Background: Endoplasmic reticulum (ER) stress is involved in the pathogenesis of atherosclerosis.
Results: Pharmacological manipulation and siRNA treatment to reduce ER stress mitigate ox-LDL-induced CD36 up-regulation, which is promoted synergistically by ER stress inducer.
Conclusion: ER stress promotes macrophage-derived foam cell formation by up-regulating CD36.
Significance: ER stress-mediated macrophage-derived foam cell formation may be a novel target in the prevention of atherosclerosis.
Keywords: Atherosclerosis, Endoplasmic Reticulum Stress, Low Density Lipoprotein (LDL), Macrophages, Unfolded Protein Response, CD36, Foam Cell, Oxidized Low-density Lipoprotein
Abstract
Oxidized low-density lipoprotein (ox-LDL) up-regulates CD36, a scavenger receptor responsible for macrophage uptake of ox-LDL without limitation. However, the precise underlying mechanism is not completely understood. Our previous study has demonstrated that ox-LDL induces endoplasmic reticulum (ER) stress in macrophages. The goal of this study was to explore the exact relationship between ER stress and macrophage-derived foam cell formation and whether ER stress would be involved in ox-LDL-induced CD36 up-regulation. Our results showed that ox-LDL-induced lipid accumulation in macrophages was promoted synergistically by ER stress inducer tunicamycin (TM), while attenuated by ER stress inhibitor 4-phenylbutyric acid (PBA). Ox-LDL caused CD36 up-regulation with concomitant activation of ER stress as assessed by phosphorylation of inositol-requiring kinase/endonuclease-1 (IRE-1) and protein kinase-like ER kinase (PERK), up-regulation of X-box-binding protein 1 (XBP1) and glucose-regulated protein 78 (GRP 78), and nuclear translocation of activating transcription factor 6 (ATF6). TM not only up-regulated CD36 alone but also synergized with ox-LDL to increase CD36 expression. Alleviation of ER stress with PBA and siRNA against ATF6, IRE1, and GRP78 mitigated ox-LDL-induced CD36 protein up-regulation. Moreover, administration of apoE−/− mice with PBA suppressed the up-regulation of CD36, phospho-IRE1, and GRP78 in macrophage-dense atherosclerotic lesions and in peritoneal macrophages. Additionally, CD36 silencing attenuated ox-LDL-induced nuclear translocation of ATF6, phosphorylation of IRE1 and up-regulation of XBP1 and GRP78. These data indicate that CD36-mediated ox-LDL uptake in macrophages triggers ER stress response, which, in turn, plays a critical role in CD36 up-regulation, enhancing the foam cell formation by uptaking more ox-LDL.
Introduction
The formation of lipid-laden foam cells, especially macrophage-derived foam cells, beneath the endothelium of vascular wall is the hallmark of atherosclerosis (AS),2 which is the leading cause of death and illness throughout most of the world (1). During atherosclerotic lesion development, circulating monocytes adhere to endothelial cells in the arterial wall, migrate into the subendothelial space where they differentiate into macrophages and ingest lipoproteins via scavenger receptors leading to the formation of foam cells. Scavenger receptors on macrophage have been proven to be critical for the foam cell formation due to their ability to bind and internalize modified lipoproteins (2). Cluster of differentiation 36 (CD36), a class B scavenger receptor, plays a quantitatively important role in uptake of oxidized low-density lipoprotein (ox-LDL) (3), which is a well-known risk factor for the development of AS. CD36 is highly expressed on lipid-laden macrophages (4), possibly as a result of a positive feedback loop mediated by ox-LDL and its lipid content (5). Inhibition of CD36 expression has been demonstrated to decrease significantly the ability of macrophages to accumulate ox-LDL and reduce the development of AS (6, 7). These studies suggest that CD36 plays a pro-atherogenic role in foam cell formation and it could be an important target for therapeutic treatment. Thus, further elucidating the mechanisms underlying the regulation of CD36 expression will be critical for the formation of novel therapeutic approaches to combat atherosclerotic lesion progression.
Although the precise mechanisms underlying ox-LDL-induced CD36 up-regulation are still not completely understood, a number of studies have shown that CD36 expression is induced by ox-LDL via peroxisome proliferator receptor gamma (PPARγ) and NFE2-related factor (Nrf2) at the transcriptional level (8, 9). However, as an 88-kDa membrane glycoprotein synthesized in endoplasmic reticulum (ER), the expression of functional CD36 may be more affected by the function of ER than the transcriptional level. ER is the primary eukaryotic organelle for the proper synthesis, post-translation modification, folding, and transportation of proteins. The correct folding of newly synthesized polypeptides, which starts in the ER lumen co-translationally, into their three-dimensional structures is critical for the precise function of many proteins. Multiple stimuli such as oxidative stress, calcium homeostasis imbalance and chronic inflammation lead to ER dysfunction characterized by the accumulation of unfolded and/or misfolded proteins and an imbalance in calcium homeostasis (ER stress) (10). To maintain the functional integrity of ER and cellular homeostasis, an evolutionarily conserved adaptive response, known as the unfolded protein response (UPR), is activated (11). The UPR responds to ER stress primarily by arresting transiently the protein translation, leading to expression of molecular chaperones such as glucose-regulated protein 78 (GRP 78), GRP94, and calreticulin that assist protein folding and directing the misfolded proteins into degradation pathways. However, when these adaptive responses are not enough or the damage is prolonged, the UPR can initiate apoptosis (10, 11). Recent studies have shown that ER stress is activated in macrophages from atherosclerotic lesions (12, 13), reduction of ER stress in macrophages alleviates AS in mice (14, 15), and ER stress controls M2 macrophage differentiation and foam cell formation (16), suggesting that macrophage ER stress may contribute to the pathogenesis of AS, especially linking macrophage apoptosis to plaque vulnerability. In addition to the role of ER stress in the induction of apoptosis, there is evidence showing that ER stress mediates the palmitate-induced up-regulation of lectin-like oxidized LDL receptor-1 (LOX-1), a scavenger receptor on macrophages (17), and Hua et al. reported that treatment of human macrophages with the ER stressor tunicamycin (TM) caused an increase in the protein levels of CD36 (18), suggesting that ER stress may regulates the expression of scavenger receptors. However, whether ER stress is involved in the ox-LDL-induced CD36 up-regulation and the precise underlying mechanisms remain to be elucidated.
