Background: Adhesion of Gram-positive bacteria to host cells is facilitated by human thrombospondin 1 and vitronectin.
Results: Repeating structures R1ab-R2ab of staphylococcal Atl interact with human thrombospondin 1 and vitronectin.
Conclusion: The staphylococcal Atl repeats possess adhesive properties for human thrombospondin 1 and vitronectin.
Significance: Repeats of Atl display multiple adhesive functions contributing to Staphylococcus-host interactions.
Keywords: Adhesion, Extracellular Matrix Proteins, Pathogenesis, Staphylococcus, Thrombospondin, Autolysin, Repeats, Vitronectin
Abstract
Human thrombospondin 1 (hTSP-1) is a matricellular glycoprotein facilitating bacterial adherence to and invasion into eukaryotic cells. However, the bacterial adhesin(s) remain elusive. In this study, we show a dose-dependent binding of soluble hTSP-1 to Gram-positive but not Gram-negative bacteria. Diminished binding of soluble hTSP-1 to proteolytically pretreated staphylococci suggested a proteinaceous nature of potential bacterial adhesin(s) for hTSP-1. A combination of separation of staphylococcal surface proteins by two-dimensional gel electrophoresis with a ligand overlay assay with hTSP-1 and identification of the target protein by mass spectrometry revealed the major staphylococcal autolysin Atl as a bacterial binding protein for hTSP-1. Binding experiments with heterologously expressed repeats of the AtlE amidase from Staphylococcus epidermidis suggest that the repeating sequences (R1ab-R2ab) of the N-acetyl-muramoyl-l-alanine amidase of Atl are essential for binding of hTSP-1. Atl has also been identified previously as a staphylococcal vitronectin (Vn)-binding protein. Similar to the interaction with hTSP-1, the R1ab-R2ab repeats of Atl are shown here to be crucial for the interaction of Atl with the complement inhibition and matrix protein Vn. Competition assays with hTSP-1 and Vn revealed the R1ab-R2ab repeats of AtlE as the common binding domain for both host proteins. Furthermore, Vn competes with hTSP-1 for binding to Atl repeats and vice versa. In conclusion, this study identifies the Atl repeats as bacterial adhesive structures interacting with the human glycoproteins hTSP-1 and Vn. Finally, this study provides insight into the molecular interplay between hTSP-1 and Vn, respectively, and a bacterial autolysin.
Introduction
Different niches of the human body are colonized by various commensals as well as by opportunistic pathogenic species. Staphylococcus aureus, Staphylococcus epidermidis, and Streptococcus pneumoniae are major human pathogens that are part of the human body flora. The moist squamous epithelium of the anterior nares is the primary habitat of S. aureus, whereas S. epidermidis can be found ubiquitously on the skin and in the nares. S. pneumoniae, however, colonizes the epithelia of the upper respiratory tract without causing any clinical symptoms (1–4). Under certain conditions, colonization with these bacteria can result in an infection accompanied with a wide variety of disease patterns, from mild wound infections, otitis media, or pneumonia to severe life-threatening infections like sepsis or meningitis (5–7). Pathogenic bacteria are endowed with numerous surface proteins interacting with structures of the human host cells and the extracellular matrix. Covalently linked microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) and non-covalently linked secretable expanded repertoire adhesive molecules (SERAMs) of different bacterial species bind to host extracellular matrix proteins like fibrinogen, fibronectin, or vitronectin (Vn)3 of host cells, mediating adherence and invasion (8–17). Additionally, in S. pneumoniae, members of the choline-binding protein family, non-covalently linked to the choline moiety of teichoic acids, are also known as adhesins interacting with host matrix proteins (18–20). Furthermore, it has been shown that non-proteinaceous surface structures like the wall teichoic acids are also important for the primary adhesion of bacteria to human host cells (21).
Human vitronectin is a multifunctional glycoprotein that can be found in a monomeric form circulating in serum as well as part of the extracellular matrix in a multimeric form (22, 23). Various bacterial species have been shown to interact with host cell-bound multimeric Vn, facilitating adherence to epithelial cells and artificial surfaces (24, 25). Multiple adhesins/MSCRAMMs for vitronectin exposed on the surface of staphylococci, pneumococci, and various other bacterial species, including the autolysins Atl and Aae from S. epidermidis, have been identified (26–28).
Human thrombospondin 1 (hTSP-1) is a homotrimeric, 420-kDa, multidomain glycoprotein belonging to the group of matricellular proteins. Matricellular proteins are defined as a group of modular, extracellular proteins whose functions are achieved by binding to matrix proteins as well as to cell surface receptors or to other host molecules, such as cytokines and proteases, that, in turn, interact with the cell surface (29). Human TSP-1 is the major protein of α granules in thrombocytes and is released in high amounts after platelet activation (30). Moreover, it is synthesized and secreted by endothelial cells and several other human cell types (31–33). Human TSP-1 is also further involved in a wide range of cellular functions and processes, such as proliferation, angiogenesis, signaling, and cell adhesion, because of its ability to bind to different cell surface receptors (34–37). Gram-positive bacteria such as S. aureus, S. epidermidis and S. pneumoniae are able to interact, via surface-exposed structures, with hTSP-1, facilitating the binding of the bacteria to various artificial surfaces (38, 39). Furthermore, adhesion to epithelial and endothelial cells is increased in the presence of host cell-bound hTSP-1, acting in this scenario as a molecular bridge between the bacterium and the host cell (40). In addition, it has been shown that hTSP-1 supports the adhesion of S. aureus and S. pneumoniae to activated platelets, which could facilitate hematogenous dissemination within the host (41, 42) and, therefore, play an important role in the development of sepsis. The activation of thrombocytes probably plays an important role in infective endocarditis and disseminated intravascular coagulation (43). The process of bacterial adherence to human epithelial and endothelial cells is a multifactorial process essential for colonization and infection of host tissues by pathogenic bacteria.
The mechanism of hTSP-1-mediated adherence of Gram-positive bacteria to different host cells is not yet fully understood. It is still a matter of debate which structures on the bacterial surface mediate binding to hTSP-1. On one hand, non-proteinaceous structures of Gram-positive cells have been proposed to be direct interaction partners (38, 40). On the other hand, surface proteins were favored as binding structures, as shown by the S. aureus extracellular adherence protein (Eap) (39, 44).
In this study, we were able to identify the major staphylococcal autolysin/adhesin Atl, a surface-associated protein, as a direct binding partner of hTSP-1. The Atl proteins of S. epidermidis (AtlE) and S. aureus (AtlA) share high similarities in sequence and domain organization. The 148-kDa (AtlE) and 137.5-kDa (AtlA) full-length proteins are processed proteolytically in vivo into an N-acetyl-l-alanine amidase domain with two repeating regions, termed R1ab and R2ab, at the C-terminal part of the enzyme and a glucosaminidase with a third repeat at the N-terminal region (28, 45). The repeats R1ab and R2ab are further subdivided into the a-type and b-type subunits, as shown recently by structural analysis (46). Each repeat consists of ∼170 amino acids folded into two SH3b domains, each containing a glycine-tryptophan (GW) dipeptide motif.
Amidase and glucosaminidase are important enzymes involved in the process of cell wall turnover of growing bacteria, in which the repeating structures interact with teichoic acids and the peptidoglycan to direct the enzymes to the site of cell separation (46). Besides this, AtlE has been shown to mediate the adherence of S. epidermidis to polystyrene surfaces, plasma proteins, the extracellular matrix protein vitronectin, and recombinant heat shock cognate protein Hsc70 (28, 47). Here we were able to narrow down the binding site of hTSP-1 and Vn to the repeats R1ab-R2ab of the Atl amidase.
EXPERIMENTAL PROCEDURES
Strains and Culture Conditions
S. pneumoniae D39Δcps (40) was cultured to mid-log phase (A600 = 0.35–0.45) in Todd-Hewitt broth (Oxoid) supplemented with 0.5% yeast extract (Roth) or grown on Columbia blood agar plates (Oxoid) at 37 °C and 5% CO2. S. aureus (SA113, SA113Δspa (48), H4862, H9053, and USA 300 (49, 50)), and S. epidermidis RP62A (ATCC 35984, 1585 (51), and 1457-M10 (52, 53)) were cultured to mid-log phase (A600 = 0.7–0.8) in basic medium (BM, 10 g of Tryptone, 5 g of yeast extract, 5 g of NaCl, 1 g of glucose, 1 g of K2HPO4 dissolved in 1 liter of water) on an environmental shaker or on BM agar plates at 37 °C. Pneumococcal and staphylococcal mutants were cultured in the presence of the appropriate antibiotic (kanamycin (150 μg/ml) or erythromycin (2.5 μg/ml)). Escherichia coli 536 (54) and Pseudomonas aeruginosa strain 6 (provided by Friedrich Schauer, Microbiology Greifswald, Germany) were cultured to mid-log-phase in Luria broth (LB) medium (A600 = 0.5) on an environmental shaker or on LB agar plates at 37 °C.
Isolation of Human TSP-1
Platelets were purified from human buffy coats (provided by the Institute for Immunology and Transfusion Medicine, Greifswald, Germany) not older than 24 h and stored at room temperature. After pH adjustment, platelets were activated with thrombin (Sigma-Aldrich) and incubated for 5 min at 37 °C. Thrombin activation was stopped by the addition of hirudin (LOXO) to prevent thrombin-mediated protein cleavage. After centrifugation at 4 °C and 2500 × g for 30 min, the supernatant was immediately deep-frozen at −80 °C for 2 h, thawed at 4 °C, and then centrifuged again to eliminate fibrin clots. The supernatant was loaded onto a HiTrapTM Heparin HP column (GE Healthcare), and the protein was eluted with increasing NaCl concentrations in Hepes buffer (10 mm Hepes, 2mMCaCl2, and 1 mm MgCl2 with 150 mm NaCl, 300 mm NaCl, 450 mm NaCl, or 600 mm NaCl, respectively (pH 7.4)) using the ÄKTA® purifier system (GE Healthcare). Human TSP-1 was dialyzed (molecular mass cut-off 12–14 kDa) overnight at 4 °C against Tris-buffered saline (pH 7.4) containing 2 mm CaCl2 and stored at −80 °C. Purity was verified by SDS-gel electrophoresis followed by Coomassie Brilliant Blue staining. Contaminations with fibronectin or vitronectin were excluded by immunoblot analysis of purified hTSP-1with primary antibodies against vitronectin (rabbit anti-human vitronectin, CompTech) and fibronectin (rabbit anti-human fibronectin, Dako) and a secondary goat anti-rabbit IgG coupled to alkaline phosphatase (1:7500, Promega). Nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt (Sigma-Aldrich) was used as a substrate (supplemental Fig. S1).
