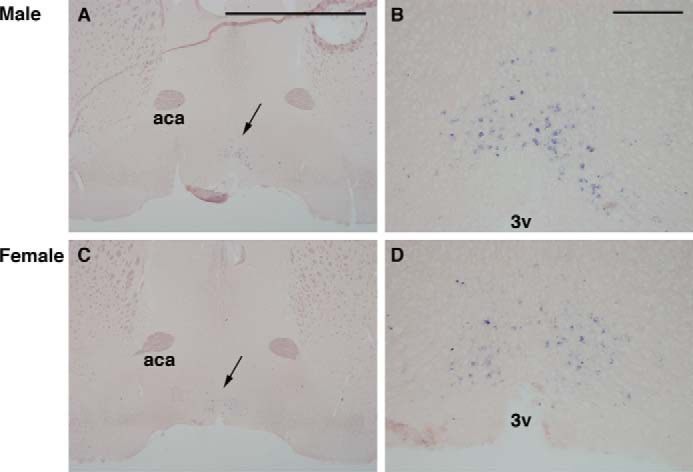
Expression of Opn5m mRNA (arrows in A and C) in the mouse anterior hypothalamus (Bregma level, 0.50 mm). A and B, from male (C57BL6 strain, 8 weeks). C and D, from female (C58BL6, 8 weeks). Higher magnification of A and C (arrows) is shown in B and D, respectively. The expression pattern appeared similar between the two sexes. aca, anterior commissure, anterior part; 3v, third ventricle. Scale bars, 2 mm in A and C and 0.2 mm in B and D.
Acknowledgments
We are grateful to Dr. Elizabeth Nakajima and Dr. Take Matsuyama for critical reading of the manuscript. We thank Prof. Rosalie K. Crouch for the generous gift of the antibody against RPE65. We also thank the Institute for Amphibian Biology of Hiroshima University for the generous gift of X. tropicalis through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology (Japan).
This work was supported by the Cooperation Research Program of Primate Research Institute, Kyoto University. This work was also supported in part by Grants-in-Aid for Scientific Research (to T. Y., K. O., H. O., S. N., K. N., and Y. S.) and the Global Center of Excellence Program “Formation of a Strategic Base for Biodiversity and Evolutionary Research: From Genome to Ecosystem” of the Ministry of Education, Culture, Sports, Science and Technology (Japan).
- DM
- n-dodecyl-β-d-maltoside
- GTPγS
- guanosine 5′-O-(thiotriphosphate)
- RPE
- retinal pigment epithelium
- Pn
- postnatal day n
- PVO
- paraventricular organ.
REFERENCES
- 1. Koyanagi M., Terakita A. (2008) Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem. Photobiol. 84, 1024–1030 [DOI] [PubMed] [Google Scholar]
- 2. Shichida Y., Matsuyama T. (2009) Evolution of opsins and phototransduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2881–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarttelin E. E., Bellingham J., Hankins M. W., Foster R. G., Lucas R. J. (2003) Neuropsin (Opn5). A novel opsin identified in mammalian neural tissue. FEBS Lett. 554, 410–416 [DOI] [PubMed] [Google Scholar]
- 4. Ohuchi H., Yamashita T., Tomonari S., Fujita-Yanagibayashi S., Sakai K., Noji S., Shichida Y. (2012) A non-mammalian type opsin 5 functions dually in the photoreceptive and non-photoreceptive organs of birds. PLoS One 7, e31534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tomonari S., Migita K., Takagi A., Noji S., Ohuchi H. (2008) Expression patterns of the opsin 5-related genes in the developing chicken retina. Dev. Dyn. 237, 1910–1922 [DOI] [PubMed] [Google Scholar]
- 6. Kuraku S., Kuratani S. (2011) Genome-wide detection of gene extinction in early mammalian evolution. Genome Biol. Evol. 3, 1449–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamashita T., Ohuchi H., Tomonari S., Ikeda K., Sakai K., Shichida Y. (2010) Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. U.S.A. 107, 22084–22089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kojima D., Mori S., Torii M., Wada A., Morishita R., Fukada Y. (2011) UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One 6, e26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai X., Conley S. M., Naash M. I. (2009) RPE65. Role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 30, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiser P. D., Palczewski K. (2010) Membrane-binding and enzymatic properties of RPE65. Prog. Retin. Eye Res. 29, 428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niwa H., Yamamura K., Miyazaki J. (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 [DOI] [PubMed] [Google Scholar]
- 12. Govardovskii V. I., Fyhrquist N., Reuter T., Kuzmin D. G., Donner K. (2000) In search of the visual pigment template. Vis. Neurosci. 17, 509–528 [DOI] [PubMed] [Google Scholar]
- 13. Lamb T. D. (1995) Photoreceptor spectral sensitivities. Common shape in the long-wavelength region. Vision Res. 35, 3083–3091 [DOI] [PubMed] [Google Scholar]
- 14. Tsutsui K., Imai H., Shichida Y. (2007) Photoisomerization efficiency in UV-absorbing visual pigments. Protein-directed isomerization of an unprotonated retinal Schiff base. Biochemistry 46, 6437–6445 [DOI] [PubMed] [Google Scholar]
- 15. Tang P. H., Wheless L., Crouch R. K. (2011) Regeneration of photopigment is enhanced in mouse cone photoreceptors expressing RPE65 protein. J. Neurosci. 31, 10403–10411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puelles L. (2007) The Chick Brain in Stereotaxic Coordinates: An Atlas Featuring Neuromeric Subdivisions and Mammalian Homologies, Academic Press, Orlando, FL [Google Scholar]
- 17. Franklin K. B., Paxinos G. (2007) The Mouse Brain in Stereotaxic Coordinates, 3rd Ed., Academic Press, Orlando, FL [Google Scholar]
- 18. Yuasa S., Nakamura K., Kohsaka S. (2010) Stereotaxic Atlas of the Marmoset Brain, Igaku Shoin, Tokyo [Google Scholar]