We have previously shown that both minimally modified LDL (mm-LDL) and ox-LDL can induce ER stress during the formation of macrophage-derived foam cells, and gene silencing of activating transcription factor 6 (ATF6) with specific small interfering RNA (siRNA) prevents ox-LDL-induced cholesterol accumulation in macrophages (19, 20). In addition, our earlier study has provided preliminary evidence that CD36 mediates potentially ox-LDL-induced ER stress through antibody neutralization tests with anti-CD36 monoclonal antibody, which inhibits the ox-LDL-induced up-regulation of phosphorylated inositol-requiring enzyme 1 (p-IRE1), X box-binding protein 1 (XBP1), and GRP94 (21). Therefore, to elucidate the exact relationship between ER stress and macrophage-derived foam cell formation, we further determined whether CD36 mediates ox-LDL-induced ER stress using gene silencing technology of CD36 and examined whether ER stress is involved in ox-LDL-induced CD36 up-regulation. Here we have demonstrated that pharmacological manipulation and siRNA treatment to reduce ER stress mitigate ox-LDL-induced CD36 up-regulation, which is promoted synergistically by ER stress inducer, and that CD36 silencing inhibits ox-LDL-induced ER stress response.
EXPERIMENTAL PROCEDURES
Reagents
Oil red O, TM, 4-phenylbutyric acid (PBA), siRNAs against ATF6, inositol-requiring kinase/endonuclease-1 (IRE-1), protein kinase-like ER kinase (PERK), GRP 78, and CD36, and rabbit antibody against β-actin were purchased from Sigma-Aldrich. Antibodies against CD36, GRP78, phospho-PERK (p-PERK), and ATF6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against p-IRE1, XBP1, and MOMA-2 were purchased from Abcam (Cambridge, MA). RIPA lysis buffer and nuclear extraction kits were purchased from Solarbio (Beijing, China). Horseradish peroxidase-labeled goat anti-rabbit IgG, rabbit anti-goat IgG and SABC-Cy3 immunohistochemistry kits were obtained from Boshide (Wuhan, China). Polyvinylidene fluoride (PVDF) membranes and enhanced chemiluminescence (ECL) kits were purchased from Millipore (Bedford) and Thermo Scientific Pierce, respectively. Real-time PCR reagent kits and tissue/cell total cholesterol assay kits were obtained from Tiangen Biological Chemistry (Beijing, China) and Applygen (Beijing, China), respectively. DiI-oxLDL was from Xiesheng Biotech (Beijing, China).
Isolation and Oxidation of Low Density Lipoprotein
Human LDL was isolated from plasma of normolipidemic donors by sequential ultracentrifugation, and incubated with 10 μmol/liter CuSO4 for 18 h at 37 °C as described recently (20).
Cell Culture
The murine RAW264.7 macrophages and human THP-1 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). RAW264.7 cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C under 5% CO2 until subconfluent, and then the medium was replaced with serum-free medium. THP-1 cells were cultured in Roswell Park Memorial Institute medium 1640 (RPMI 1640, Hyclone) containing 10% fetal bovine serum and 2 mm l-glutamine. Phorbol 12-myristate 13-acetate (PMA, Sigma, 100 nm) was added to THP-1 cells for 3 days to induce a macrophage phenotype of differentiation.
siRNA Transfection
RAW264.7 cells were transfected with specific siRNA oligomers directed against ATF6, IRE-1, PERK, GRP78, and CD36 (80 nm) using Lipofectaminqe 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Negative control siRNA oligomers were used as a negative control. After transfection for 48 h, the cells were exposed to ox-LDL (50 mg/liter) for 12 h. The silencing of target genes was validated by quantitative real-time PCR. The siRNA sequences are listed in supplemental Table S1.
Lipid Uptake Assay
Three different methods were used to assess the uptake of lipid by macrophages. In the first assay, oil red O staining was used to stain intracellular lipid deposits in macrophages and analyzed with Image-Pro Plus software (version 6.0, Media Cybernetics, LP, USA) as described previously (20). For the second method, the intracellular total cholesterol (TC) level was measured using a tissue/cell TC assay kit according to the manufacturer's instruction and the concentration of TC was determined using a standard curve and normalized to level of total cellular protein. For the third method, macrophages were incubated with Dil-labeled ox-LDL, and the extent of ox-LDL uptake was quantitated by fluorescence measurement as reported previously (18). Briefly, after incubation with Dil-ox-LDL (50 μg/ml) for 4 h at 37 °C, cells were washed with phosphate-buffered saline (PBS) and then lysed in 200 μl of lysis buffer. Protein concentration of each sample was determined using bicinchoninic acid assay. Fluorescence was detected with excitation and emission wavelengths of 530 and 590 nm, respectively, using an Infinite F200 microplate reader (Tecan, Switzerland). Fluorescence standard curve was prepared by diluting 0, 1, 2, 3, 4, 5, 10, and 20 μg Dil-ox-LDL in lysis buffer. Dil-ox-LDL uptake by macrophages was calculated as amount of Dil-ox-LDL per milligram of cellular protein.
Immunofluorescence Assay for CD36 Expression
The treated cells grown on glass coverslips were fixed with PBS containing 4% (w/v) paraformaldehyde for 20 min and blocked in 3% BSA for 30 min, and then incubated with CD36 antibody (1:100) for 1 h at room temperature. Following incubation with the corresponding secondary FITC-conjugated antibody and 4′,6-diamidino-2-phenylindole (DAPI), the samples were then washed with PBS, mounted in antifade reagent and observed using an Olympus BX51 microscope (Tokyo, Japan).
Immunofluorescence Assay for ATF6 Nuclear Relocation
The treated cells grown on glass coverslips were washed with PBS, fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton-X 100 for 5 min, blocked in 3% BSA for 30 min, and then incubated with ATF6 antibody (1:100, Santa Cruz, catalogue no. sc-22799) for 1 h at room temperature. Following incubation with the appropriate secondary antibody (1:200), the samples were incubated with SABC-Cy3 complex and DAPI, and then observed using an Olympus BX51 microscope as described previously (20).
Western Blotting
Total proteins and nuclear proteins from the treated cells were extracted using RIPA lysis buffer and nuclear extraction kits, respectively, following the manufacturer's instructions. Equal amounts of protein were separated by SDS-PAGE and probed with various primary antibodies as indicated. Immunoblots were visualized using ECL reagent, and the integrated optical density (IOD) of immunoreactive bands was measured using Image-Pro Plus software and normalized by house-keeping protein (β-actin or histone) as described previously (20).
Quantitative Real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen). 2 μg of total RNA was reversely transcripted using MuLV Reverse Transcriptase (Applied Biosystems). Real-time PCR was performed on a Rotor-Gene Q real-time PCR cycler (Qiagen, Shanghai, China) using SYBR-green PCR master mix kits as described previously (20). The data were analyzed using the Rotor-Gene Q software (version 1.7, Qiagen), and relative mRNA levels were calculated using the 2−−▵▵Ct method and normalized against β-actin. The primers used for real-time PCR were synthesized by Sangon Biotech (Shanghai, China) and listed in supplemental Table S2.
