Background: The nucleotide-binding leucine-rich repeat (NLR) family of innate immune genes are important regulators of inflammatory responses in mammals.
Results: The NLR gene, Nlrx1, suppresses neuroinflammation in vivo and inhibits microglial (Mg) activation.
Conclusion: NLRX1 immunosuppressive function in Mg correlates with suppression of neuroinflammation during mouse models of Multiple Sclerosis (MS).
Significance: NLR genes can have protective roles during neuroinflammation.
Keywords: Cytokine, Immunology, Innate immunity, Interleukin, Neuroimmunology
Abstract
The nucleotide binding domain and leucine-rich repeat-containing (NLR) family of proteins is known to activate innate immunity, and the inflammasome-associated NLRs are prime examples. In contrast, the concept that NLRs can inhibit innate immunity is still debated, and the impact of such inhibitory NLRs in diseases shaped by adaptive immune responses is entirely unexplored. This study demonstrates that, in contrast to other NLRs that activate immunity, NLRX1 plays a protective role in experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis. When compared with wild-type controls, Nlrx1−/− mice have significantly worsened clinical scores and heightened CNS tissue damage during EAE. NLRX1 does not alter the production of encephalitogenic T cells in the peripheral lymphatic tissue, but Nlrx1−/− mice are more susceptible to adoptively transferred myelin-reactive T cells. Analysis of the macrophage and microglial populations indicates that NLRX1 reduces activation during both active and passive EAE models. This work represents the first case of an NLR that attenuates microglia inflammatory activities and protects against a neurodegenerative disease model caused by autoreactive T cells.
Introduction
Multiple sclerosis (MS)5 is an autoimmune disease that primarily affects adults between 20–40 years of age (1). In this disease, T cells infiltrate and induce inflammation within the CNS. The enhanced inflammatory environment in the CNS contributes to neuronal demyelination and axonal damage. Neuronal pathology causes the physical and mental disabilities associated with MS. The mouse model of experimental autoimmune encephalomyelitis (EAE) mimics many aspects of MS and has led directly to the development of several therapeutic treatments (2). As with MS, the characteristics of EAE include T cell immune responses to myelin antigens, neuronal pathology, and CNS inflammation.
The nucleotide binding domain and leucine-rich repeat-containing (NLR) protein family consists of intracellular sensors that regulate inflammatory responses (3). Previous studies have shown that NLRs and their adaptors can positively influence the development of EAE (4–6). Deletion of the “inflammasome” Nlrp3, the adaptor-encoding Asc, or the caspase-1 gene resulted in better clinical outcomes. ASC and Caspase-1 have been shown to influence the production of encephalitogenic T cells in lymphoid tissue, mostly by influencing the expression of IL-1β and IL-18 (4, 5). NLRP3 has also been shown to modulate worse outcomes in the EAE model by enhancing T cell migration and TH1/TH17 development. Additionally, Nod1 and Nod2 contribute to pathogenesis in EAE by regulation of CNS-infiltrating dendritic cells (12). These findings collectively demonstrate that NLR proteins and adaptors can exacerbate EAE.
Although NLRs have been shown to be important in peripheral myeloid monocytic cells, their roles in microglia (Mg) are less studied. Mg are the chief immune cells in the CNS and have important roles in numerous neurodegenerative diseases. Microglial activation results in cytokine/chemokine secretion, induction of MHC class II molecules, and production of nitric oxide. Pharmacological targeting of microglia has been demonstrated to suppress the clinical symptoms of EAE (7, 8). Additionally, Mg become activated and promote neuroinflammation during the EAE model as well as in MS patients (9). Therefore, Mg-mediated CNS inflammation is significant in the context of both EAE and MS.
NLRX1 is a mitochondrially localized NLR protein (10) that has been characterized as a non-inflammasome NLR because it does not affect IL-1β production (11). Previous studies have demonstrated that NLRX1 functions to repress inflammatory responses to microbes (10, 11). Recent work increasingly recognizes the significance of innate immune sensors/receptors in “sterile inflammation,” defined as inflammation that occurs in the absence of an obvious pathogen (12). Inflammation in the CNS can fall into this category and occurs in diseases with immunologic associations, such as MS as well as those without an immunologic cause such as Alzheimer disease and Parkinson disease. In this work, we demonstrate that NLRX1 functions as a protective factor against EAE by suppressing CNS inflammation and macrophage/microglial activation. This is the first report of an NLR that functions to repress inflammation and protect against neurological disease.
EXPERIMENTAL PROCEDURES
Mouse Generation
The Nlrx1−/− mice were generated as described previously (11). 2D2 Tg mice (13) were purchased from The Jackson Laboratory. All mouse studies were used in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Institutional Animal Care and Use Committee guidelines of the University of North Carolina Chapel Hill.
Active Induction of EAE
Induction of EAE was performed as described (14). Briefly, MOG(35–55) peptide (Genemed Synthesis Inc.) was emulsified in complete Freund adjuvant and mixed with a Luer lock syringe system (Cadence, Inc). The dosage of heat-killed Mycobacterium tuberculosis in the emulsion was 4 mg/ml. The emulsion was subcutaneously injected to sites adjacent to the mouse tails. For the experiment depicted in Fig. 1A, 200 ng of pertussis toxin was injected intraperitoneally at 0 and 2 days post-immunization. For Fig. 1B, 100 ng of pertussis toxin was injected at 0 and 2 days post-immunization. The following scale was used to assess clinical scores: 0, normal mouse, no overt sign of disease; 0.5, partial tail paralysis (loss of tip tail tonus); 1, limp tail or hind limb weakness but not both; 2, limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; and 5, moribund state, sacrifice for humane reasons. Animals were scored by two independent investigators. All additional experiments describing the active EAE model were performed under the conditions depicted in Fig. 1A.