Antibody Production
Polyclonal antibodies against human TSP-1 and repeats R1ab-R2ab of AtlE from S. epidermidis were raised in mice using routine immunization protocols. Briefly, 20 μg of purified protein and Freund incomplete adjuvant (50:50 v/v) (Sigma-Aldrich) were injected intraperitoneally. Mice were boosted twice (at days 14 and 28) with 20 μg of protein and Freund incomplete adjuvant (50:50 v/v) (Sigma-Aldrich). After bleeding, the polyclonal IgGs from serum were purified using protein A-Sepharose (Sigma-Aldrich).
Fluorescein Isothiocyanate Labeling of Human TSP-1
Human TSP-1 was labeled with FITC (Sigma-Aldrich) using standard protocols. Briefly, hTSP-1 was incubated with FITC (dissolved in carbonate buffer (pH 9.2)) in a molar ratio of 1:30 for 1 h at 37 °C. Unbound FITC was removed by dialysis (12–14 kDa molecular mass cut-off) against PBS (pH 7.4) overnight at 4 °C.
Digestion of Surface Proteins and Oxidation of Surface Carbohydrate Structures
S. aureus SA113 was grown to exponential phase, harvested, and washed twice with PBS (pH 7.4). Aliquots of 100 μl containing 2 × 108 bacteria/well in 96-well microtiter plates were incubated with either Pronase E (1 mg/ml, Sigma-Aldrich) to digest surface-exposed proteins or with sodium periodate (NaIO4, 0.05 mg/ml, Sigma-Aldrich) to oxidize surface-exposed carbohydrate structures. Plates were incubated for 15 min at 37 °C. After washing with PBS, treated and untreated bacteria were employed in binding assays with FITC-hTSP-1. The binding of FITC-hTSP-1 to bacteria was measured by flow cytometry (FACSCaliburTM (BD Biosciences).
Heterologous Expression of AtlE Fragments from S. epidermidis
DNA fragments of atlE encoding the N-acetylmuramoyl-l-alanine amidase with repeats R1ab-R2ab (Ami-R1ab-R2ab), N-acetylmuramoyl-l-alanine amidase (Ami), AtlE repeats R1ab-R2ab, and AtlE repeat R1ab residues, respectively, were PCR-amplified from S. epidermidis O-47 genomic DNA and cloned into the pGEX 4T-3 expression plasmid (GE Healthcare). Expression of protein fragments fused to a thrombin-cleavable GST tag was induced in E. coli BL21 (DE3) as described earlier (46, 55). For R1ab-R2ab, protein expression was induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside. Bacteria were cultured for a further 12 h at 22 °C. The soluble GST-tagged fusion protein was purified using a glutathione-Sepharose column (Novagen), followed by an on-column cleavage with thrombin (Sigma-Aldrich). The eluted fractions were loaded onto a HiTrapTM Benzamidine FF column (GE Healthcare) to get rid of thrombin. Proteins were purified by size exclusion chromatography using a Superdex 75 column (GE Healthcare) (46). Finally, Vivaspin® columns (10-kDa molecular mass cut-off, Sartorius) were used for buffer exchange and concentration of the purified protein.
Enrichment of Soluble Surface Proteins from S. aureus and S. epidermidis
S. aureus SA113 and S. epidermidis RP62A were grown to exponential phase (A600 0.5–0.7), centrifuged, and washed twice with 20 mm Tris-HCl (pH 8). Cell wall-associated proteins were solubilized by bacterial treatment with various concentrations of the cell wall lytic enzyme lysostaphin (Genmedics, Tübingen, Germany) in the presence of 20% sucrose. Resulting protoplasts were separated by centrifugation at 2500 × g for 30 min. The supernatant containing the cell wall-associated proteins was supplemented with proteinase inhibitors (Complete®, Roche) and immediately deep-frozen and stored at - 80 °C.
Immobilization of hTSP-1 for Surface Plasmon Resonance (SPR) Spectroscopy
To analyze direct protein-protein interactions, human TSP-1 was immobilized on a CM5 biosensor (Biacore, GE Healthcare) using amine coupling as described earlier (57). Briefly, the dextran surface was activated with 1-ethyl-3-(3-dimethylpropyl)-carbodiimide (0.2 m) and N-hydroxysuccinimide (0.05 m). Human TSP-1, diluted at 10 μg/ml in 10 mm acetate buffer (pH 4.0), was injected for surface immobilization (flow rate, 10 μl/min), followed by deactivation of residual activated groups with 1 m ethanolamine. The values of the bound protein (RU) were between 2500–7500 RU (see figure legends). The control flow cell was prepared in the same way but without protein injection. Samples were measured in PBS (pH 7.4) containing 0.05% Tween 20 at 25 °C with a flow rate of 10 μl/min. Regeneration of the affinity surface was carried out with 12.5 mm NaOH. The given RU in the sensorgrams represent the RU values after subtraction of the values measured in the blank chamber.
Ligand Overlay Assay with Human TSP-1
Surface proteins of S. aureus SA113 and purified AtlE proteins were separated by SDS-gel electrophoresis and transferred on a nitrocellulose membrane by semi-dry-blotting (15 V, 1.5 h). The membrane was blocked with 5% skim milk (blotting grade, Roth) and, after washing, incubated with 50 μg/ml hTSP-1 for 2–3 h at 37 °C. After washing, the membrane was incubated with our primary polyclonal mouse anti-hTSP-1 antibody (1:500). Binding was visualized using a secondary goat anti-mouse IgG coupled to alkaline phosphatase (1:7500, Promega) and nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt (Sigma-Aldrich) as a substrate.
Two-dimensional Gel Electrophoresis and Mass Spectrometry
Two samples of cell wall-associated proteins of S. aureus SA113 were prepared for two-dimensional gel electrophoresis. 40 μg of bacterial protein was incubated for 30 min at 20 °C in a total volume of 400 μl of two-dimensional sample buffer containing 12.5 μl 10× rehydration solution (80 mg of CHAPS, 17.5 mg of DTT, 52.5 μl of Pharmalytes (pH 3–10) (GE Healthcare), and 400 μl of 8 m urea/2 m thiourea). After centrifugation, supernatants were loaded on 7-cm internal pH gradient strips (pH 4–7) (GE Healthcare) for rehydration for 18–24 h. After equilibration of the samples, isoelectric focusing was performed in a three-step procedure using the Multiphor II system (GE Healthcare). For the second dimension, internal pH gradient strips were placed on a SDS-polyacrylamide gel (5% stacking gel, 12% resolving gel) and separated for 1.5 h with 20 mA. One gel was used for a ligand overlay blot with hTSP-1 and the other for corresponding silver nitrate staining (58). Protein spots showing hTSP-1 binding were excised for LC/MS analysis.
Briefly, gel spots from the silver-stained gel were destained with 30 mm K3[Fe(CN)6] and 100 mm Na2S2O3 and rinsed with water and 200 mm NH4HCO3 in 50% acetonitrile. After dehydration with acetonitrile, proteins were digested using trypsin at 37 °C overnight. Peptides were extracted from the gel matrix by sonication at 30 °C for 30 min in a 0.5% formic acid, 50% acetonitrile solution.
After nanoLC separation, the samples were measured on an LTQ-Velos Orbitrap (Thermo Fisher Scientific) as described previously (59). The database search was performed using the Sorcerer system and an in-house database of S. aureus (Uniprot-SwissProt 11/2010). Up to two missed cleavages, a mass tolerance of 10 ppm, the variable modification of cysteine by carbamidomethylation or the addition of propionamide, and the oxidation of methionines were considered. Search results were filtered and visualized using Scaffold (Proteome Software).
Flow Cytometry Analysis of hTSP-1 Binding
Bacteria were grown to mid-exponential phase, harvested, and washed twice with PBS (pH 7.4). Bacteria (5 × 108) were incubated in 100 μl of PBS for 30 min at 37 °C with increasing concentrations (0–50 μg/ml) of hTSP-1. After three washing steps, bacteria were incubated with protein A-Sepharose-purified mouse anti-hTSP-1 IgG (1:500). Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) was used as secondary fluorescence-labeled antibody (1:500). Flow cytometric analysis was performed to measure binding of hTSP-1 after fixation of the bacteria with 1% paraformaldehyde using a FACSCaliburTM (BD Biosciences). Bacteria were detected using log-forward and log-side scatter dot plots. Gating was set to exclude debris and aggregates of bacteria. 50,000 events were counted and analyzed for fluorescence using log-scale amplification. As a measure for binding activity, the geometric mean fluorescence activity (GMFI) multiplied by the percentage of labeled bacteria was recorded. Data acquisition was conducted using CellQuest Pro software v. 6.0 (BD Biosciences), and data analysis was performed using WinMDI 2.9 software (written by J. Trotter).
Binding of hTSP after Incubation with R1ab-R2ab
S. aureus Sa113Δspa was grown to mid-exponential phase (A600 = 0.7–0.8), harvested, and washed twice with PBS (pH 7.4). Bacteria (5 × 108/well) were incubated with 3 μg of heterologously expressed R1ab-R2ab for 30 min at 37 °C. After washing, bacteria were incubated with various concentrations of hTSP-1 and measured by flow cytometry as described.