- 19. Murakami M., Kouyama T. (2008) Crystal structure of squid rhodopsin. Nature 453, 363–367 [DOI] [PubMed] [Google Scholar]
- 20. Okada T., Fujiyoshi Y., Silow M., Navarro J., Landau E. M., Shichida Y. (2002) Functional role of internal water molecules in rhodopsin revealed by x-ray crystallography. Proc. Natl. Acad. Sci. U.S.A. 99, 5982–5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jäger S., Palczewski K., Hofmann K. P. (1996) Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry 35, 2901–2908 [DOI] [PubMed] [Google Scholar]
- 22. Vogel R., Siebert F. (2001) Conformations of the active and inactive states of opsin. J. Biol. Chem. 276, 38487–38493 [DOI] [PubMed] [Google Scholar]
- 23. Bellingham J., Chaurasia S. S., Melyan Z., Liu C., Cameron M. A., Tarttelin E. E., Iuvone P. M., Hankins M. W., Tosini G., Lucas R. J. (2006) Evolution of melanopsin photoreceptors. Discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 4, e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blackshaw S., Snyder S. H. (1999) Encephalopsin. A novel mammalian extraretinal opsin discretely localized in the brain. J. Neurosci. 19, 3681–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieto P. S., Valdez D. J., Acosta-Rodríguez V. A., Guido M. E. (2011) Expression of novel opsins and intrinsic light responses in the mammalian retinal ganglion cell line RGC-5. Presence of OPN5 in the rat retina. PLoS One 6, e26417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vigh B., Manzano M. J., Zádori A., Frank C. L., Lukáts A., Röhlich P., Szél A., Dávid C. (2002) Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol. Histopathol. 17, 555–590 [DOI] [PubMed] [Google Scholar]
- 27. Liu L., Gudas L. J. (2005) Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J. Biol. Chem. 280, 40226–40234 [DOI] [PubMed] [Google Scholar]
- 28. Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., Ebihara S., Kubo Y., Yoshimura T. (2010) A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. U.S.A. 107, 15264–15268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oishi T., Yamao M., Kondo C., Haida Y., Masuda A., Tamotsu S. (2001) Multiphotoreceptor and multioscillator system in avian circadian organization. Microsc. Res. Tech. 53, 43–47 [DOI] [PubMed] [Google Scholar]
- 30. Dacey D. M., Liao H. W., Peterson B. B., Robinson F. R., Smith V. C., Pokorny J., Yau K. W., Gamlin P. D. (2005) Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 [DOI] [PubMed] [Google Scholar]
- 31. Provencio I., Rodriguez I. R., Jiang G., Hayes W. P., Moreira E. F., Rollag M. D. (2000) A novel human opsin in the inner retina. J. Neurosci. 20, 600–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wade P. D., Taylor J., Siekevitz P. (1988) Mammalian cerebral cortical tissue responds to low-intensity visible light. Proc. Natl. Acad. Sci. U.S.A. 85, 9322–9326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oishi T., Ohashi K. (1993) Effects of wavelengths of light on the photoperiodic gonadal response of blinded-pinealectomized Japanese quail. Zool. Sci. 10, 757–762 [Google Scholar]
- 34. Kim J. E., Tauber M. J., Mathies R. A. (2001) Wavelength dependent cis-trans isomerization in vision. Biochemistry 40, 13774–13778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forger N. G. (2009) Control of cell number in the sexually dimorphic brain and spinal cord. J. Neuroendocrinol. 21, 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morrison S. F., Nakamura K., Madden C. J. (2008) Central control of thermogenesis in mammals. Exp. Physiol. 93, 773–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cajochen C., Münch M., Kobialka S., Kräuchi K., Steiner R., Oelhafen P., Orgül S., Wirz-Justice A. (2005) High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab 90, 1311–1316 [DOI] [PubMed] [Google Scholar]
- 38. Hattar S., Lucas R. J., Mrosovsky N., Thompson S., Douglas R. H., Hankins M. W., Lem J., Biel M., Hofmann F., Foster R. G., Yau K. W. (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panda S., Provencio I., Tu D. C., Pires S. S., Rollag M. D., Castrucci A. M., Pletcher M. T., Sato T. K., Wiltshire T., Andahazy M., Kay S. A., Van Gelder R. N., Hogenesch J. B. (2003) Melanopsin is required for non-image-forming photic responses in blind mice. Science 301, 525–527 [DOI] [PubMed] [Google Scholar]
- 40. Hoffman R. A., Reiter R. J. (1965) Pineal gland. Influence on gonads of male hamsters. Science 148, 1609–1611 [DOI] [PubMed] [Google Scholar]
- 41. Sung C. H., Schneider B. G., Agarwal N., Papermaster D. S., Nathans J. (1991) Functional heterogeneity of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. U.S.A. 88, 8840–8844 [DOI] [PMC free article] [PubMed] [Google Scholar]