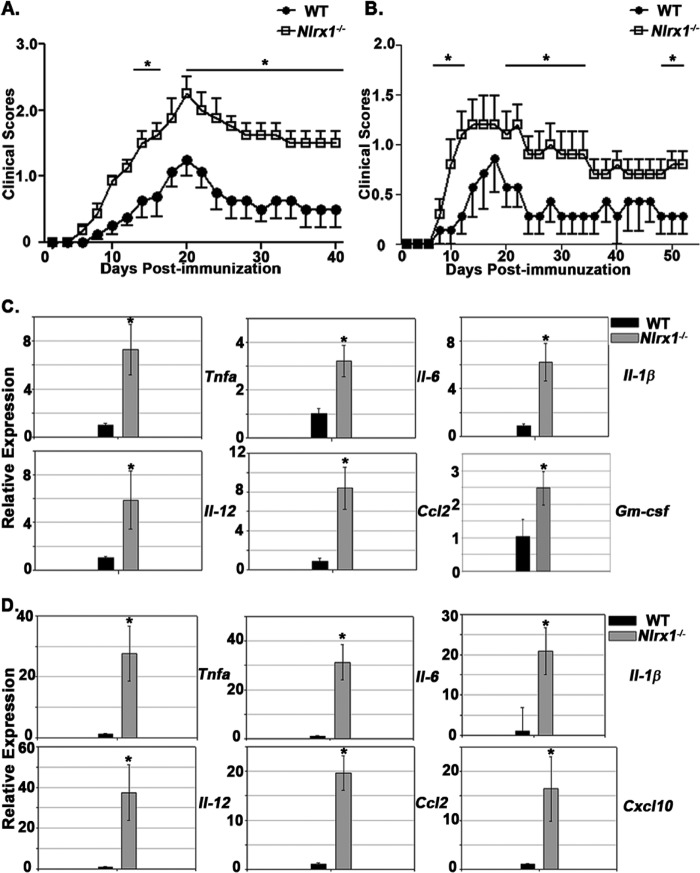
FIGURE 1.
Nlrx1−/− mice suffer worse outcomes during EAE. A and B, clinical scores of WT and Nlrx1−/− mice during EAE. n = 8 per group (A) and 7(WT) and 10 (Nlrx1−/−) (B). The experiment in A was performed with 200 ng of pertussis toxin, whereas the experiment in B was performed with 100 ng of pertussis toxin. All additional experiments describing the active EAE model were performed under the conditions used in A. C, real-time PCR analysis of various inflammatory genes in spinal cord tissue at 14 dpi. n = 6 (WT) and 7 (Nlrx1−/−). Relative expression levels were normalized to the WT (WT = 1). D, real-time PCR analysis of various inflammatory genes in spinal cord tissue at 63 dpi. n = 5mice/group. Relative expression levels were normalized to the WT (WT = 1). Error bars represent mean ± S.E. *, p < 0.05.
Real-time PCR Analysis
Total RNA was extracted from the spinal cord using TRIzol (Invitrogen). cDNA synthesis was performed using a reverse transcriptase kit (Promega). RNA accumulation was measured using TaqMan primers against various inflammatory mediators. The accumulation of inflammatory mediators was determined on the basis of actin expression, and levels of inflammatory mediator expression was determined by a standard ΔΔCt method. Actb (Mm00607939_s1), Ifngr-1(Mm00599890_m1), Il-17a (Mm00439618_m1), Cxcl10 (Mm00445235), Nlrx1 (Mm00617978_m1), Tnfa (Mm00443260_m1), Il-1b (Mm01226189_m1), Ccl2 (Mm00441297_m1), Il-12a (Mm00434165_m1), Il-17f (Mm00521423_m1), Gm-csf (Mm01290062_m1), Il-22 (Mm01226722_g1), Il-23a (Mm00518984_m1), Il-6 (Mm00446190_m1). Il-4 (Mm00445259_m1), and Il-13 (Mm00434204_m1) were used.
Immunoblot Analysis
Analysis was performed with either the indicated cell or spinal cord lysates using antibodies against NLRX1 (15), myelin basic protein (Santa Cruz Biotechnology, catalog no. sc-13914), glial fibrillary acidic protein (Thermo Scientific, catalog no. OPA1-06100), HSP90 (Santa Cruz Biotechnology, catalog no. sc-7947), NOS2 (Millipore, catalog no. NGY63), GST-Pi (Enzo, catalog no. ADI-MSA-102), GAPDH (Cell Signaling Technology, catalog no. 3683S), and MHC class II molecules (Millipore, catalog no. MAB222).
Immunohistochemical Analysis
Animals were perfused with PBS and 4% formaldehyde (Sigma-Aldrich). Spinal cords were embedded in paraffin and cut into 5-μm sections. Myelin was measured using a Luxol fast blue (LFB) periodic stain or a Luxol fast blue-periodic acid-Schiff (PAS) base-hematoxylin stain.