Enzyme-linked Immunosorbent Assays
To assess binding of R1ab-R2ab or R1ab to immobilized hTSP-1, 0.5 μg of hTSP/well was immobilized on 96-well Nunc MaxiSorp® plates (Thermo Scientific) at 4 °C overnight. After washing with PBS supplemented with 0.05% Tween 20 (Roth) and blocking with 1% BSA (Roth), wells were washed again with PBS-Tween (0.05%) and incubated with increasing molecular ratios of AtlE repeats. After washing, wells were incubated with polyclonal mouse anti-AtlE repeat IgG (1:500), washed, and incubated with a secondary goat anti-mouse IgG coupled to horseradish peroxidase (1:2500, Jackson ImmunoResearch Laboratories, Inc.). 2,2′-Azino-di-3-ethylbenzthiazoline sulfonate (6) (Roche) or O-Phenylenediamine dihydrochloride (Dako) was used as substrate, and absorbance at 405 and 492 nm was detected using a FLUOstar Omega Fluoreader (BMG Labtech). Binding of hTSP-1 and Vn to immobilized R1ab-R2ab was tested in a similar way using a polyclonal mouse anti-hTSP-1 or polyclonal rabbit anti-Vn IgG (Complement Technology). Detection of hTSP-1 and Vn binding was measured using a secondary goat anti-mouse or anti-rabbit IgG coupled with horseradish peroxidase (Dianova) and O-phenylenediamine dihydrochloride as a substrate.
In competitive ELISAs, binding of hTSP-1 to immobilized R1ab-R2ab (0.5 μg) in the presence of Vn (and the other way around) was analyzed using a constant concentration of one binding partner and increasing molecular ratios of the other binding partner.
RESULTS
Gram-positive Bacteria Bind Soluble hTSP-1
Binding of soluble hTSP-1 was analyzed in different Gram-positive and Gram-negative bacterial species, which included laboratory strains and clinical isolates. Bacteria were incubated with increasing concentrations of purified hTSP-1 (0–50 μg/ml), and binding of soluble hTSP-1 was analyzed by flow cytometry using a primary hTSP1-specific polyclonal mouse antibody and a secondary Alexa Fluor 488-conjugated antibody. Binding of hTSP-1 to clinical isolates of S. aureus (H4862, H9053, and USA300) was analyzed using FITC-labeled TSP-1 because of the presence of protein A (Fig. 1 C).
FIGURE 1.
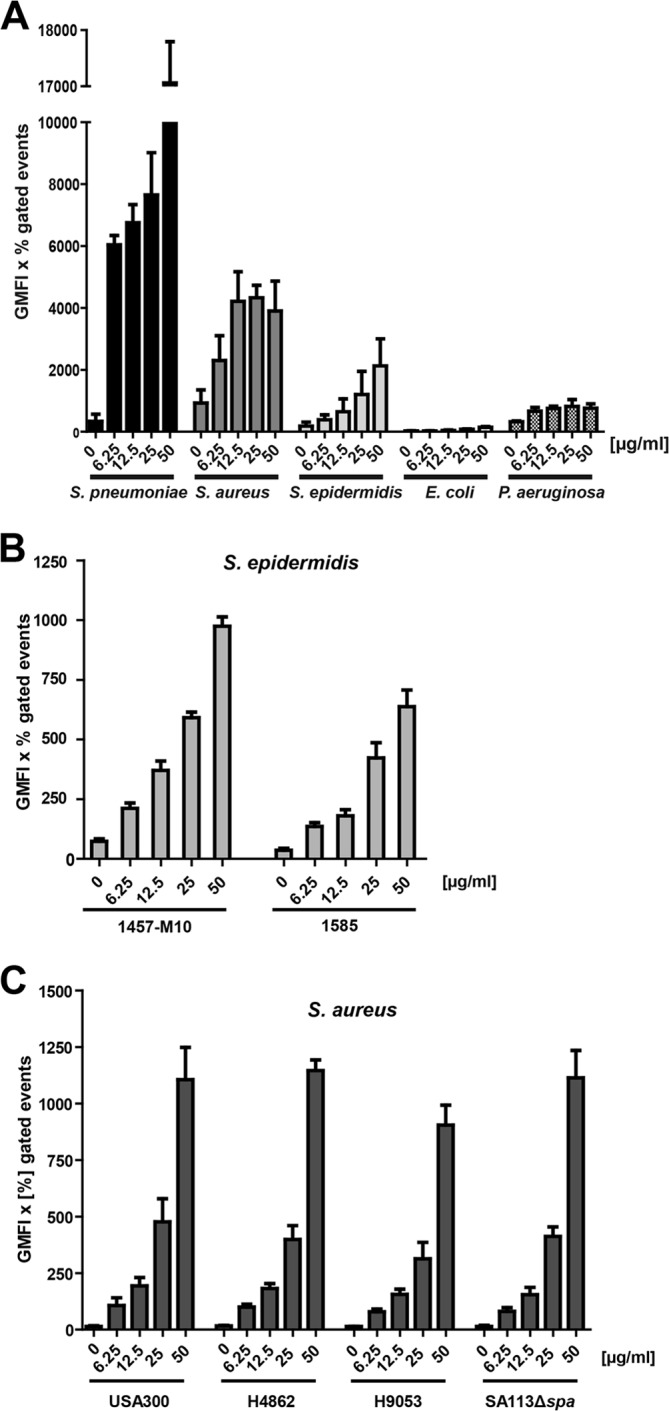
Binding of soluble hTSP-1 by bacteria. A, dose-dependent binding of hTSP-1 to Gram-positive S. pneumoniae D39Δcps, S. aureus SA113Δspa, S. epidermidis RP62A, and Gram-negative E. coli 536 and P. aeruginosa strain 6, respectively, was analyzed by flow cytometry. Bacterial suspensions of 5 × 108 bacteria in 100 μl of PBS were incubated with increasing concentrations of hTSP-1 (0–50 μg/ml) and analyzed for surface-bound hTSP-1 using a specific mouse anti-TSP-1 antibody and secondary Alexa Fluor 488 conjugated anti-mouse IgG. The values are represented as the geometrical mean fluorescence intensity multiplied with the percent of gated events (GMFI × % gated events). B, dose-dependent binding of hTSP-1 to clinical isolates S. epidermidis 1457-M10 (pia-negative mutant of a 1457 catheter infection isolate) and S. epidermidis 1585 (liquor shunt infection isolate). The values are represented as the geometrical mean fluorescence intensity multiplied with percent gated events. C, dose-dependent binding of FITC-labeled hTSP-1 to different clinical isolates of S. aureus (H4862 and H9053, furuncle isolates, and caMRSA USA300). Bacterial suspensions of 5 × 108 bacteria in 100 μl of PBS were incubated with increasing concentrations of FITC-labeled hTSP-1 (0–50 μg/ml) and analyzed for surface-bound hTSP-1 using flow cytometry. The values are represented as the geometrical mean fluorescence intensity multiplied with percent gated events.
The Gram-positive bacteria S. pneumoniae, S. aureus, and S. epidermidis showed a dose-dependent binding of hTSP-1, whereas only a moderate but dose-independent binding of hTSP-1 to Gram-negative bacteria such as E. coli or P. aeruginosa was measured (Fig. 1A). These data suggest that the bacterial adhesin for hTSP-1 is either specific for Gram-positive bacteria or only accessible in the Gram-positive cell wall.
TSP-1 Recruitment Depends on Protein(s)
To assess the chemical nature of the bacterial adhesin(s) for hTSP-1 on the bacterial surface, S. aureus SA113 was pretreated either with Pronase E to degrade potential surface-associated hTSP-1 binding proteins or with sodium periodate to oxidize potential carbohydrate structures interacting with hTSP-1. Pronase E- and sodium periodate-pretreated bacteria were incubated with two different concentrations of FITC-labeled hTSP-1 to compare their ability to bind hTSP-1 to their surfaces with untreated bacteria. The proteolytic treatment of staphylococci significantly decreased binding of human TSP-1, whereas pretreatment of the bacteria with sodium periodate had no effect on their hTSP-1 binding capacity (Fig. 2). These results suggest that surface-exposed proteins of Gram-positive bacteria represent hTSP-1 adhesin(s) and recruit soluble hTSP-1 to the bacterial surface.
FIGURE 2.
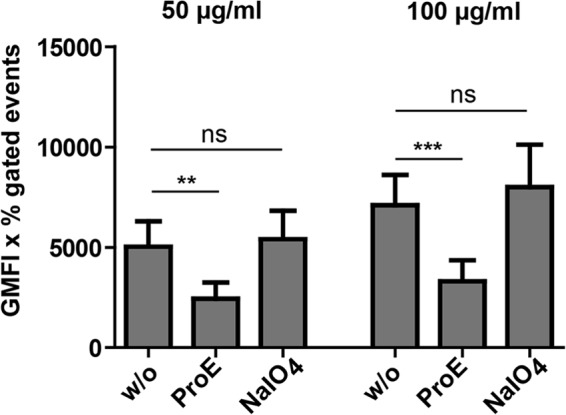
Proteolytic treatment diminishes TSP-1 binding activity of S. aureus. Binding of different concentrations of FITC-labeled hTSP-1 (50 and 100 μg/ml) to 2 × 108 S. aureus SA113. Bacteria were pretreated with either Pronase E (ProE, 1 mg/ml) to digest potential proteinaceous binding partners on the bacterial surface or with sodium periodate (NalO4, 0.05 mg/ml) to oxidize potential carbohydrate binding partners. Binding of FITC-labeled human TSP-1 was analyzed (n = 3) by flow cytometry in comparison with untreated bacteria (w/o). The values are represented as the geometrical mean fluorescence intensity multiplied with percent gated events (GMFI x % gated events). **, p < 0.01; ***, p < 0.001; ns, not significant.