Animals
Male apolipoprotein E knock-out (apoE−/−) mice (7 weeks of age) and male C57BL/6 wild-mice were obtained from Huafukang Bio-Technology Company (Beijing, China). 16 apoE−/− mice were fed a high-fat diet (15.8% fat and 1.25% cholesterol) for 8 weeks, and given saline (model group, n = 8) or PBA (100 mg/kg per day) (PBA group, n = 8) by intraperitoneal injection during the final 4 weeks. Male C57BL/6 mice injected intraperitoneally with saline were maintained on normal chow diet as a control group (n = 8). All experiments were approved by the laboratory animals' ethical committee of Taishan Medical University and followed national guidelines for the care and use of animals.
Isolation of Peritoneal Macrophages
Peritoneal macrophages were harvested from PBA or saline-treated apoE−/− mice and C57BL/6J mice, and then immediately frozen at −80 °C until protein extraction for Western blotting analysis.
Immunohistochemistry Analysis
Serial aortic root cryosections (6 μm) were fixed in acetone, peroxidase-blocked and incubated with specific antibodies against macrophages (MOMA-2), GRP78 and CD36 following manufacturer's recommendations. The sections were overlaid with biotinylated secondary antibodies, visualized with a streptavidin- peroxidase/diaminobenzidine system and counterstained with hematoxylin. Images were captured using microscope (Olympus, Tokyo, Japan). Color threshold and planimetry were used for quantitation. All quantitations were determined by calculating the percentage of the antigen positive area to the total cross-sectional vessel wall area by using Image-Pro Plus software.
Statistical Analysis
Results were expressed as mean ± S.E. Statistical analysis was performed using one-way analysis of variance with Student-Newmann-Keuls test for multiple comparisons and Student's t test for comparison between two groups. p < 0.05 was considered statistically significant.
RESULTS
ER Stress Mediates Ox-LDL-induced Lipid Accumulation in Macrophages
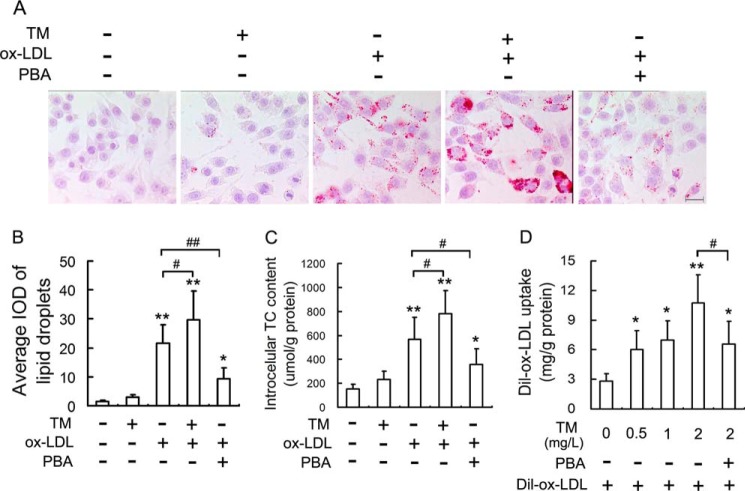
Because macrophage uptake of ox-LDL is the event that triggers the formation of lipid-laden foam cells, we examined the effect of ER stress on lipid accumulation in macrophages. The oil red O staining (Fig. 1, A and B) and intracellular TC quantitative assay (Fig. 1C) indicated that lipid content in RAW264.7 cells was significantly increased by ox-LDL in the presence or absence of TM (a classical ER stress inducer), and macrophages treated with ox-LDL and TM together had higher level of intracellular lipids compared with those treated with ox-LDL alone. However, the reported ER stress inhibitor, PBA (14), attenuated significantly ox-LDL-induced lipid accumulation.
FIGURE 1.
ER stress mediates ox-LDL-induced lipid accumulation in macrophages. A, RAW264.7 cells were treated with 50 mg/liter ox-LDL in the presence or absence of tunicamycin (TM) (0.5 mg/liter) and 4-phenylbutyric acid (PBA) (20 mm) for 12 h, and the extent of lipid loading was assessed by oil red O staining. Representative lipid droplet staining images are shown. Scale bar, 20 μm. B, the average integrated optical density (IOD) of lipid droplets stained with oil red O from differentiated macrophages. C, under the same conditions as in A, the intracellular total cholesterol (TC) content was measured using a tissue/cell TC assay kit. D, Dil-ox-LDL fluorescence intensity in cells incubated with TM at the indicated concentrations in the presence or absence of PBA (20 mm) for 4 h, followed by incubation with Dil-labeled ox-LDL (50 mg/liter) for 4 h. Data are presented as the mean ± S.E. of at least four independent experiments. *, p < 0.05, **, p < 0.01 versus vehicle-treated control, #, p < 0.05, ##, p < 0.01.
To further determine the role of ER stress in lipid accumulation in macrophages, we examined the effect of TM on ox-LDL uptake by macrophages. As shown in Fig. 1D, TM promoted uptake of ox-LDL in a concentration-dependent manner (0.5–2 mg/liter), and PBA prevented this enhancement of ox-LDL uptake induced by TM. These data suggested that ER stress enhanced the ox-LDL-induced lipid accumulation in macrophages.
ER Stress Caused by TM Promotes Ox-LDL-induced CD36 Expression in Macrophages
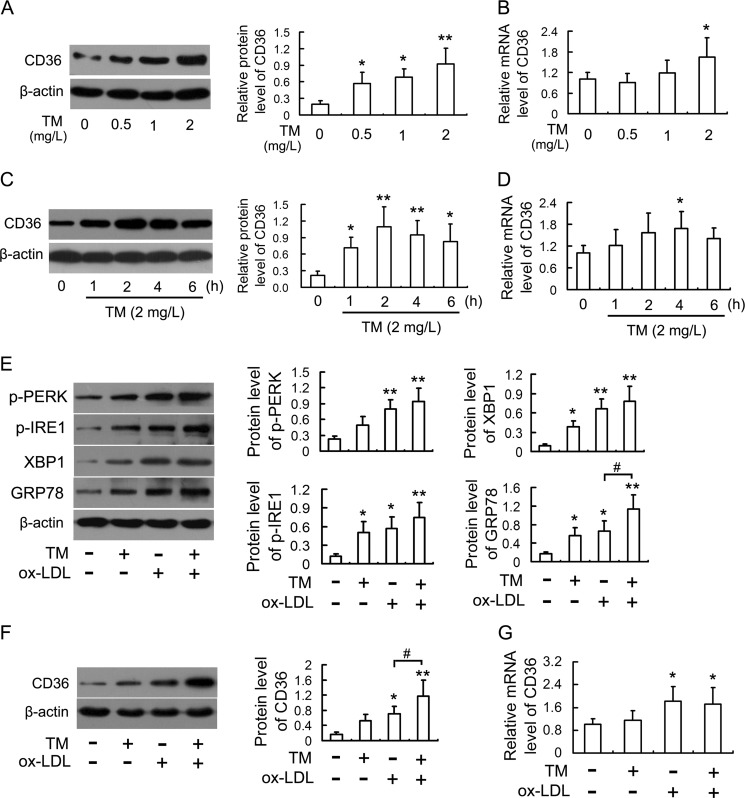
CD36 is one of scavenger receptors responsible for ox-LDL uptake by macrophages and accounts for about 60–70% of macrophage-derived foam cell formation (22). To understand the potential mechanisms involved in the promotion of macrophage lipid accumulation by ER stress, we first examined whether TM could induce CD36 expression in macrophages. As shown in Fig. 2, A–D, treatment of RAW264.7 cells with TM up-regulated the levels of CD36 protein and mRNA in a concentration-dependent and time-dependent manner. Interestingly, low concentrations (0.5 and 1 mg/liter) of TM induced markedly the CD36 protein expression but had no significant effect on CD36 mRNA. In addition, comparing the time course of up-regulation of CD36 protein with mRNA, CD36 protein became evident at an earlier time point than CD36 mRNA up-regulation, suggesting CD36 protein up-regulation preceding mRNA induction.