Characterization of Inflammatory Cell Infiltration
Flow cytometry with single cell suspensions from spinal cord tissue (4) was performed with antibodies to detect CD11b (ebioscience, catalog no. 15-0112-81), CD3 (ebioscience, catalog no. 11-0031-82), CD45 (ebioscience, catalog no. 13-0451-81), CD4 (Biolegend, catalog no. 100428), I-Ab (BD Biosciences, catalog no. 553552), and CD39 (ebioscience, catalog no. 50-0391-80). Flow cytometry analysis was performed on a CYAN flow cytometer.
Primary Glial Cultures
Primary glial cultures were generated following an established protocol (16), with differences in microglia isolation. After ∼2.5 weeks in glial culture, Mg were isolated using an anti-CD11b antibody fused with magnetic beads (MACS). Mg were stimulated with Ultrapure LPS (Invivogen) (500 ng/ml) and IFNγ (Peprotech) (20 ng/ml). LPS and IFNγ were used to stimulate Mg to induce both early (LPS, NF-κB signaling) and late (IFNγ, JAK/STAT signaling) signaling pathways for induction of NOS2 and MHC class II molecule expression.
Intracellular Cytokine Staining
Single cell suspensions were generated from the inguinal lymph nodes of mice 12 days post-immunization. Cells were stimulated with 50 μg/ml MOG35–55 peptide for 48 h. Cytokine secretion was inhibited with GolgiStop(BD) or GolgiPlug(BD) according to the instructions of the manufacturer. Cells were stained for CD3 (ebioscience, catalog no. 11-0031-82), CD4 (Biolegend, catalog no. 100428), CD8 (Biolegend, catalog no. 100714), IFNγ (Biolegend, catalog no. 505826), or IL-17A (Biolegend, catalog no. 506964) and fixed in 1% paraformaldehyde. Flow cytometry analysis was performed on a CYAN flow cytometer.
Adoptive Transfer of CD4+ Encephalitogenic T Cells
The passive transfer of EAE was performed as described previously (17). Briefly, active EAE induction was performed in Tg 2D2 mice (13). 12 days post-immunization, CD4+ T cells were isolated from the spleen and draining lymph nodes using anti-CD4+ magnetic beads (MACS). 5 × 106 cells were transferred intravenously to recipient mice, and pertussis toxin (200 ng) was administered at days 0 and 2 post-CD4+ T cell transfer.
RESULTS
Nlrx1−/− Mice Suffer Worse Outcomes during EAE
To test whether NLRX1 has a role in EAE development, we immunized WT and Nlrx1−/− mice with MOG(35–55) peptide (see “Experimental Procedures”) and evaluated the development of limb paralysis. To minimize artifacts, Nlrx1−/− mice were produced on a C57BL/6 genetic background, and the control mice were bred in-house. Under a standard protocol, Nlrx1−/− mice suffered enhanced limb paralysis during both the acute and chronic phases of EAE (Fig. 1A). Additionally, Nlrx1−/− mice suffered enhanced limb paralysis when using a lower dosage of pertussis toxin (100 ng) (Fig. 1B). In both experiments, the enhancement of limb paralysis in the Nlrx1−/− mice was sustained throughout the course of the disease (Fig. 1, A and B). RNA analysis of spinal cord tissue revealed that Nlrx1−/− mice displayed enhanced expression of numerous inflammatory cytokines and chemokines (Tnfa, Il-6, Il-1β, Ccl2, Gm-csf, and Il-12) during the acute stage of EAE (Fig. 1C). Analysis of the chronic stage of EAE revealed sustained and even more substantial changes in the expression of inflammatory cytokines and chemokines than the acute phase (Fig. 1D). These findings collectively indicate that during EAE, NLRX1 suppressed disease symptoms and reduced the expression of inflammatory cytokines and chemokines in CNS tissue.
Nlrx1−/− Mice Suffer Enhanced Demyelination and Peripheral Immune Cell Accumulation during EAE
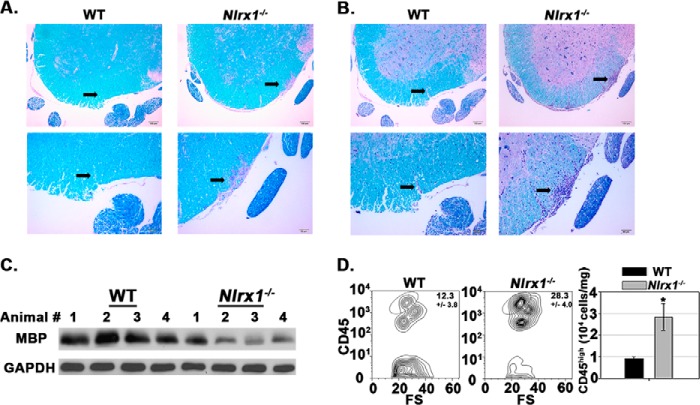
We next evaluated the influence of NLRX1 on CNS damage during EAE. Immunohistochemical analysis of spinal cord tissue revealed that Nlrx1−/− specimens exhibited enhanced demyelination, as indicated by less LFB and more PAS staining in white matter tissue (Fig. 2, A and B). Demyelination in the Nlrx1−/− mice was quantified by performing an immunoblot analysis for myelin basic protein in spinal cord tissues (Fig. 2C). Additionally, LFB-PAS-hematoxylin staining demonstrated enhanced immune cell infiltration into the spinal cord tissue of Nlrx1−/− mice relative to the WT control (Fig. 2B). Flow cytometry analysis of spinal cord tissue further showed that Nlrx1−/− mice suffered enhanced accumulation of peripheral immune cells (CD45high) cells in the CNS (Fig. 2D). Collectively, these findings indicate that the worsened symptoms of the Nlrx1−/− mice during EAE correlated with enhanced demyelination and peripheral immune cell accumulation in the CNS.