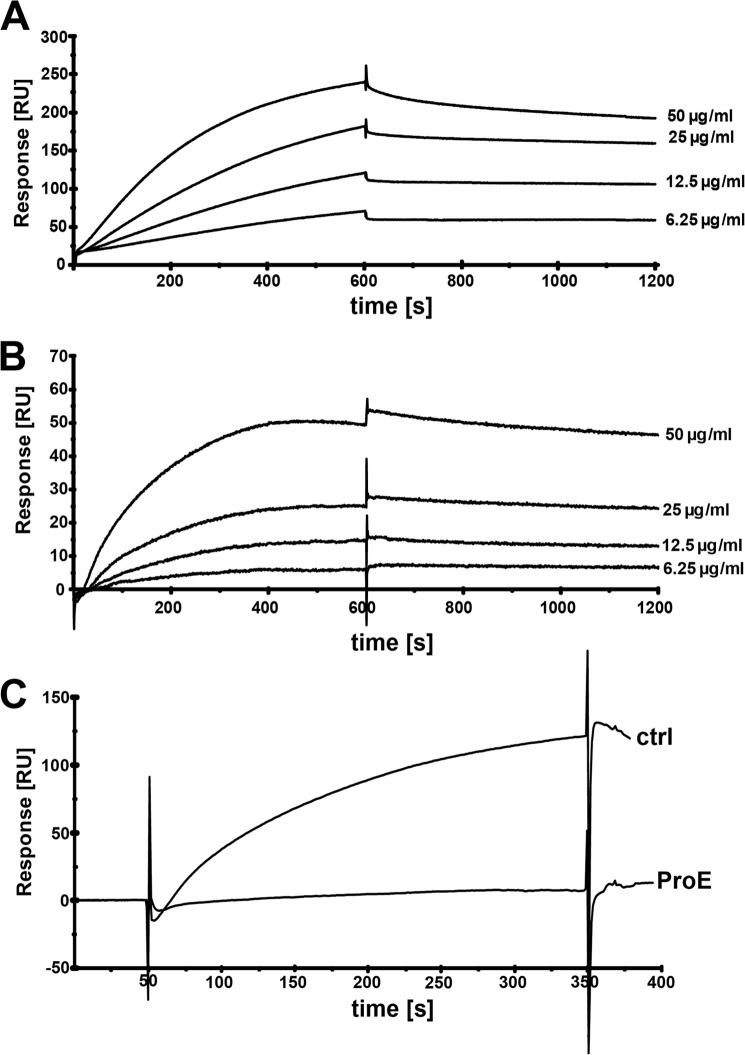
To assess the role of surface-exposed proteins in hTSP-1 binding, SPR studies were employed to demonstrate that enriched bacterial surface protein fractions contain adhesin(s) for hTSP-1. Human TSP-1 was immobilized on a CM5 biosensor, whereas the extracted cell wall-associated proteins were used as analytes in SPR studies. Indeed, the sensorgrams revealed a dose-dependent binding of cell wall-associated staphylococcal proteins to immobilized hTSP-1 (Fig. 3, A and B). To confirm the proteinaceous nature of the bacterial hTSP-1 adhesin, the enriched surface components were proteolytically treated prior to their use as analyte and, after heat inactivation of the protease (20 min, 80 °C), employed again in binding studies (Fig. 3C). Proteolytic pretreatment of protein samples diminished binding to hTSP-1, confirming that hTSP-1 interacts with surface protein(s).
FIGURE 3.
Binding of S. aureus surface proteins to hTSP-1 as analyzed by surface plasmon resonance. A and B, surface plasmon resonance sensorgrams demonstrating a dose-dependent binding of surface proteins enriched from S. epidermidis RP62A (A) and S. aureus SA113 (B) to hTSP-1 immobilized on a CM5 biosensor. C, binding of surface proteins of S. aureus SA113 (100 μg/ml) to immobilized hTSP-1 before (ctrl) and after incubation with Pronase E (ProE). The CM5 biosensor was coated with hTSP-1 (∼7500 RU), and enriched surface proteins were used as analytes at a flow rate of 10 μl/min. The affinity surface was regenerated between subsequent sample injections with 12.5 mm sodium hydroxide. The values of the control flow cell were subtracted from each sensorgram.
Identification of Atl by Two-dimensional SDS-Gel Electrophoresis and Mass Spectrometry
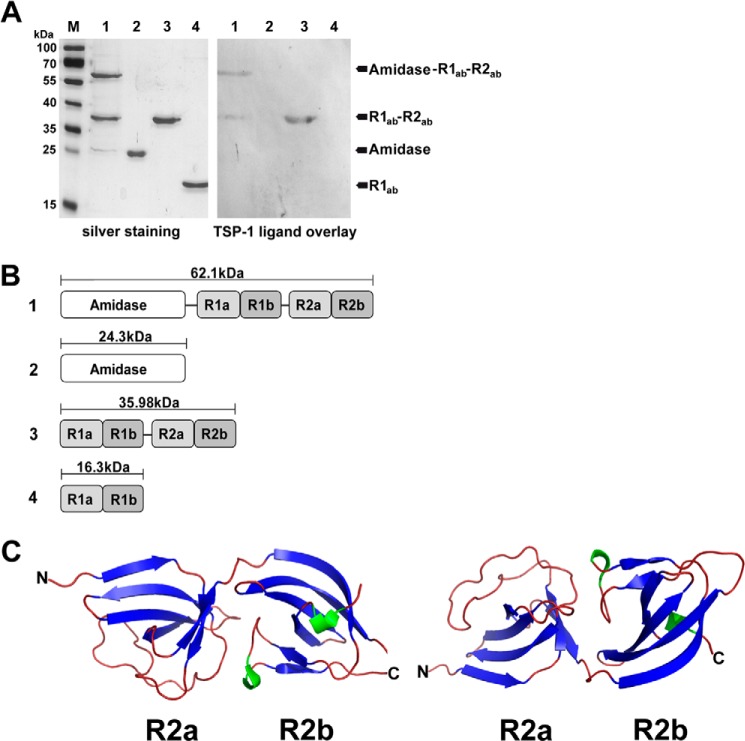
To identify staphylococcal surface protein(s) interacting specifically with hTSP-1, a proteomic approach was used. Cell surface proteins extracted from S. aureus SA113 were separated by two-dimensional SDS-gel electrophoresis, and a ligand overlay assay with hTSP-1 was performed after transfer of the proteins onto nitrocellulose. In parallel, protein patterns were visualized by staining with silver nitrate. The hTSP-1 ligand overlay assay revealed protein spots interacting with hTSP-1. After matching positive signals of the hTSP-1 ligand overlay with the protein pattern of the silver nitrate stain, candidate protein spots were picked from the corresponding silver-stained polyacrylamide gel (Fig. 4), digested with trypsin, and analyzed by mass spectrometry. The database search identified AtlA, among others, as one potential hTSP-1 binding protein of S. aureus SA113 (supplemental Table S1). Six of the eight identified peptides of AtlA (FYLVQDYNSGNK, EGDVVYNTAK, SPVNVNQSYSYSIKPGTK, SIYLYGSVNGK, AYLVDTAKPTPTPTPK, and AYLAVPAAPK) were located within the repeats R1ab-R2ab. A spot pattern similar to the hTSP-1 ligand overlay assay was also demonstrated by immunoblot analysis after two-dimensional gel electrophoresis of the extracted S. aureus surface proteins when using anti-R1ab-R2ab antibodies (supplemental Fig. S2), confirming the results of the MS analysis.
FIGURE 4.
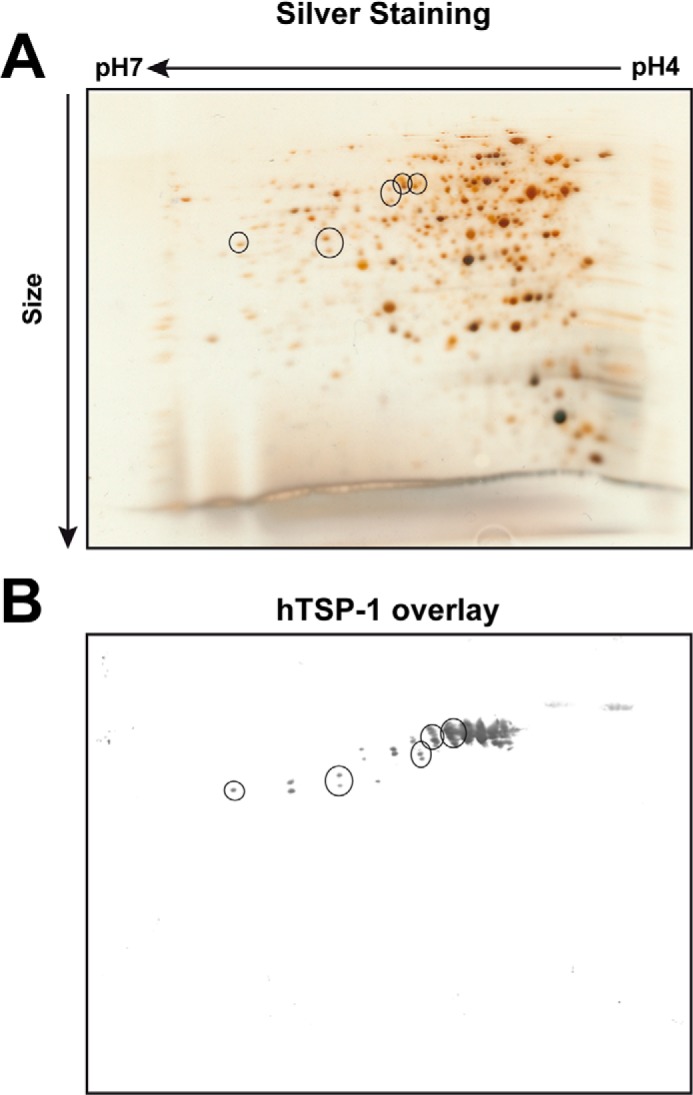
Identification of the major staphylococcal autolysin as TSP-1 binding protein. A, representative silver-stained protein gel (12%) after two-dimensional separation of purified cell wall-associated and secreted proteins (40 μg) from S. aureus SA113. Proteins were subjected to isoelectric focusing with 7-cm IPG strips (pH 4–7), followed by SDS-PAGE. Single spots were excised after matching (circles) with a corresponding overlay blot with hTSP-1 (B). After digestion with trypsin, peptides of the spots were analyzed using mass spectrometry.