FIGURE 2.
ER stress caused by TM up-regulates CD36 expression in macrophages. A and B, expression of CD36 protein and mRNA in RAW264.7 cells incubated with TM for 4 h at the indicated concentrations were analyzed by Western blot and real-time PCR, respectively. C and D, cells were treated with 2 mg/L TM for the indicated periods, and the expression of CD36 protein and mRNA were analyzed by Western blot and real-time PCR, respectively. E, Western blot analysis of ER stress markers in cells stimulated with 50 mg/liter ox-LDL in the presence or absence of TM (0.5 mg/liter) for 12 h. F and G, effects of TM on ox-LDL-induced CD36 up-regulation. Cells were treated as described in E, and the expression of CD36 protein and mRNA were analyzed by Western blot and real-time PCR, respectively. Data are presented as the mean ± S.E. of at least three independent experiments. *, p < 0.05, **, p < 0.01 versus vehicle-treated control, #, p < 0.05.
Since we confirmed TM induces CD36 expression in macrophages, we next examined whether TM could promote ox-LDL-induced CD36 up-regulation. As indicated in Fig. 2E, exposure of cells with TM and ox-LDL together or alone all induced ER stress, as determined by phosphorylation of PERK and IRE-1, up-regulation of XBP1 and GRP78 as well as nuclear translocation of ATF6 (supplemental Fig. S1). Furthermore, macrophages treated with ox-LDL and TM together had higher levels of GRP78 compared with those treated with ox-LDL alone. Consistent with the facilitative effects on ox-LDL-induced ER stress, TM further increased the level of CD36 protein (Figs. 2F and 3A) but had no significant effect on CD36 mRNA in the ox-LDL-treated cells (Fig. 2G).
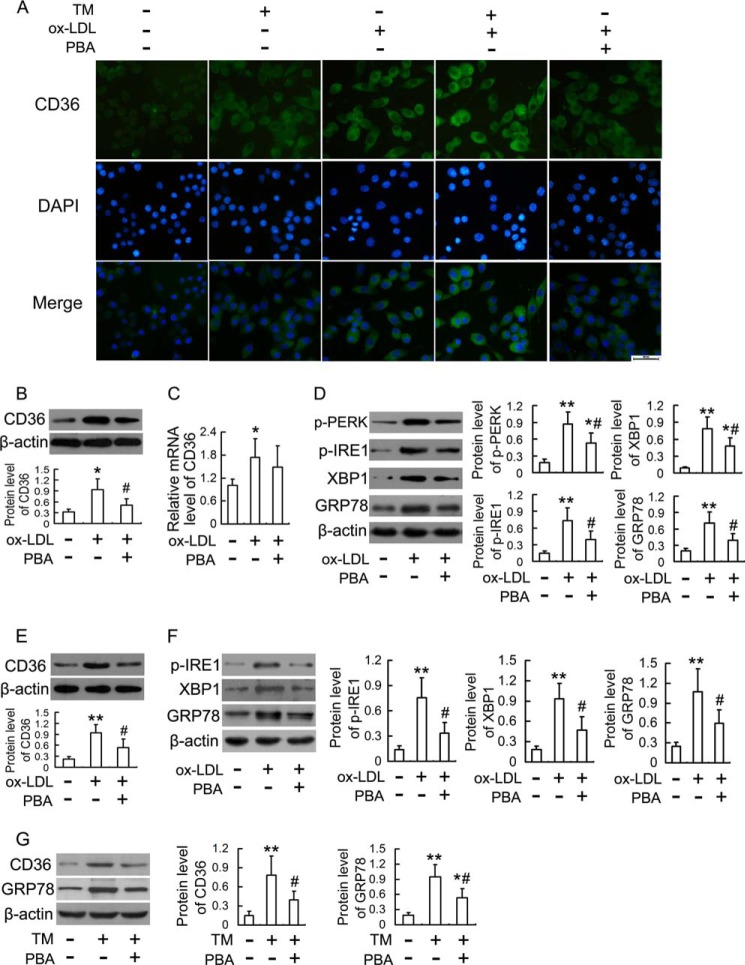
FIGURE 3.
Effects of TM and PBA on ox-LDL-induced CD36 expression in macrophages. A, immunofluorescence experiments show CD36 visualized by FITC labeling (green) and nuclei stained with DAPI (blue). RAW264.7 cells were treated with 50 mg/liter ox-LDL in the presence or absence of TM (0.5 mg/liter) and PBA (20 mm) for 12 h. Representative fluorescent images are shown. Scale bar, 20 μm. B and C, RAW264.7 cells were stimulated with 50 mg/liter ox-LDL in the presence or absence of PBA (20 mm) for 12 h, and the expression of CD36 protein and mRNA were analyzed by Western blot and real-time PCR, respectively. D, Western blot analysis of ER stress markers in RAW264.7 cells stimulated with 50 mg/liter ox-LDL in the presence or absence of PBA (20 mm) for 12 h. E and F, Western blot analysis of CD36 and ER stress markers in human THP-1-derived macrophages treated as described in D. G, Western blot analysis of CD36 and GRP78 in THP-1-derived macrophages incubated with TM (2 mg/liter) in the presence or absence of PBA (20 mm) for 4 h. Data are presented as the mean ± S.E. of at least three independent experiments. *, p < 0.05, **, p < 0.01 versus vehicle-treated control, #, p < 0.05 versus ox-LDL-treated group.