FIGURE 2.
Nlrx1−/− mice suffer enhanced CNS damage and peripheral immune cell accumulation during EAE. A, sections of lumbar spinal cord tissue stained with LFB-PAS at 18 dpi. Top row, ×100 magnification. Scale bars = 100 μm. Bottom row, ×200 magnification. Scale bars = 50 μm. Arrows point to a lesion in the white matter. B, sections of lumbar spinal cord tissue stained with LFB-PAS-hematoxylin at 18 dpi. Top row, ×100 magnification. Scale bars = 100 μm. Bottom row, ×200 magnification. Scale bars = 50 μm. Arrows point to a lesion in the white matter that contains immune cell infiltrates. C, quantification of demyelination by immunoblot analysis with anti-myelin basic protein (MBP) and anti-GAPDH antibody at 18dpi. GAPDH was used as a control for total protein loading. The lanes represent individual mice. 4 mice/strain were tested. D, flow cytometric analysis of peripheral immune cell (CD45high) infiltration into spinal cord tissue at 18 dpi. Left panel, contour map depicting the selection of the CD45high cell population. Right panel, total number of CD45high cells in the spinal cord. FS, forward scatter (103). n = 6 mice/group. Error bars represent mean ± S.E. *, p < 0.05.
Encephalitogenic T Cell Accumulation in the CNS Is Heightened in Nlrx1−/− Mice
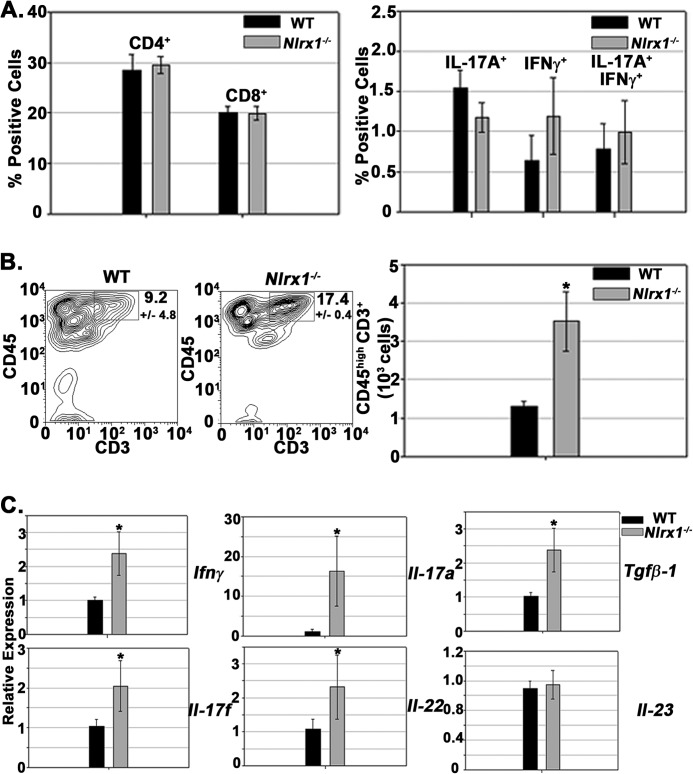
We next evaluated whether NLRX1 influenced T cell development in the peripheral lymphatic tissue. Flow cytometry analysis revealed that NLRX1 did not influence the frequency of CD4+ and CD8+ T cells within the draining lymph nodes (Fig. 3A, left panel). Additionally, intracellular cytokine staining for IFNγ and IL-17A after restimulation with the MOG peptide ex vivo demonstrated that NLRX1 did not significantly affect the expression of either cytokine within CD4+ T cells (Fig. 3A, right panel). These results indicate that NLRX1 did not significantly affect the production of pathogenic TH-1 or TH-17 effector cells in the peripheral immune system. Conversely, flow cytometry analysis revealed enhanced accumulation of CD45high CD3+ cells in the spinal cord tissue of Nlrx1−/− mice (Fig. 3B). Additionally, analysis of spinal cord tissue revealed that Nlrx1−/− mice had enhanced expression of cytokine transcripts associated with inflammatory T cells, including Tgfb-1, Ifnγ, Il-17a, Il-17f, and Il-22 (Fig. 3C). However, Il-23 expression was not increased, indicating that a global gene induction did not occur (Fig. 3C). These findings collectively indicate that NLRX1 represses T cell accumulation in the CNS during EAE.
FIGURE 3.
Encephalitogenic T cell accumulation in the CNS is heightened in Nlrx1−/− mice. A, left panel, frequency of CD4+ and CD8+ T cell in the draining lymph nodes during EAE at 12 dpi. Cells were gated on CD3+ CD4+ or CD3+ CD8+. n = 6 mice/group. Right panel, intracellular cytokine staining in CD3+ CD4+ T cells 48 h after stimulation with MOG(35–55) peptide. B, analysis of T cell infiltration during active EAE. Left panel, contour map depicting the selection of the CD45high CD3+ population in spinal cord tissue. Right panel, quantification of the CD45high CD3+ cell populations in WT and Nlrx1−/− mice. n = 6 mice/group. C, real-time PCR analysis of various inflammatory genes in spinal cord tissue at 14 dpi. n = 6 (WT) and 7 (Nlrx1−/−). Relative expression levels were normalized to the WT (WT = 1). Error bars represent mean ± S.E. *, p < 0.05.