Repeating Structures of Atl are Essential for Binding Activity
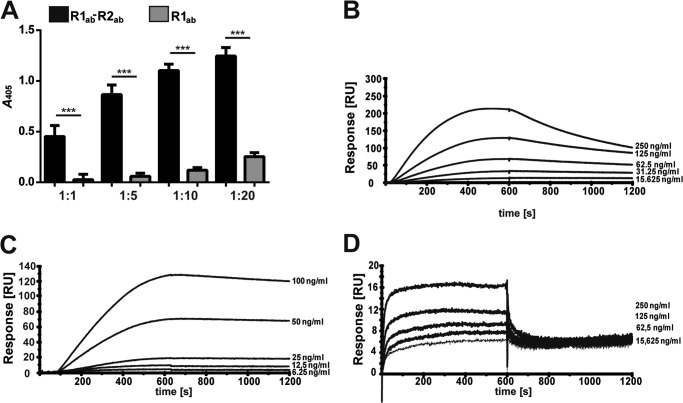
AtlE is highly similar to AtlA (61% identity to AtlA of S. aureus NCTC 8325) and interchangeable between S. epidermidis and S. aureus, as shown previously (60). Recently, the crystal structures of the catalytic domain of AtlE amidase and of the repetitive structures R1ab-R2ab were solved (46, 61). In our study, heterologously expressed fragments containing the amidase domain of AtlE together with repeats R1ab-R2ab, repeats R1ab-R2ab, and the R1ab domain were used in a ligand overlay assay with hTSP-1. The amidase domain with and without R1ab-R2ab, repeats R1ab-R2ab, and the single repeat R1ab were separated by SDS-PAGE and stained with silver nitrate. A corresponding SDS gel was used for a ligand overlay blot with human TSP-1 (Fig. 5A). The protein domain composed of the amidase and repeats R1ab-R2ab and the similar repeats R1ab-R2ab showed binding of hTSP-1, whereas the amidase domain without repeats R1ab-R2ab and the repeat R1ab showed no binding to hTSP-1. These results were confirmed in further binding studies using the repeats R1ab-R2ab and the single repeat R1ab. The results of an ELISA showed a dose-dependent binding of R1ab-R2ab to immobilized hTSP-1, whereas the single repeat R1ab showed only a low hTSP-1-binding activity (Fig. 6A). Surface plasmon resonance with immobilized hTSP-1 showed similar results. The analyte R1ab-R2ab bound in a dose-dependent manner to immobilized hTSP-1, and dissociation after stopping the injection of the analyte was moderate (Fig. 6B). In contrast, the single repeat R1ab exhibited only a low binding activity with a fast association but also an immediate dissociation, suggesting a low hTSP-1 binding affinity (Fig. 6D). These data suggest that the repeats R1ab-R2ab are the minimal domain required for the interaction of Atl with hTSP-1. In contrast, a single repeat, represented here by R1ab, was not sufficient to mediate the interaction of Atl with hTSP-1.
FIGURE 5.
Human TSP-1 binding to heterologously expressed AtlE derivates. A, silver-stained SDS gel (12%) of heterologously expressed part structures of AtlE and corresponding ligand overlay blot with hTSP-1. SDS-PAGE-separated proteins were blotted on a nitrocellulose membrane, incubated with hTSP-1 (50 μg/ml), and then binding was detected using a mouse polyclonal anti-hTSP-1 IgG followed by incubation with an alkaline phosphatase-coupled secondary anti-mouse antibody. M, marker PageRuler-prestained (Fermentas); lane 1, heterologously expressed N-acetyl-muramoyl-l-alanine amidase with repeats R1ab-R2ab (62.1 kDa); lane 2, amidase (24.3 kDa); lane 3, repeats R1ab-R2ab (35.98 kDa); lane 4, repeat R1ab (16.3 kDa). B, domain arrangement and molecular weight of the heterologously expressed AtlE part structures used in this study. C, schematic of the crystal structure of repeat R2ab of the AtlE amidase (PDB code 4EPC) generated with PyMOL. R2ab consists of ∼170 amino acids folded into two SH3b domains, each containing a GW dipeptide motif. Blue, β strands; green, α helices.
FIGURE 6.
Human TSP-1 binds preferentially to repeats R1ab-R2ab as shown by surface plasmon resonance studies. A, human TSP-1 (0.1 μg) was immobilized on 96-well plates (MaxiSorp) and incubated with various molecular ratios of AtlE R1ab-R2ab or AtlE R1ab. The binding of repeats was detected using a polyclonal anti-AtlE-R1ab-R2ab IgG followed by incubation with a peroxidase-coupled secondary antibody. Results are expressed as means ± S.D. (n = 3). **, p < 0.01; ***, p < 0.001; ns, not significant. B, surface plasmon resonance sensorgrams of heterologously expressed AtlE R1ab-R2ab show a dose-dependent binding to immobilized hTSP-1. A CM5 biosensor was coated with hTSP-1 (∼4000 response units), and the heterologously expressed repeats R1ab-R2ab of AtlE were used as analytes. The values of the control flow cells were subtracted from each sensorgram. C, surface plasmon resonance sensorgrams of heterologously expressed AtlE R1ab-2ab show a dose-dependent binding to immobilized human vitronectin. Vn was immobilized on the CM5 biosensor (∼2500 response units), and the heterologously expressed repeats R1ab-R2ab of AtlE were used as analytes. The values of the control flow cells were subtracted from each sensorgram. D, low binding activity of heterologously expressed AtlE repeat R1ab (25 μg/ml) to immobilized hTSP-1 as analyzed by an SPR study. Shown is an SPR sensorgram of a manual run.
Preincubation of S. aureus with R1ab-R2ab Increased Recruitment of hTSP-1
Recent studies showed the ability of the isolated AtlE repeats R1ab-R2ab to bind to isolated peptidoglycan as well as to whole bacteria (55, 60). S. aureus Sa113Δspa was preincubated with heterologously expressed R1ab-R2ab from AtlE to assess whether the acquisition of hTSP-1 is increased in the presence of higher amounts of cell surface-bound repeats R1ab-R2ab. Flow cytometric analysis confirmed that the repeats R1ab-R2ab have the capability to reassociate to the staphylococcal cell surface (data not shown). Indeed, the increased availability of the repeats bound to the surface of the bacteria resulted in an increased ability to recruit soluble hTSP-1 to the bacterial surface (Fig. 7). Because the repeats were able to bind to the bacterial cell surface and hTSP-1, these data further suggest that the binding sites for peptidoglycan and hTSP-1 are located in different parts of the repeats.
FIGURE 7.
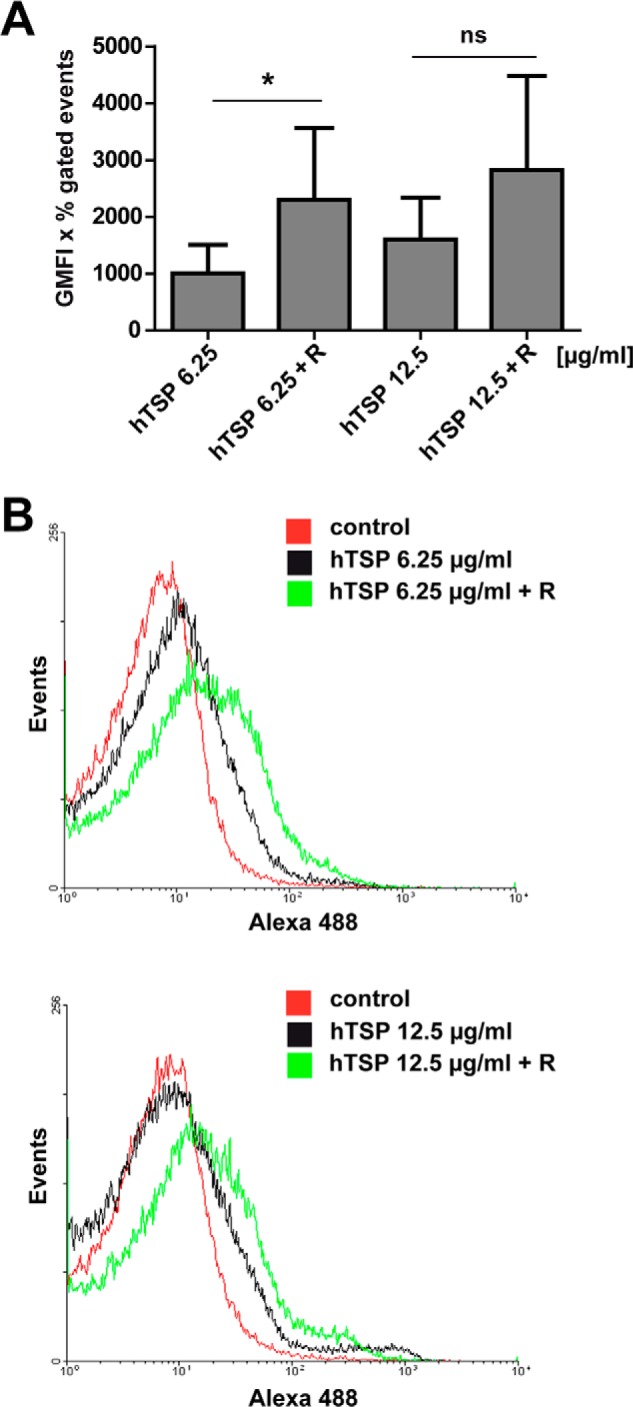
Reassociation of Atl repeats to S. aureus increases hTSP-1 binding. A, binding of hTSP-1 (6.25 and 12.5 μg/ml) to S. aureus SA113Δspa was analyzed using 5 × 108 of untreated or 3 μg of AtlE repeats R1ab-R2ab (TSP 6.25 + R and TSP 12.5 + R) pretreated bacteria. Binding was analyzed by flow cytometry using a specific mouse anti-TSP-1 antibody and secondary Alexa Fluor 488-conjugated anti-mouse IgG. The values represent the geometrical mean fluorescence intensity multiplied with the percent of gated events (GMFI × % gated events) (n = 3). *, p < 0.05; ns, not significant. B, representative overlay histograms showing binding of hTSP to untreated S. aureus Sa113Δspa (black) and increased binding of hTSP-1 to S. aureus Δspa pretreated with 3 μg of AtlE repeats R1ab-R2ab (green).