Alleviation of ER Stress Mitigates Ox-LDL-induced CD36 Up-regulation in Macrophages
To further elucidate the contribution of ER stress to ox-LDL-induced CD36 up-regulation, we examined whether alleviation of ER stress using PBA could mitigate ox-LDL-induced CD36 up-regulation. As shown in Fig. 3, A and B, PBA significantly inhibited ox-LDL-induced CD36 protein up-regulation but had no significant effect on CD36 mRNA expression (Fig. 3C). Consistent with the inhibitory effect on CD36 protein up-regulation, PBA reduced phosphorylation of PERK and IRE1, up-regulation of XBP-1 and GRP78 as well as nuclear translocation of ATF6 induced by ox-LDL (Fig. 3D and supplemental Fig. S1). It should be noted that similar results were obtained in human THP-1-derived macrophages, as assessed by inhibition of ox-LDL- or TM-induced up-regulation of CD36 and ER stress markers by PBA (Fig. 3, E–G). These results indicated that alleviation of ER stress could mitigate ox-LDL-induced CD36 up-regulation.
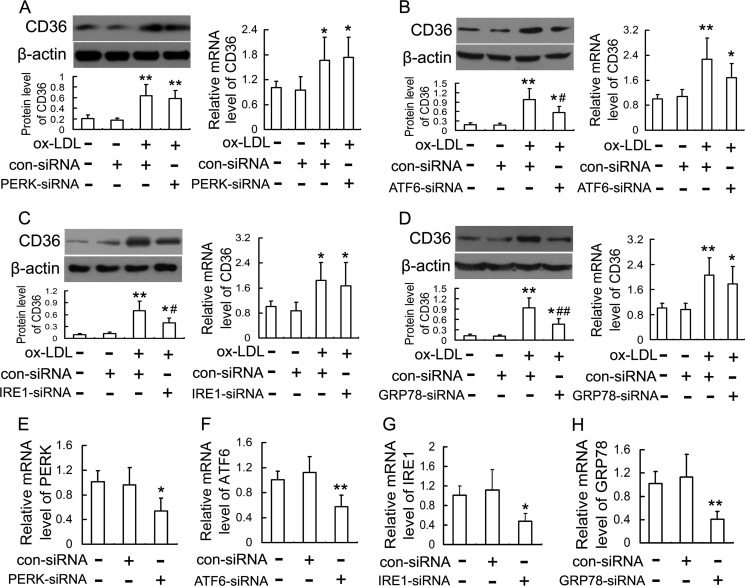
ATF6, IRE1, and GRP78 Are Involved in Ox-LDL-induced CD36 Up-regulation
Because studies using TM (ER stress inducer) and PBA (ER stress inhibitor) suggested the contribution of ER stress to CD36 up-regulation, we next investigated the effects of gene silencing of three major UPR pathways (PERK, ATF6, and IRE1) and their downstream molecule, GRP78, on ox-LDL-induced CD36 up-regulation. As shown in Fig. 4A, PERK silencing by PERK siRNA failed to down-regulate ox-LDL-induced CD36 protein and mRNA significantly. However, siRNAs directed against ATF6, IRE1, and GRP78 reduced ox-LDL-induced CD36 protein by 42.0, 44.4, and 50.5%, respectively, but had no significant effect on CD36 mRNA (Fig. 4, B–D). All siRNAs used in this study showed efficient inhibition of each target gene expression (Fig. 4, E–H). These results suggest that the ATF6 and IRE1 pathways and molecular chaperone GRP78 mainly contribute to ox-LDL-induced CD36 protein up-regulation.
FIGURE 4.
Effects of siRNAs against PERK, ATF6, IRE1, and GRP78 on CD36 expression in ox-LDL-incubated RAW264.7 cells. Cells were transfected with siRNA directed against PERK (A), ATF6 (B), IRE1 (C), and GRP78 (D), followed by treatment with 50 mg/liter ox-LDL for 12 h. Expression of CD36 protein and mRNA were evaluated by Western blot and quantitative real-time PCR, respectively. Expression of the target gene of each siRNA, PERK (E), ATF6 (F), IRE1 (G), and GRP78 (H), was also quantified by real-time PCR. Data are expressed as mean ± S.E. of at least three independent experiments. *, p < 0.05, **, p < 0.01 versus control group transfected with con-siRNA; #, p < 0.05, ##, p < 0.01 versus ox-LDL group transfected with con-siRNA.
PBA Reduces the Up-regulation of CD36 in Atherosclerotic Lesions of ApoE−/− Mice
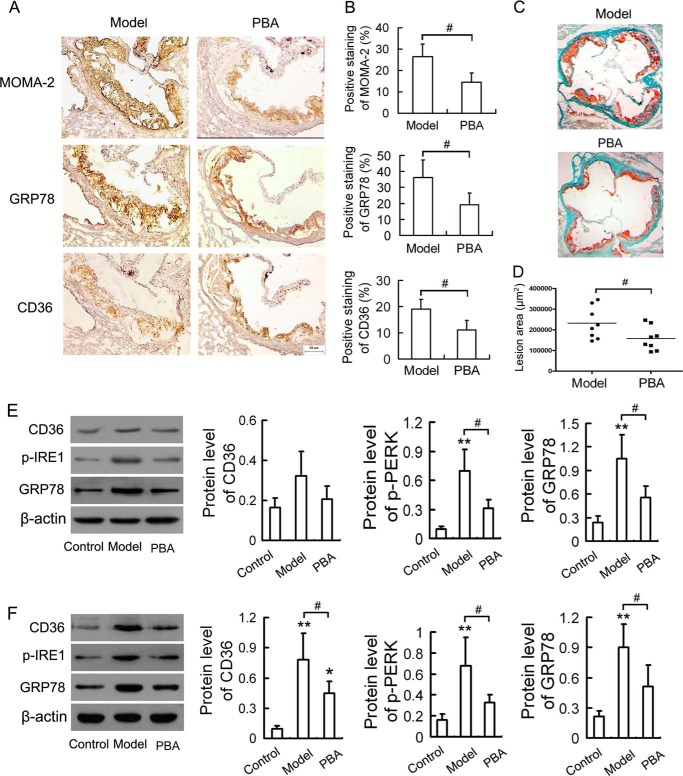
It has been reported that mitigation of ER stress with PBA results in marked reduction in macrophage apoptosis in atherosclerotic lesions (14). To further elucidate the contribution of ER stress to CD36 expression in vivo, we analyzed the expression of CD36 in aortic roots and peritoneal macrophages of atherosclerotic mice treated with PBA. PBA administration had no significant effect on the body weights, serum cholesterol, and triglycerides levels compared with model group (supplemental Table S3). Immunohistochemical analysis (Fig. 5, A and B) revealed that there was dramatic reduction in macrophage content of atherosclerotic lesions (shown by staining with MOMA-2) with decreased lesions as measured by oil red O staining of the aortic sinus sections (Fig. 5, C and D) in PBA-treated apoE−/− mice when compared with untreated apoE−/− (model) mice. The atherosclerotic lesions of model mice stained positive for CD36 and GRP78 in macrophage-dense areas, which were significantly diminished by PBA administration. Western blot analysis of peritoneal macrophages (Fig. 5E) showed that there was no significant change in the CD36 expression. However, administration of apoE−/− mice with PBA suppressed remarkably the activation of ER stress as assessed by diminished p-IRE1 and GRP78 expression in peritoneal macrophages. To further confirm the effect of PBA on CD36 expression in vivo, total tissue protein lysates from the aortic arch were extracted for Western blotting analysis. As seen in Fig. 5F, CD36 expression was markedly increased with concomitant up-regulation of p-IRE1 and GRP78 in model group compared with the control group. However, the changes above were significantly inhibited by PBA administration. Altogether, these results demonstrate that PBA may reduce CD36 up-regulation via inhibition of macrophage ER stress in vivo.