Nlrx1−/− Mice Are More Susceptible to Adoptively Transferred Encephalitogenic T Cells
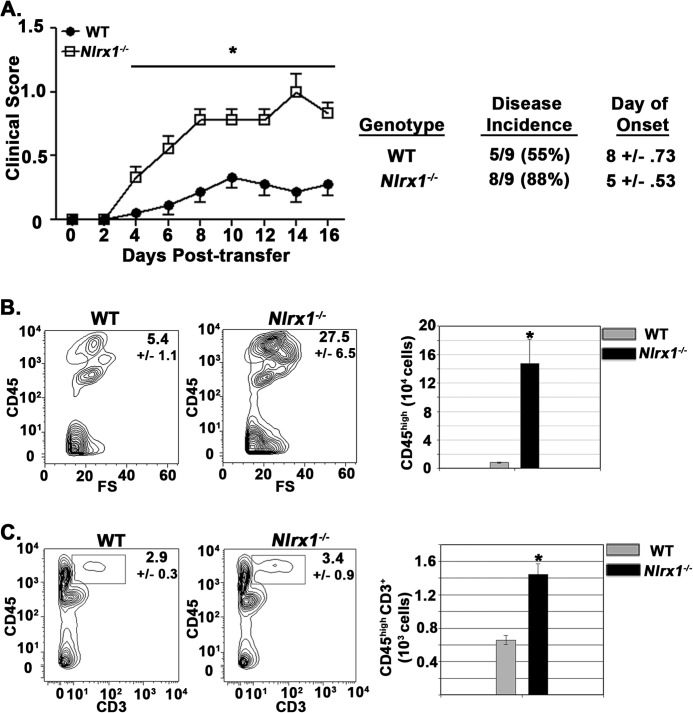
To directly address the impact of NLRX1 downstream of encephalitogenic T cell production, we performed adoptive transfer experiments with MOG(35–55)-specific T cell receptor transgenic T cells isolated from 2D2 Tg mice (13). After the transfer of encephalitogenic T cells into WT and Nlrx1−/− mice, the Nlrx1−/− mice displayed enhanced limb paralysis, disease incidence, and onset of disease symptoms (Fig. 4A). The worsened symptoms of the Nlrx1−/− mice correlated with a heightened accumulation of peripheral immune cells (CD45high) in spinal cord tissue (Fig. 4B). Additionally, T cell infiltration into spinal cord tissue was enhanced significantly in the Nlrx1−/− mice (Fig. 4C). Because the same encephalitogenic T cells were introduced into WT and Nlrx1−/− animals, these findings demonstrated that NLRX1-mediated protection occurred in response to autoreactive T cells.
FIGURE 4.
Nlrx1−/− mice suffer worse outcomes in response to encephalitogenic T cells. A, clinical scores of WT and Nlrx1−/− mice after transfer of encephalitogenic T cells. n = 9 mice/group. B, flow cytometric analysis of peripheral immune cell (CD45high) infiltration into spinal cord tissue at 16 dpi. Left panel, contour map depicting the selection of the CD45high cell population. Right panel, total number of CD45high cells per spinal cord. FS, forward scatter (103). n = 5 mice/group. C, analysis of T cell infiltrates into spinal cord tissue after encephalitogenic T cell transfer. Left panel, contour map depicting the selection of CD45high CD3+ cells. Right panel, total numbers of CD45high CD3+ cells in WT and Nlrx1−/− mice. n = 9 mice/group. Error bars represent mean ± S.E. *, p < 0.05.
Peripheral Innate Immune Cells and Resident Microglial Populations Are Hyperactivated in Nlrx1−/− Mice during EAE
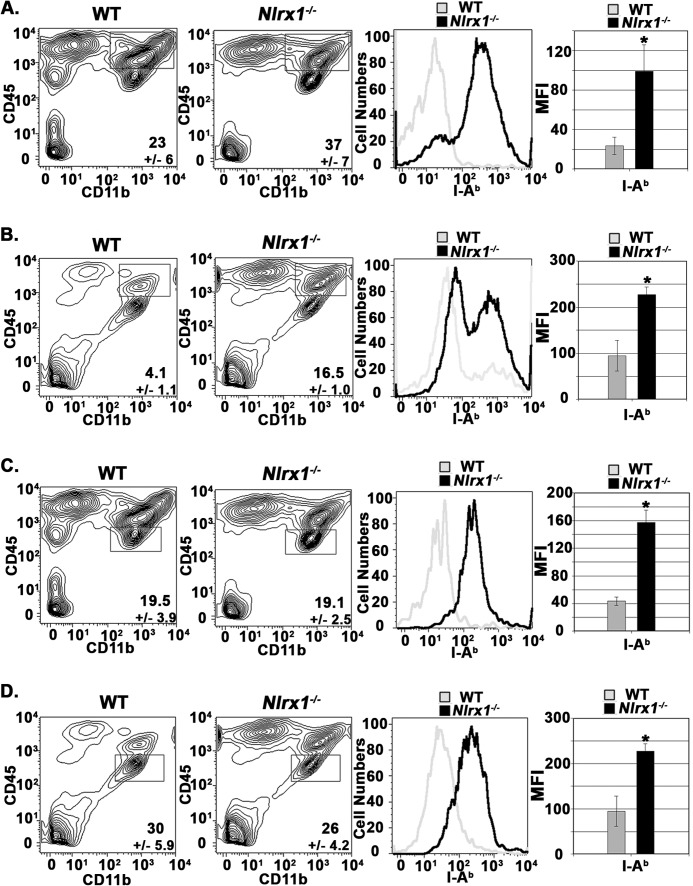
To gain further insight into the influence of NLRX1 on the local CNS environment, we evaluated the activation status of peripheral innate immune cells and microglia during both active and passive EAE models by flow cytometric analyses. The activation status of CD45high CD11b+ cells isolated from spinal cord tissue was assessed by measuring the surface expression of the MHC class II molecule I-A (18, 19). Typically, the CD45high CD11b+ population is primarily comprised of macrophages/monocytes but also includes dendritic cells and granulocytes. Our results demonstrate that the presence of NLRX1 reduced the expression of I-A on peripheral CD11b+ cells during both active EAE and passive EAE (Fig. 5, A and B). Analysis of the CD45low CD11b+ population cells in spinal cord tissue have been used by many groups to identify CNS-resident Mg populations (8, 12, 18, 20, 21) (Fig. 5, C and D). Mg populations also displayed a significant up-regulation of I-A expression in the Nlrx1−/− mice during active EAE (Fig. 5C) and after transfer of encephalogenic T cells (Fig. 5D). Our results collectively demonstrate that expression of MHC class II molecules on both peripheral innate immune cells and Mg populations was increased dramatically in the Nlrx1−/− mice during both active and passive EAE.