Human TSP-1 and Vitronectin Compete for the Binding Site within AtlE
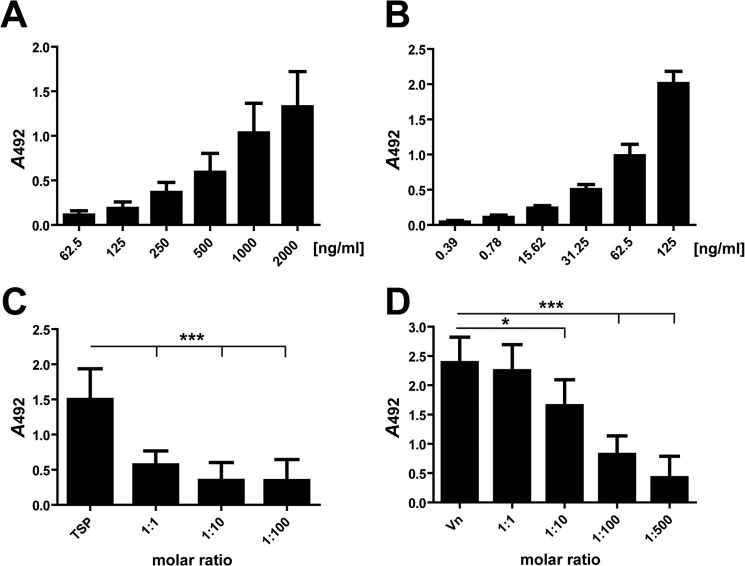
The amidase repeats R1ab-R2ab immobilized in Maxisorp® microtiter plates showed a dose-dependent binding of either hTSP-1 or Vn (Fig. 8, A and B). However, lower amounts of Vn seem to be necessary for the detection of binding to R1ab-R2ab compared with TSP-1 in ELISA assays.
FIGURE 8.
Human TSP-1 and vitronectin bind dose-dependently to the R1ab-R2ab repeats of Atl and compete for binding. A, binding of hTSP-1 to immobilized Atl repeats R1ab-R2ab. The heterologously expressed repeats R1ab-R2ab (0.5 μg) were immobilized on microtiter plates (MaxiSorp) and incubated with increasing amounts of hTSP-1. B, binding of human vitronectin to immobilized Atl repeats R1ab-R2ab. The heterologously expressed repeats R1ab-R2ab (0.5 μg) were immobilized on microtiter plates (MaxiSorp) and incubated with increasing amounts of Vn. C and D, human TSP-1 competes with human vitronectin for binding to the immobilized Atl repeats R1ab-R2ab. The heterologously expressed repeats R1ab-R2ab (0.5 μg) were immobilized on microtiter plates (MaxiSorp) and incubated with hTSP-1 (1000 ng/well) in the presence of increasing molecular ratios of Vn (C) or with Vn (125 ng/well) in the presence of increasing molecular ratios of hTSP-1 (D). Bound hTSP was detected using a polyclonal mouse anti-hTSP-1 IgG antibody followed by incubation with a peroxidase-coupled secondary anti-mouse antibody, and bound Vn was detected using a polyclonal rabbit anti-Vn IgG followed by incubation with a peroxidase-coupled secondary anti-rabbit antibody. Results are expressed as means ± S.D. (n = 3). *, p < 0.05; ***, p < 0.001; ns, not significant.
To prove whether the same binding site within the AtlE repeats was occupied by Vn and hTSP-1, competition assays were conducted. In the presence of increasing molecular ratios of TSP-1, Vn binding to R1ab-R2ab was inhibited dose-dependently. Vice versa, increasing molecular ratios of Vn also showed a dose-dependent inhibition of hTSP-1 binding (Fig. 8, C and D). Interestingly, lower molecular ratios of Vn seem to be necessary to inhibit hTSP-1 binding to R1ab-R2ab, suggesting a stronger binding affinity of Vn to R1ab-R2ab compared with hTSP-1. We hypothesize, therefore, that Vn and hTSP-1 share the same or proximal binding sites within the repeats R1ab-R2ab.
DISCUSSION
S. aureus and S. epidermidis are facultative human pathogens causing a wide range of diseases, preferentially in hospitalized and immunocompromised persons but also in the community. A characteristic feature of these bacteria is their ability to colonize host tissues and persist on abiotic surfaces, including the formation of biofilms on medical devices (5, 6, 62). Staphylococci produce a variety of adhesins interacting with the host cell matrix or serum proteins such as fibrinogen, fibronectin, vitronectin, collagen, and thrombospondin 1. The recruitment and binding of these host proteins has been demonstrated to be pivotal for the pathogenesis of staphylococci and facilitates bacterial attachment to host tissues or inert surfaces (8–10, 63). Human TSP-1, a matricellular glycoprotein produced by platelets and different cell types, has been shown to promote binding of Gram-positive bacteria, including S. aureus, to artificial surfaces. TSP-1 further mediates adhesion to and invasion of Gram-positive bacteria into various epithelial and endothelial cells (38, 40). However, the adhesive bacterial structure and binding partner for hTSP-1 remained elusive, and it is still a matter of debate whether the bacterial adhesin is a carbohydrate structure or a surface-exposed protein (38, 39, 40, 44).
In this study, we demonstrate that only Gram-positive bacteria have the capability to recruit soluble hTSP-1 in a dose-dependent manner to their surfaces (Fig. 1). Importantly, proteolytic treatment of the staphylococcal cell surface impaired the interaction with hTSP1, suggesting (a) proteinaceous structure(s) as adhesin(s) for hTSP-1. Binding of soluble hTSP-1 was diminished to S. aureus pretreated with Pronase E, as shown by flow cytometry (Fig. 2), and, similarly, proteolytic treatment of enriched surface proteins of S. aureus and S. epidermidis, respectively, abolished binding to hTSP-1 immobilized on a biosensor (Fig. 3C). The characterization of a proteinaceous bacterial binding partner for hTSP-1 is in agreement with the data from Yanagisawa and colleagues (39) favoring surface proteins of S. aureus and S. epidermidis as a bacterial adhesin for hTSP-1.
By applying a proteome-based approach in combination with a ligand overlay assay and using soluble hTSP-1, we identified Atl, the major autolysin of S. aureus and S. epidermidis, as a hTSP-1-binding protein. Atl is a multifunctional protein of staphylococci and, in addition to its amidase and glucosaminidase activity, Atl and homologues have been shown to act as binding partners for the extracellular host glycoproteins fibronectin, fibrinogen, and vitronectin. Atl is further involved in the internalization of staphylococci by endothelial cells (28, 47, 64). The protein domains of Atl essential for the interaction with host proteins have not been characterized in detail so far. However, recently it has been shown that the amidase containing R1ab-R2ab repeats is able to bind to fibrinogen, fibronectin, and Vn (47). To narrow down the binding domain of hTSP-1 within the Atl amidase, heterologously produced AtlE derivatives of S. epidermidis were tested for their ability to bind hTSP-1. The binding domain of hTSP-1 was localized to the repeats R1ab-2ab of the amidase of Atl. In contrast, the amidase domain without R1ab-R2ab of Atl showed no hTSP-1-binding activity. Because Atl has also been shown to bind Vn (28), we were interested whether the repeats R1ab-2ab of Atl also mediate binding to Vn. Indeed, Vn binding was also localized to the repeatsR1ab-2ab, which are widely distributed among staphylococcal amidases and show conserved amino acids near the GW motifs (46). Importantly, only R1ab-R2ab showed a high hTSP-1 binding activity, whereas the sole repeat R1ab showed only moderate binding to hTSP-1, suggesting that at least two repeats represent the minimal motif required for an efficient interaction with hTSP-1 or Vn. Therefore, we conclude that the repeats R1ab-R2ab of staphylococcal Atl proteins possess the adhesive capacity and represent the domain interacting with host-derived glycoproteins such as hTSP-1 and Vn. Strikingly, Vn is able to compete with TSP-1 for binding to Atl repeats R1ab-R2ab and vice versa, suggesting that the modes of interaction are similar and that steric hindrance avoids binding of the other host protein to R1ab-R2ab. In addition, recruitment of TSP-1 to staphylococci pretreated with the exogenously added repeats R1ab-2ab is enhanced, as is the surface abundance of repeats, suggesting that the peptide sequence involved in reassociation of Atl repeats to the bacterial cell wall is different from the binding sites for TSP-1 and Vn, respectively. One can further speculate that these repeats are also involved in Atl-mediated adhesion of staphylococci to epithelial cells. Recently, a gene expression study of S. aureus in human nasal colonization showed that atlA is up-regulated during nasal colonization, emphasizing a potential in vivo role of Atl as an adhesin in the process of adhesion and colonization of host tissues (65). In this regard, it is noteworthy that a known bacterial interaction partner of hTSP-1, the extracellular adherence protein Eap of S. aureus, which also binds to hTSP-1 and vitronectin, does not share sequence similarities with R1ab-R2ab. However, Eap also exhibits repeating sequences that are involved in binding to hTSP-1, Vn, and other extracellular matrix proteins (13). Similarly, pneumococcal proteins composed of repetitive sequences, such as the PspC and PavB proteins, interact via their repeats with Vn and fibronectin, respectively (27, 66).