FIGURE 5.
PBA reduces the up-regulation of CD36 and ER stress markers in apoE−/− mice. Male apoE−/− mice were fed a high-fat diet for 8 weeks, and given saline (model group) or 100 mg/kg of PBA (PBA group) per day by intraperitoneal injection during the final 4 weeks. Male C57BL/6J mice were maintained on normal chow diet as a control group. A, representative immunostained aortic sinus sections using specific antibodies against MOMA-2, GRP78, and CD36. Scale bar, 50 μm. B, densitometric quantification of MOMA-2, GRP78, and CD36 positive cells per field of view. C, representative sections of aortic roots stained with oil red O. D, quantitative analysis of lesion area in oil red O-stained aortic sections by Image-Pro Plus software. E, Western blot analysis of CD36 and ER stress markers in peritoneal macrophages. F, Western blot analysis of CD36 and ER stress markers in aortic arch. Data are presented as the mean ± S.E. of at least three independent experiments. *, p < 0.05, **, p < 0.01 versus control group; #, p < 0.05.
CD36 Mediates Ox-LDL-induced ER Stress
Since CD36 is regulated by ox-LDL and responsible for ox-LDL uptake by macrophages, and our previous study has shown that ox-LDL can induce ER stress during the formation of macrophage-derived foam cells (20), then we evaluated whether CD36 could mediate ox-LDL-induced ER stress through gene silencing of CD36 in RAW264.7 cells. As seen in Fig. 6A, CD36 siRNA inhibited significantly the ox-LDL-induced up-regulation of p-IRE1, XBP1, and GRP78. The effect of CD36 siRNA on ATF6 nuclear translocation induced by ox-LDL was also determined by immunofluorescence analysis and Western blotting. As illustrated in Fig. 6B, ATF6 showed a diffused cytoplasmic localization in control cells, whereas clusters of ATF6 were detected in the nuclear region in ox-LDL-treated cells. The ATF6 translocation from the cytoplasm to the nucleus induced by ox-LDL was significantly supressed by CD36 siRNA. Consistently, the immunoblotting analysis also indicated that the ox-LDL-induced up-regulation of ATF6 in nuclei was inhibited by CD36 siRNA (Fig. 6C). The target gene expression was inhibited efficiently by CD36 siRNA (Fig. 6D).
FIGURE 6.
CD36 silencing inhibits ox-LDL-induced ER stress response in RAW264.7 cells. Cells were transfected with a siRNA against CD36 or a negative control siRNA, followed by treatment with 50 mg/liter ox-LDL for 12 h. A, ER stress markers were evaluated by Western blot and normalized to β-actin. B, immunofluorescence experiments show ATF6 visualized by Cy3 labeling (red) and nuclei stained with DAPI (blue). Representative fluorescent images are shown. Scale bar, 20 μm. C, the protein level of ATF6 in nuclear extracts was analyzed by Western blot and normalized to histone level. D, the silencing of CD36 was validated by quantitative real-time PCR. Data are expressed as mean ± S.E. of at least three independent experiments. *, p < 0.05, **, p < 0.01 versus control group transfected with con-siRNA; #, p < 0.05 versus ox-LDL group transfected with con-siRNA.
DISCUSSION
It has been suggested that CD36 plays an important role in foam cell formation and progression of AS. However, the precise mechanism underlying ox-LDL-induced CD36 up-regulation has not been completely clarified. Our results in the present study suggested that ER stress response contributes to the ox-LDL-induced up-regulation of CD36, especially at the the post-transcriptional level, which was supported by a number of observations. First, ox-LDL-induced lipid accumulation in macrophages was promoted synergistically by the ER stress inducer TM, while attenuated by the ER stress inhibitor PBA. Second, treatment of macrophages with ox-LDL resulted in activation of ER stress markers with concomitant up-regulation of CD36. Third, TM not only up-regulated CD36 alone but also synergistically promoted the ox-LDL-induced CD36 expression, especially at the protein level. Fourth, pharmacological manipulation to reduce the ER stress with PBA alleviated ox-LDL-induced CD36 protein up-regulation but had no significant effect on CD36 mRNA expression. Fifth, siRNA directed against ATF6, IRE1, and GRP78, important molecules in ER stress response pathways, alleviated ox-LDL- induced CD36 protein up-regulation. Sixth, PBA reduced the up-regulation of CD36 in atherosclerotic lesions of apoE−/− mice. These results demonstrate that ox-LDL causes up-regulation of CD36 via activation of the ER stress response.
The uptake of ox-LDL leading to focal accumulation of lipid in macrophage-derived foam cells is intimately involved in the formation of early fatty streak lesions, as well as in the evolution of more complex atherosclerotic plaques (23–25). We (19) and others (26, 27) have demonstrated that cholesterol accumulation in the ER membrane triggers ER stress and activation of the UPR in cultured macrophages. Accumulating evidence reveals that ER stress is a cross-point to link cellular processes with multiple risk factors that exist in all stages of AS (10) and believed to play a critical role in lipid metabolic disorder (28), activation of inflammatory reactions (29), and cellular apoptosis (30). Activation of ER stress pathways is also a characteristic of lipid-laden macrophages in both early and advanced atherosclerotic lesions and is proposed to play a role in plaque vulnerability and acute cardiac death (13, 26, 31), while reduction of ER stress in macrophages alleviates AS in mice (14, 15). Our previous work (20) has demonstrated that ATF6, a sensor to UPR, mediates ox-LDL-induced cholesterol accumulation and apoptosis in macrophages, suggesting that macrophage ER stress may contribute to the pathogenesis of AS. In this study, we showed that ER stress inducer TM not only increased uptake of ox-LDL in a concentration-dependent manner, but also synergistically promoted the ox-LDL-induced lipid accumulation. However, ER stress inhibitor, PBA, attenuated significantly ox-LDL-induced lipid accumulation and the enhancement of ox-LDL uptake induced by TM. Accumulating studies (3–7, 22, 32) point to a significant role of CD36, the major macrophage scavenger receptor responsible for ox-LDL uptake, in progression of AS and suggest it could be an important target for therapeutic treatment. Our data in the present study demonstrated that ox-LDL activated ER stress with concomitant up-regulation of CD36. TM not only up-regulated CD36 alone but also synergistically promoted the ox-LDL-induced CD36 expression, especially at the protein level. Additionally, reduction of ER stress with PBA alleviated ox-LDL-induced CD36 protein up-regulation but had no significant effect on CD36 mRNA expression. These data suggested that ER stress may mediate the ox-LDL-induced lipid accumulation in macrophages via modulation of CD36 expression at the post-transcriptional level.