FIGURE 5.
Peripheral innate immune cells and resident microglial populations are hyperactivated in Nlrx1−/− mice during EAE. A, flow cytometry analysis of CD45high CD11b+ cell populations in spinal cord tissue at 18 dpi during active EAE. Left panels, contour maps depicting the selection of CD45high CD11b+ cell populations. Center panel, I-Ab expression on CD45high CD11b+ WT and Nlrx1−/− cells. Right panel, mean fluorescence intensity (MFI) was used to quantify surface expression of I-Ab. n = 6 mice/group. B, flow cytometry analysis of CD45high CD11b+ cell populations at 16 dpi during passive EAE. The panels are the same as in A. n = 5 mice/group. C, flow cytometry analysis of microglia at 16 dpi during active EAE. Left panels, contour maps depicting selection of CD45lo CD11b+ cell populations. Center panel, I-Ab expression on CD45lo, CD11b+ WT, and Nlrx1−/− cells. Right panel, mean fluorescence intensity was used to quantify the surface expression of I-Ab. n = 5 mice/group. D, flow cytometry analysis of microglia at 16 dpi during passive EAE. The panels are the same as in C. n = 5 mice/group. Error bars represent mean ± S.E. *, p < 0.05.
Nlrx1−/− Microglia Have Enhanced Inflammatory Responses
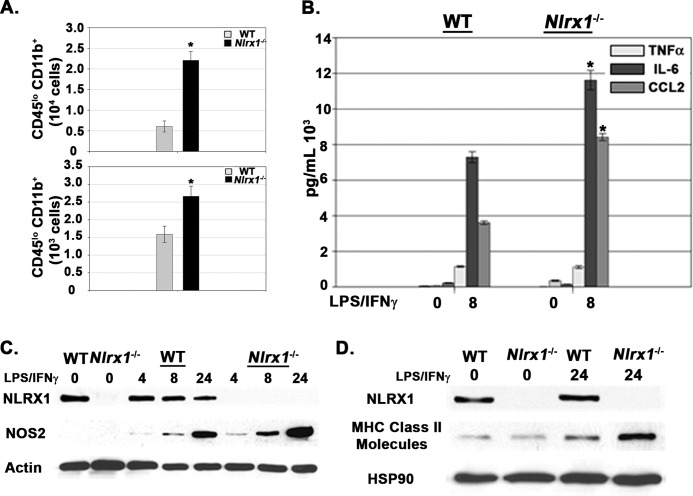
Examination of CNS tissue revealed that enhanced microgliosis occurred in Nlrx1−/− mice during both active and passive EAE (Fig. 6A). To further evaluate whether NLRX1 functioned intrinsically within microglia, we prepared a standard glial culture from neonatal mice (16). Importantly, Nlrx1−/− Mg produced more IL-6 and CCL2 after stimulation (Fig. 6B). Enhanced activation of Nlrx1−/− Mg was also demonstrated by increased expression of NOS2 and MHC class II molecules (Fig. 6, C and D). Both NOS2 and MHC class II molecule expression represent standard markers for microglia/macrophage activation (see below) (19). Collectively, our results demonstrate that NLRX1 attenuates Mg activation both in vivo and in vitro.
FIGURE 6.
Nlrx1−/− microglia have enhanced inflammatory responses. A, top panel, total numbers of CD45lo CD11b+ populations in spinal cord tissue 18 dpi during active EAE. n = 6 mice/group. Bottom panel, total numbers of CD45lo CD11b+ populations in spinal cord tissue 16 dpi during passive EAE. n = 5 mice/group. B, ELISA analysis of cytokines and chemokines produced by primary WT and Nlrx1−/− microglia after stimulation with LPS/IFNγ. C, immunoblot analyses of NOS2 in primary WT and Nlrx1−/− microglia after stimulation with LPS/IFNγ. Actin was used as a total protein loading control. D, Immunoblot analyses of MHC class II molecule expression in primary WT and Nlrx1−/− microglia after stimulation with LPS/IFNγ. HSP90 was used as a total protein loading control. Error bars represent mean ± S.E. *, p < 0.05.