It remains obscure why hTSP-1 is bound only by Gram-positive bacteria. The restriction of hTSP-1 acquisition to the surface of Gram-positive bacteria could be due to the fundamentally different architecture of the cell wall of Gram-positive and Gram-negative bacteria. The structure of the outer membrane of the Gram-negative cell wall with its high amounts of lipopolysaccharide may interfere with the binding of hTSP-1. Putative adhesins existing on the bacterial cell surface can be masked by lipopolysaccharides. Such a phenomenon has been shown recently for the interaction of platelet factor 4 with Gram-negative bacteria (67).
Although a previous study also suggested that peptidoglycan is involved in binding of bacteria to cell-bound hTSP-1 (40), this study indicates that surface proteins interact specifically with hTSP-1. In the previous study, peptidoglycan purified from S. aureus (40) was used to inhibit the attachment of bacteria to hTSP-1-preincubated host epithelial cells. However, Atl interacts with the teichoic acids (55) and was abundant in the used peptidoglycan fraction because no proteolytic treatment has been carried out to degrade proteins (data not shown) (40).
Human TSP-1 is composed of three identical multidomain monomers with different binding sites for multiple known ligands. Although the results suggest that the repeats R1ab-R2ab of Atl amidase interact with repetitive sequences of TSP-1, the binding domain within hTSP-1 and mechanism of the protein-protein interaction has to be elucidated in further studies, which then also allows to determine affinity constants in SPR studies. A strategy to gain comprehensive insight into the Atl-hTSP1 interaction will be the structural analysis of protein complexes consisting of the Atl amidase repeats R1ab-R2ab and the TSP-1 domain interacting with Atl R1ab-R2ab. This will also indicate the amino acids critical for this interaction. Because Atl and Eap, both containing repeating structures (28, 56), interact specifically with Vn and TSP-1, it will be of interest whether other bacterial vitronectin-binding proteins also have the ability to bind TSP-1. Atl and Eap, sharing only minor sequence homology, interact via their repeats with TSP-1 and Vn, respectively, pointing to the fact that not the primary sequences but probably the structures of the adhesins share similarities and confer binding activity for Vn and TSP-1. To identify further microbial TSP-1 or Vn-binding proteins, candidate proteins consisting of repeats with ∼100 amino acid residues should be tested for their ability to interact with TSP-1, Vn, or other adhesive glycoproteins. Furthermore, the isolation and analysis of surface proteins from S. aureus and different Gram-positive bacteria using native two-dimensional gel electrophoresis and ligand overlay blots may reveal additional proteins mediating the binding of hTSP-1 and Vn.
Taken together, we showed that Gram-positive bacteria dose-dependently recruit human TSP-1 to their surfaces. We identified AtlE, the major autolysin of S. epidermidis, as a direct binding partner of hTSP-1. The analysis of heterologously expressed protein fragments of the AtlE amidase revealed that hTSP-1 and Vn recognize the repeating structures R1ab-R2ab. The preincubation of S. aureus with R1ab-R2ab leads to an increase in binding of hTSP-1 to the surface of S. aureus. Furthermore, we were able to show that hTSP-1 as well as Vn share the same binding site within the Atl amidase repeats R1ab-R2ab.
Acknowledgments
We thank Leif Steil (Ernst Moritz Arndt Universität Greifswald) for handling mass spectrometry data, Gottfried Palm (Ernst Moritz Arndt Universität Greifswald) for support in data generation with PyMOL, Barbara Bröker (Ernst Moritz Arndt Universität Greifswald) and Holger Rohde (University Medical Center Hamburg-Eppendorf) for S. aureus and S. epidermidis strains, and Simone Thomsen (Leibnitz Center for Medicine and Biosciences, Borstel, Germany) and Peggy Stremlow (Ernst Moritz Arndt Universität Greifswald) for technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB TRR 34, Project C10 (to S. H.), DFG HA 3125/2-1, and ZA 149/6-1.

This article contains supplemental Figs. S1 and S2 and Table S1.
- Vn
- vitronectin
- hTSP
- human thrombospondin-1
- SPR
- surface plasmon resonance
- RU
- response unit(s)
- GMFI
- geometrical mean fluorescence intensity.
REFERENCES
- 1. Williams R. E. (1963) Healthy carriage of Staphylococcus aureus. Its prevalence and importance. Bacteriol. Rev. 27, 56–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kluytmans J., van Belkum A., Verbrugh H. (1997) Nasal carriage of Staphylococcus aureus. Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10, 505–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kloos W. E., Musselwhite M. S. (1975) Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl. Microbiol. 30, 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bogaert D., De Groot R., Hermans P. W. (2004) Streptococcus pneumoniae colonisation. The key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154 [DOI] [PubMed] [Google Scholar]
- 5. Foster T. J. (2004) The Staphylococcus aureus “superbug.” J. Clin. Invest. 114, 1693–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogers K. L., Fey P. D., Rupp M. E. (2009) Coagulase-negative staphylococcal infections. Infect. Dis. Clin. North Am. 23, 73–98 [DOI] [PubMed] [Google Scholar]
- 7. Musher D. M. (1992) Infections caused by Streptococcus pneumoniae. Clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14, 801–807 [DOI] [PubMed] [Google Scholar]
- 8. Foster T. J., Höök M. (1998) Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6, 484–488 [DOI] [PubMed] [Google Scholar]
- 9. Clarke S. R., Foster S. J. (2006) Surface adhesins of Staphylococcus aureus. Adv. Microb. Physiol. 51, 187–224 [DOI] [PubMed] [Google Scholar]
- 10. Chavakis T., Wiechmann K., Preissner K. T., Herrmann M. (2005) Staphylococcus aureus interactions with the endothelium. The role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb. Haemost. 94, 278–285 [DOI] [PubMed] [Google Scholar]
- 11. Paterson G. K., Orihuela C. J. (2010) Pneumococcal microbial surface components recognizing adhesive matrix molecules targeting of the extracellular matrix. Mol. Microbiol. 77, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. (1988) Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158, 693–701 [DOI] [PubMed] [Google Scholar]
- 13. Hussain M., Haggar A., Peters G., Chhatwal G. S., Herrmann M., Flock J. I., Sinha B. (2008) More than one tandem repeat domain of the extracellular adherence protein of Staphylococcus aureus is required for aggregation, adherence, and host cell invasion but not for leukocyte activation. Infect. Immun. 76, 5615–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voss S., Gámez G., Hammerschmidt S. (2012) Impact of pneumococcal microbial surface components recognizing adhesive matrix molecules on colonization. Mol. Oral Microbiol. 27, 246–256 [DOI] [PubMed] [Google Scholar]
- 15. Kang M., Ko Y. P., Liang X., Ross C. L., Liu Q., Murray B. E., Höök M. (2013) Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J. Biol. Chem. 288, 20520–20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johannessen M., Sollid J. E., Hanssen A. M. (2012) Host- and microbe determinants that may influence the success of S. aureus colonization. Front. Cell. Infect. Microbiol. 2, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vazquez V., Liang X., Horndahl J. K., Ganesh V. K., Smeds E., Foster T. J., Hook M. (2011) Fibrinogen is a ligand for the Staphylococcus aureus microbial surface components recognizing adhesive matrix molecules (MSCRAMM) bone sialoprotein-binding protein (Bbp). J. Biol. Chem. 286, 29797–29805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mann B., Orihuela C., Antikainen J., Gao G., Sublett J., Korhonen T. K., Tuomanen E. (2006) Multifunctional role of choline binding protein G in pneumococcal pathogenesis. Infect. Immun. 74, 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Attali C., Frolet C., Durmort C., Offant J., Vernet T., Di Guilmi A. M. (2008) Streptococcus pneumoniae choline-binding protein E interaction with plasminogen/plasmin stimulates migration across the extracellular matrix. Infect. Immun. 76, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammerschmidt S. (2006) Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol. 9, 12–20 [DOI] [PubMed] [Google Scholar]
- 21. Weidenmaier C., Kokai-Kun J. F., Kristian S. A., Chanturiya T., Kalbacher H., Gross M., Nicholson G., Neumeister B., Mond J. J., Peschel A. (2004) Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10, 243–245 [DOI] [PubMed] [Google Scholar]
- 22. Preissner K. T., Jenne D. (1991) Vitronectin. A new molecular connection in haemostasis. Thromb. Haemost. 66, 189–194 [PubMed] [Google Scholar]
- 23. Preissner K. T., Reuning U. (2011) Vitronectin in vascular context. Facets of a multitalented matricellular protein. Semin. Thromb. Hemost. 37, 408–424 [DOI] [PubMed] [Google Scholar]
- 24. Chhatwal G. S., Preissner K. T., Müller-Berghaus G., Blobel H. (1987) Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect. Immun. 55, 1878–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lundberg F., Schliamser S., Ljungh A. (1997) Vitronectin may mediate staphylococcal adhesion to polymer surfaces in perfusing human cerebrospinal fluid. J. Med. Microbiol. 46, 285–296 [DOI] [PubMed] [Google Scholar]
- 26. Li D. Q., Lundberg F., Ljungh A. (2001) Characterization of vitronectin-binding proteins of Staphylococcus epidermidis. Curr. Microbiol. 42, 361–367 [DOI] [PubMed] [Google Scholar]