Since ER is the primary site for protein synthesis, folding and trafficking, the existence of an ER quality control system ensures that only correctly folded, newly synthesized proteins are transported to the plasma membrane or secreted. The ER quality control system includes a number of chaperones and folding enzymes localized in the lumen or in the membrane of the ER (33). Under a variety of stressful conditions, the accumulation of unfolded or misfolded proteins in ER triggers an evolutionarily conserved mechanism, termed the UPR, to compensate for ER stress. The UPR is mediated by three ER transmembrane proteins: PERK, IRE1 and ATF6. Upon activation of UPR, PERK phosphorylates the subunit of eukaryotic translation initiation factor 2α (eIF2α) and transiently attenuates general protein synthesis. IRE1 mediates the splicing of XBP1 mRNA and results in the production of the XBP1s transcription factor. ATF6 undergoes proteolytic cleavage by proteases, allowing cytosolic domain p50ATF6 translocation to the nucleus and activation of UPR target genes. GRP78, which is regulated by p50ATF6 and XBP1s, is a major ER chaperone in the quality control of newly synthesized proteins and plays a critical role in regulating ER homeostasis (34). Several pieces of evidence suggest a connection between expressing regulation of the scavenger receptors and the UPR. For example, IRE-1 is involved in the palmitate-induced LOX-1 up-regulation in macrophages (17) and treatment of macrophages with tunicamycin up-regulates the CD36 protein levels (18). Our data in the present study showed that siRNA directed against ATF6, IRE1, and GRP78, not PERK, alleviated ox-LDL-induced CD36 protein up-regulation but had no significant effect on CD36 mRNA expression, suggesting that ATF6/IRE1-GRP78-mediated UPR regulates CD36 expression at the protein level and promotes foam cell formation. In other words, GRP78, which is up-regulated to a certain extent under the induction of ox-LDL or TM via UPR signal pathway, may enhance macrophage uptake of ox-LDL by assisting the correct folding, post-translational modification and transportation of CD36.
Another interesting finding in the present study was that CD36 mediated ox-LDL-induced ER stress. Cholesterol uptake is a pathway by which extracellular modified LDL are ingested by macrophages via receptors-mediated phagocytosis and pinocytosis (35). CD36 plays a critical role in foam cell formation and occurrence of many signaling responses such as oxidative stress and inflammation by mediating recognition and uptake of ox-LDL (3, 36, 37). Consistent with our previous study through antibody neutralization tests with anti-CD36 monoclonal antibody (21), the results of the present study showed that siRNA against CD36 attenuated ox-LDL-induced ER stress response as assessed by less nuclear translocation of ATF6, lower phosphorylation of IRE1 and down-regulation of XBP1 and GRP78, suggesting that CD36 may mediate ox-LDL-induced macrophage ER stress.
In conclusion, our results indicated that CD36-mediated ox-LDL uptake in macrophages triggered ER stress response, which, in turn, played a critical role in CD36 up-regulation mainly at the protein level, enhancing the foam cell formation by uptaking more ox-LDL. Our findings may offer a new mechanism to explain in part the macrophage uptake of ox-LDL without limitation and further elucidate the relationship between ER stress and macrophage-derived foam cell formation. Because activation of the ER stress response occurs at all stages of atherosclerotic lesion development (31), and there are correlations between ER stress and plaque vulnerability (13), the elevation of macrophage CD36 caused by ER stress may contribute to the progression of atherosclerosis by enhancement of foam cell formation and plaque vulnerability.
This work was supported by the Taishan Scholars Foundation of the Shandong Province (zd056, zd057) and National Natural Science Foundation of China (81202949, 81370381, 81070247).

This article contains supplemental Tables S1–S3 and Fig. S1.
- AS
- atherosclerosis
- CD36
- cluster of differentiation 36
- ox-LDL
- oxidized low-density lipoprotein
- apoE−/−
- apolipoprotein E knockout
- PPARγ
- peroxisome proliferator receptor gamma
- Nrf2
- NFE2-related factor
- ER
- endoplasmic reticulum
- UPR
- unfolded protein response
- GRP 78
- glucose-regulated protein 78
- LOX-1
- lectin-like oxidized LDL receptor-1
- mm-LDL
- minimally modified LDL
- siRNA
- small interfering RNA
- TM
- tunicamycin
- PBA
- 4-phenylbutyric acid
- ATF6
- activating transcription factor 6
- IRE-1
- inositol-requiring kinase/endonuclease-1
- PERK
- protein kinase-like ER kinase
- XBP1
- X-box-binding protein 1
- PVDF
- polyvinylidene fluoride
- ECL
- enhanced chemiluminescence
- DAPI
- 4′, 6-diamidino-2-phenylindole
- IOD
- integrated optical density
- TC
- total cholesterol.