DISCUSSION
EAE is a model for MS that represents a breakdown in immunological tolerance. Unfortunately, the targeted tissue in these disorders is the CNS, an area of the body that has a low tolerance capacity for damage (22). The CNS is initially attacked by T cells that target CNS-derived antigens such as myelin peptides. Antigen-presenting cells from the peripheral tissues play an important role in this disease (4, 6). Additionally, Mg respond to CNS damage and are the primary CNS-located cell type that initiates inflammatory responses (1, 2). In the EAE model, Mg activation precedes limb paralysis and high accumulation of peripheral immune cell infiltrates (18). Therefore, Mg are key players in maintaining CNS homeostasis after the initial T cell-mediated damage. Our findings demonstrate that NLRX1 represses excessive activation of innate immune cells from the periphery and also Mg immune responses, both of which contribute to CNS inflammation during EAE. Dysregulated inflammatory responses in the Nlrx1−/− mice are associated with exacerbated limb paralysis, tissue damage, and peripheral immune cell accumulation into the CNS.
During EAE, the NLR family and adaptor components NLRP3, ASC, Nod1, Nod2, and Caspase-1 have all been reported to exacerbate limb paralysis, inflammation, and CNS damage (4–6). NLRP3 and ASC function in regulating cytokines that then promote the development of encephalitic TH-1 and TH-17 subsets (4) and promote T cell migration (23). Nod1 and Nod2 have been shown to regulate the activation of CNS-infiltrating dendritic cells (6). These studies indicate that the function of NLR proteins in innate immune cells promotes worse outcomes in the EAE model. In contrast to those findings, our work demonstrates that NLRX1 represses disease progression. We show that NLRX1 functioned in Mg to inhibit excessive production of IL-6 and CCL2. IL-6 has a pathogenic role in EAE (24) and promotes the survival of activated CD4+ T cells, the recall responses of memory CD4+ T cells, and the migration of activated CD4+ T cells to tissues (25). We also demonstrate that NLRX1 inhibits the production of CCL2. CCL2 recruits immune cells into damaged tissues (26) and promotes T cell migration to the CNS during EAE (27). Our findings show that enhanced expression of CCL2 in Nlrx1−/− mice correlates with heightened T cell infiltration into the CNS. Hence, in contrast to other studies characterizing the function NLR proteins in the EAE model, NLRX1 represses the activation of CNS-infiltrating innate immune cells and CNS-resident Mg populations to extrinsically modulate encephalitogenic T cell activity. To this point, the passive transfer of encephalitogenic T cells induced enhanced disease in Nlrx1−/− mice, indicating that NLRX1 functions to protect against autoreactive T cells.
Although numerous studies have characterized the role of the Toll-like receptor family in regulating Mg responses (28), work with the NLR family of proteins has been more limited. The inflammasome NLR protein NLRP3 and the NLR adaptors ASC and Caspase-1 have been shown to positively regulate Mg immune responses (29). Using microglial cell lines, it was demonstrated that NLRP3 and ASC are required for IL-1β production in response to amyloid-β (29). Furthermore, it was shown that Caspase-1 contributes to the production of chemokines and neurotoxic molecules (29). NLRP3 has also been shown to promote NOS2 expression and M1 status in microglia (30). These results collectively demonstrate a pathogenic role for NLRP3, ASC, and Caspase-1 in microglial inflammatory responses. Our results provide the reciprocal finding that NLRX1 represses the production of inflammatory cytokines/chemokines, NOS2 accumulation, and expression of MHC class II molecules. Mg activation provides for tissue defense, whereas the subsequent alternative/deactivation states modulate tissue repair (19). We demonstrate that Nlrx1−/− Mg have enhanced expression of NOS2 and MHC class II molecules, both of which are standard markers for Mg activation. Therefore, our work is the first to demonstrate that an NLR protein can inhibit Mg immune responses through regulating Mg activation status.
Almost all neurological disorders lead to acute inflammation, in which microglia function to regulate tissue defense and repair (31). Excessive activation of these cells promotes a chronic, perpetual inflammatory cycle that ultimately results in neurodegeneration (32). Our findings demonstrate that an NLR protein can inhibit not only peripheral innate immune cells but also Mg inflammatory responses and reduce CNS pathology during EAE. Because the majority of therapeutic treatments for MS are targeted to the peripheral immune system (33), a further characterization of Mg activation in disease progression represents an understudied aspect of this disease. Additionally, Mg activation and dysregulation of inflammatory responses are hallmarks for numerous neurological diseases including Parkinson disease, Alzheimer disease, and amyotrophic lateral sclerosis (31). Therefore, further studies elucidating the role of NLR proteins in CNS inflammation should yield valuable insight into the broader field of neurodegeneration.
This work was supported by National Multiple Sclerosis Society (NMSS) Grants CA-10530A-8 and RG-1785G9/2 (to J. P. Y. T.).
- MS
- multiple sclerosis
- EAE
- experimental autoimmune encephalomyelitis
- NLR
- human NBD-LRR or NOD-like receptor
- TH
- T helper cell
- Mg
- microglia
- LFB
- Luxol fast blue
- PAS
- periodic acid-Schiff
- MOG
- myelin oligodendrocyte glycoprotein
- dpi
- days post-immunization.