- 27. Voss S., Hallström T., Saleh M., Burchhardt G., Pribyl T., Singh B., Riesbeck K., Zipfel P. F., Hammerschmidt S. (2013) The choline-binding protein PspC of Streptococcus pneumoniae interacts with the C-terminal heparin-binding domain of vitronectin. J. Biol. Chem. 288, 15614–15627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heilmann C., Hussain M., Peters G., Götz F. (1997) Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24, 1013–1024 [DOI] [PubMed] [Google Scholar]
- 29. Bornstein P. (1995) Diversity of function is inherent in matricellular proteins. An appraisal of thrombospondin 1. J. Cell Biol. 130, 503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baenziger N. L., Brodie G. N., Majerus P. W. (1971) A thrombin-sensitive protein of human platelet membranes. Proc. Natl. Acad. Sci. U.S.A. 68, 240–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raugi G. J., Lovett D. H. (1987) Thrombospondin secretion by cultured human glomerular mesangial cells. Am. J. Pathol. 129, 364–372 [PMC free article] [PubMed] [Google Scholar]
- 32. Asch A. S., Leung L. L., Shapiro J., Nachman R. L. (1986) Human brain glial cells synthesize thrombospondin. Proc. Natl. Acad. Sci. U.S.A. 83, 2904–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaffe E. A., Ruggiero J. T., Falcone D. J. (1985) Monocytes and macrophages synthesize and secrete thrombospondin. Blood 65, 79–84 [PubMed] [Google Scholar]
- 34. Tang M., Zhou F., Zhang W., Guo Z., Shang Y., Lu H., Lu R., Zhang Y., Chen Y., Zhong M. (2011) The role of thrombospondin-1-mediated TGF-β1 on collagen type III synthesis induced by high glucose. Mol. Cell. Biochem. 346, 49–56 [DOI] [PubMed] [Google Scholar]
- 35. Calzada M. J., Sipes J. M., Krutzsch H. C., Yurchenco P. D., Annis D. S., Mosher D. F., Roberts D. D. (2003) Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by α6β1 integrin. J. Biol. Chem. 278, 40679–40687 [DOI] [PubMed] [Google Scholar]
- 36. Isenberg J. S., Jia Y., Fukuyama J., Switzer C. H., Wink D. A., Roberts D. D. (2007) Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J. Biol. Chem. 282, 15404–15415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Narizhneva N. V., Razorenova O. V., Podrez E. A., Chen J., Chandrasekharan U. M., DiCorleto P. E., Plow E. F., Topol E. J., Byzova T. V. (2005) Thrombospondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 19, 1158–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herrmann M., Suchard S. J., Boxer L. A., Waldvogel F. A., Lew P. D. (1991) Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect. Immun. 59, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yanagisawa N., Li D. Q., Ljungh A. (2001) The N-terminal of thrombospondin-1 is essential for coagulase-negative staphylococcal binding. J. Med. Microbiol. 50, 712–719 [DOI] [PubMed] [Google Scholar]
- 40. Rennemeier C., Hammerschmidt S., Niemann S., Inamura S., Zähringer U., Kehrel B. E. (2007) Thrombospondin-1 promotes cellular adherence of gram-positive pathogens via recognition of peptidoglycan. FASEB J. 21, 3118–3132 [DOI] [PubMed] [Google Scholar]
- 41. Niemann S., Spehr N., Van Aken H., Morgenstern E., Peters G., Herrmann M., Kehrel B. E. (2004) Soluble fibrin is the main mediator of Staphylococcus aureus adhesion to platelets. Circulation 110, 193–200 [DOI] [PubMed] [Google Scholar]
- 42. Niemann S., Kehrel B. E., Heilmann C., Rennemeier C., Peters G., Hammerschmidt S. (2009) Pneumococcal association to platelets is mediated by soluble fibrin and supported by thrombospondin-1. Thromb. Haemost. 102, 735–742 [DOI] [PubMed] [Google Scholar]
- 43. Bertling A., Niemann S., Hussain M., Holbrook L., Stanley R. G., Brodde M. F., Pohl S., Schifferdecker T., Roth J., Jurk K., Müller A., Lahav J., Peters G., Heilmann C., Gibbins J. M., Kehrel B. E. (2012) Staphylococcal extracellular adherence protein induces platelet activation by stimulation of thiol isomerases. Arterioscler. Thromb. Vasc. Biol. 32, 1979–1990 [DOI] [PubMed] [Google Scholar]
- 44. Hussain M., von Eiff C., Sinha B., Joost I., Herrmann M., Peters G., Becker K. (2008) eap Gene as novel target for specific identification of Staphylococcus aureus. J. Clin. Microbiol. 46, 470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oshida T., Sugai M., Komatsuzawa H., Hong Y. M., Suginaka H., Tomasz A. (1995) A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain. Cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. U.S.A. 92, 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zoll S., Schlag M., Shkumatov A. V., Rautenberg M., Svergun D. I., Götz F., Stehle T. (2012) Ligand-binding properties and conformational dynamics of autolysin repeat domains in staphylococcal cell wall recognition. J. Bacteriol. 194, 3789–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hirschhausen N., Schlesier T., Schmidt M. A., Götz F., Peters G., Heilmann C. (2010) A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc70 as host cell receptor. Cell. Microbiol. 12, 1746–1764 [DOI] [PubMed] [Google Scholar]
- 48. Herbert S., Ziebandt A. K., Ohlsen K., Schäfer T., Hecker M., Albrecht D., Novick R., Götz F. (2010) Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 78, 2877–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Masiuk H., Kopron K., Grumann D., Goerke C., Kolata J., Jursa-Kulesza J., Giedrys-Kalemba S., Bröker B. M., Holtfreter S. (2010) Association of recurrent furunculosis with Panton-Valentine leukocidin and the genetic background of Staphylococcus aureus. J. Clin. Microbiol. 48, 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDougal L. K., Steward C. D., Killgore G. E., Chaitram J. M., McAllister S. K., Tenover F. C. (2003) Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States. Establishing a national database. J. Clin. Microbiol. 41, 5113–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rohde H., Burdelski C., Bartscht K., Hussain M., Buck F., Horstkotte M. A., Knobloch J. K., Heilmann C., Herrmann M., Mack D. (2005) Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55, 1883–1895 [DOI] [PubMed] [Google Scholar]
- 52. Mack D., Nedelmann M., Krokotsch A., Schwarzkopf A., Heesemann J., Laufs R. (1994) Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production. Genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect. Immun. 62, 3244–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mack D., Riedewald J., Rohde H., Magnus T., Feucht H. H., Elsner H. A., Laufs R., Rupp M. E. (1999) Essential functional role of the polysaccharide intercellular adhesin of Staphylococcus epidermidis in hemagglutination. Infect. Immun. 67, 1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berger H., Hacker J., Juarez A., Hughes C., Goebel W. (1982) Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J. Bacteriol. 152, 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schlag M., Biswas R., Krismer B., Kohler T., Zoll S., Yu W., Schwarz H., Peschel A., Götz F. (2010) Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol. Microbiol. 75, 864–873 [DOI] [PubMed] [Google Scholar]
- 56. Geisbrecht B. V., Hamaoka B. Y., Perman B., Zemla A., Leahy D. J. (2005) The crystal structures of EAP domains from Staphylococcus aureus reveal an unexpected homology to bacterial superantigens. J. Biol. Chem. 280, 17243–17250 [DOI] [PubMed] [Google Scholar]
- 57. Bergmann S., Wild D., Diekmann O., Frank R., Bracht D., Chhatwal G. S., Hammerschmidt S. (2003) Identification of a novel plasmin(ogen)-binding motif in surface displayed α-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49, 411–423 [DOI] [PubMed] [Google Scholar]
- 58. Nebrich G., Herrmann M., Sagi D., Klose J., Giavalisco P. (2007) High MS-compatibility of silver nitrate-stained protein spots from 2-DE gels using ZipPlates and AnchorChips for successful protein identification. Electrophoresis 28, 1607–1614 [DOI] [PubMed] [Google Scholar]
- 59. Thiele T., Iuga C., Janetzky S., Schwertz H., Gesell Salazar M., Fürll B., Völker U., Greinacher A., Steil L. (2012) Early storage lesions in apheresis platelets are induced by the activation of the integrin αIIbβ3 and focal adhesion signaling pathways. J. Proteomics 76, 297–315 [DOI] [PubMed] [Google Scholar]
- 60. Biswas R., Voggu L., Simon U. K., Hentschel P., Thumm G., Götz F. (2006) Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 259, 260–268 [DOI] [PubMed] [Google Scholar]
- 61. Zoll S., Pätzold B., Schlag M., Götz F., Kalbacher H., Stehle T. (2010) Structural basis of cell wall cleavage by a staphylococcal autolysin. PLoS Pathog. 6, e1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 [DOI] [PubMed] [Google Scholar]
- 63. Heilmann C. (2011) Adhesion mechanisms of staphylococci. Adv. Exp. Med. Biol. 715, 105–123 [DOI] [PubMed] [Google Scholar]
- 64. Hirschhausen N., Schlesier T., Peters G., Heilmann C. (2012) Characterization of the modular design of the autolysin/adhesin Aaa from Staphylococcus aureus. PLoS ONE 7, e40353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Burian M., Wolz C., Goerke C. (2010) Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS ONE 5, e10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jensch I., Gámez G., Rothe M., Ebert S., Fulde M., Somplatzki D., Bergmann S., Petruschka L., Rohde M., Nau R., Hammerschmidt S. (2010) PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol. Microbiol. 77, 22–43 [DOI] [PubMed] [Google Scholar]
- 67. Krauel K., Weber C., Brandt S., Zähringer U., Mamat U., Greinacher A., Hammerschmidt S. (2012) Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood 120, 3345–3352 [DOI] [PubMed] [Google Scholar]