REFERENCES
- 1. Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Borden W. B., Bravata D. M., Dai S., Ford E. S., Fox C. S., Franco S., Fullerton H. J., Gillespie C., Hailpern S. M., Heit J. A., Howard V. J., Huffman M. D., Kissela B. M., Kittner S. J., Lackland D. T., Lichtman J. H., Lisabeth L. D., Magid D., Marcus G. M., Marelli A., Matchar D. B., McGuire D. K., Mohler E. R., Moy C. S., Mussolino M. E., Nichol G., Paynter N. P., Schreiner P. J., Sorlie P. D., Stein J., Turan T. N., Virani S. S., Wong N. D., Woo D., Turner M. B. (2013) Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127, 143–152 [DOI] [PubMed] [Google Scholar]
- 2. Goyal T., Mitra S., Khaidakov M., Wang X., Singla S., Ding Z., Liu S., Mehta J. L. (2012) Current concepts of the role of oxidized LDL receptors in atherosclerosis. Curr. Atheroscler. Rep. 14, 150–159 [DOI] [PubMed] [Google Scholar]
- 3. Rahaman S. O., Lennon D. J., Febbraio M., Podrez E. A., Hazen S. L., Silverstein R. L. (2006) A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakata A., Nakagawa Y., Nishida M., Nozaki S., Miyagawa J., Nakagawa T., Tamura R., Matsumoto K., Kameda-Takemura K., Yamashita S., Matsuzawa Y. (1999) CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler. Thromb. Vasc. Biol. 19, 1333–1339 [DOI] [PubMed] [Google Scholar]
- 5. Han J., Hajjar D. P., Febbraio M., Nicholson A. C. (1997) Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J. Biol. Chem. 272, 21654–21659 [DOI] [PubMed] [Google Scholar]
- 6. Kuchibhotla S., Vanegas D., Kennedy D. J., Guy E., Nimako G., Morton R. E., Febbraio M. (2008) Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 78, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Febbraio M., Podrez E. A., Smith J. D., Hajjar D. P., Hazen S. L., Hoff H. F., Sharma K., Silverstein R. L. (2000) Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105, 1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagy L., Tontonoz P., Alvarez J. G., Chen H., Evans R. M. (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 93, 229–240 [DOI] [PubMed] [Google Scholar]
- 9. Ishii T., Itoh K., Ruiz E., Leake D. S., Unoki H., Yamamoto M., Mann G. E. (2004) Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 94, 609–616 [DOI] [PubMed] [Google Scholar]
- 10. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 16, 396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou A. X., Tabas I. (2013) The UPR in atherosclerosis. Semin. Immunopathol. 35, 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han S., Liang C. P., DeVries-Seimon T., Ranalletta M., Welch C. L., Collins-Fletcher K., Accili D., Tabas I., Tall A. R. (2006) Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 3, 257–266 [DOI] [PubMed] [Google Scholar]
- 13. Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., Bochaton-Piallat M. L., Mochizuki N., Kitakaze M. (2007) Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116, 1226–1233 [DOI] [PubMed] [Google Scholar]
- 14. Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. (2009) Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metab. 9, 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh J., Riek A. E., Weng S., Petty M., Kim D., Colonna M., Cella M., Bernal-Mizrachi C. (2012) Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J. Biol. Chem. 287, 11629–11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishiyama J., Taguchi R., Akasaka Y., Shibata S., Ito M., Nagasawa M., Murakami K. (2011) Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J. Lipid Res. 52, 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hua Y., Kandadi M. R., Zhu M., Ren J., Sreejayan N. (2010) Tauroursodeoxycholic acid attenuates lipid accumulation in endoplasmic reticulum-stressed macrophages. J. Cardiovasc. Pharmacol. 55, 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao S., Yang N., Song G., Sang H., Tian H., Miao C., Zhang Y., Qin S. (2012) Minimally modified low-density lipoprotein induces macrophage endoplasmic reticulum stress via toll-like receptor 4. Biochim. Biophys. Acta 1821, 954–963 [DOI] [PubMed] [Google Scholar]
- 20. Yao S., Zong C., Zhang Y., Sang H., Yang M., Jiao P., Fang Y., Yang N., Song G., Qin S. (2013) Activating transcription factor 6 mediates oxidized LDL-induced cholesterol accumulation and apoptosis in macrophages by up-regulating CHOP expression. J. Atheroscler. Thromb. 20, 94–107 [DOI] [PubMed] [Google Scholar]
- 21. Yao S. T., Sang H., Yang N. N., Kang L., Tian H., Zhang Y., Song G. H., Qin S. C. (2010) Oxidized low density lipoprotein induces macrophage endoplasmic reticulum stress via CD36. Sheng. Li. Xue. Bao. 62, 433–440 [PubMed] [Google Scholar]
- 22. Kunjathoor V. V., Febbraio M., Podrez E. A., Moore K. J., Andersson L., Koehn S., Rhee J. S., Silverstein R., Hoff H. F., Freeman M. W. (2002) Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277, 49982–49988 [DOI] [PubMed] [Google Scholar]
- 23. Seimon T., Tabas I. (2009) Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 50, S382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabas I. (2005) Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 [DOI] [PubMed] [Google Scholar]
- 25. Tiwari R. L., Singh V., Barthwal M. K. (2008) Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 28, 483–544 [DOI] [PubMed] [Google Scholar]
- 26. Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., Marks A. R., Ron D., Tabas I. (2003) The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5, 781–792 [DOI] [PubMed] [Google Scholar]
- 27. Li Y., Ge M., Ciani L., Kuriakose G., Westover E. J., Dura M., Covey D. F., Freed J. H., Maxfield F. R., Lytton J., Tabas I. (2004) Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J. Biol. Chem. 279, 37030–37039 [DOI] [PubMed] [Google Scholar]
- 28. Pineau L., Colas J., Dupont S., Beney L., Fleurat-Lessard P., Berjeaud J. M., Bergès T., Ferreira T. (2009) Lipid-induced ER stress: synergistic effects of sterols and saturated fatty acids. Traffic. 10, 673–690 [DOI] [PubMed] [Google Scholar]
- 29. Gora S., Maouche S., Atout R., Wanherdrick K., Lambeau G., Cambien F., Ninio E., Karabina S. A. (2010) Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 24, 3284–3297 [DOI] [PubMed] [Google Scholar]
- 30. Cash J. G., Kuhel D. G., Basford J. E., Jaeschke A., Chatterjee T. K., Weintraub N. L., Hui D. Y. (2012) Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J. Biol. Chem. 287, 27876–27884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou J., Lhoták S., Hilditch B. A., Austin R. C. (2005) Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111, 1814–1821 [DOI] [PubMed] [Google Scholar]
- 32. Ashraf M. Z., Gupta N. (2011) Scavenger receptors: Implications in atherothrombotic disorders. Int. J. Biochem. Cell Biol. 43, 697–700 [DOI] [PubMed] [Google Scholar]
- 33. Araki K., Nagata K. (2011) Protein folding and quality control in the ER. Cold Spring Harb. Perspect. Biol. 3, a007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jørgensen M. M., Jensen O. N., Holst H. U., Hansen J. J., Corydon T. J., Bross P., Bolund L., Gregersen N. (2000) Grp78 is involved in retention of mutant low density lipoprotein receptor protein in the endoplasmic reticulum. J. Biol. Chem. 275, 33861–33868 [DOI] [PubMed] [Google Scholar]
- 35. Yu X. H., Fu Y. C., Zhang D. W., Yin K., Tang C. K. (2013) Foam cells in atherosclerosis. Clin. Chim. Acta. 424, 245–252 [DOI] [PubMed] [Google Scholar]
- 36. Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., Moore K. J. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou M. S., Chadipiralla K., Mendez A. J., Jaimes E. A., Silverstein R. L., Webster K. A., Raij L. (2013) Nicotine potentiates oxLDL-induced proatherogenic effects via stimulating and upregulating macrophage CD36 signaling. Am. J. Physiol. Heart. Circ. Physiol. 305, H563–H574 [DOI] [PMC free article] [PubMed] [Google Scholar]