REFERENCES
- 1. McFarland H. F., Martin R. (2007) Multiple sclerosis. A complicated picture of autoimmunity. Nat. Immunol. 9, 913–919 [DOI] [PubMed] [Google Scholar]
- 2. Steinman L., Zamvil S. (2006) How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann. Neurol. 60, 12–21 [DOI] [PubMed] [Google Scholar]
- 3. Ting J. P., Willingham S. B., Bergstralh D. T. (2008) NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8, 372–379 [DOI] [PubMed] [Google Scholar]
- 4. Gris D., Ye Z., Iocca H. A., Wen H., Craven R. R., Gris P., Huang M., Schneider M., Miller S. D., Ting J. P. (2010) NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaw P. J., Lukens J., Burns S., Chi H., McGargill M. A., Kanneganti T. D. (2010) Cutting edge. Critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J. Immunol. 9, 4610–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw P. J., Barr M. J., Lukens J. R., McGargill M. A., Chi H., Mak T. W., Kanneganti T. D. (2011) Signaling via the RIP2 adaptor protein in central nervous system-infiltrating dendritic cells promotes inflammation and autoimmunity. Immunity 34, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Popovic N., Schubart A., Goetz B. D., Zhang S. C., Linington C., Duncan I. D. (2002) Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann. Neurol. 51, 215–223 [DOI] [PubMed] [Google Scholar]
- 8. Ponomarev E. D., Veremeyko T., Barteneva N., Krichevsky A. M., Weiner H. L. (2011) MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 1, 67–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramaglia V., Hughes T. R., Donev R. M., Ruseva M. M., Wu X., Huitinga I., Baas F., Neal J. W., Morgan B. P. (2012) C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 109, 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore C. B., Bergstralh D. T., Duncan J. A., Lei Y., Morrison T. E., Zimmermann A. G., Accavitti-Loper M. A., Madden V. J., Sun L., Ye Z., Lich J. D., Heise M. T., Chen Z., Ting J. P. (2008) NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451, 573–577 [DOI] [PubMed] [Google Scholar]
- 11. Allen I. C., Moore C. B., Schneider M., Lei Y., Davis B. K., Scull M. A., Gris D., Roney K. E., Zimmermann A. G., Bowzard J. B., Ranjan P., Monroe K. M., Pickles R. J., Sambhara S., Ting J. P. (2011) NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dann A., Poeck H., Croxford A. L., Gaupp S., Kierdorf K., Knust M., Pfeifer D., Maihoefer C., Endres S., Kalinke U., Meuth S. G., Wiendl H., Knobeloch K. P., Akira S., Waisman A., Hartmann G., Prinz M. (2012) Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat. Neurosci. 15, 98–106 [DOI] [PubMed] [Google Scholar]
- 13. Bettelli E., Pagany M., Weiner H. L., Linington C., Sobel R. A., Kuchroo V. K. (2003) Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller S. D., Karpus W., Davidson T. S. (2007) Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. Unit 15.1, 1–26 [DOI] [PubMed] [Google Scholar]
- 15. Lei Y., Wen H., Yu Y., Taxman D. J., Zhang L., Widman D. G., Swanson K. V., Wen K. W., Damania B., Moore C. B., Giguère P. M., Siderovski D. P., Hiscott J., Razani B., Semenkovich C. F., Chen X., Ting J. P. (2012) The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36, 933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Floden A. M., Li S., Combs C. K. (2005) β-Amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor α and NMDA receptors. J. Neurosci. 25, 2566–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inoue M., Willilams K., Oliver T., Vandenabeele P., Rajan J. V., Miao E. A., Shinohara M. L. (2012) Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci. Signal. 5, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ponomarev E. D., Shriver L., Maresz K., Dittel B. N. (2005) Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neuro. Res. 81, 374–389 [DOI] [PubMed] [Google Scholar]
- 19. Colton C. A. (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune. Pharmacol. 4, 399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Locatelli G., Wörtge S., Buch T., Ingold B., Frommer F., Sobottka B., Krüger M., Karram K., Bühlmann C., Bechmann I., Heppner F. L., Waisman A., Becher B. (2012) Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat. Neurosci. 15, 543–550 [DOI] [PubMed] [Google Scholar]
- 21. Schreiner B., Bailey S. L., Shin T., Chen L., Miller S. D. (2008) PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur. J. Immunol. 38, 2706–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medzhitov R., Schneider D. S., Soares M. P. (2012) Disease tolerance as a defense strategy. Science 335, 936–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue M., Williams K. L., Gunn M. D., Shinohara M. L. (2012) NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 109, 10480–10485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Serada S., Fujimoto M., Mihara M., Koike N., Ohsugi Y., Nomura S., Yoshida H., Nishikawa T., Terabe F., Ohkawara T., Takahashi T., Ripley B., Kimura A., Kishimoto T., Naka T. (2008) IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. 105, 9041–9046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dienz O., Ricon M. (2006) The effects of IL-6 on CD4 T cell responses. Clin. Immunol. 30, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conductier G., Blondeau N., Guyon A., Nahon J. L., Rovère C. (2010) The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 224, 93–100 [DOI] [PubMed] [Google Scholar]
- 27. Brini E., Ruffini F., Bergami A., Brambilla E., Dati G., Greco B., Cirillo R., Proudfoot A. E., Comi G., Furlan R., Zaratin P., Martino G. (2009) Administration of a monomeric CCL2 variant to EAE mice inhibits inflammatory cell recruitment and protects from demyelination and axonal loss. J. Neuroimmunol. 209, 33–39 [DOI] [PubMed] [Google Scholar]
- 28. Lehnardt S., (2010) Innate immunity and neuroinflammation in the CNS. The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 58, 253–263 [DOI] [PubMed] [Google Scholar]
- 29. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T. C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D. T. (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polazzi E., Monti B. (2010) Microglia and neuroprotection. From in vitro studies to therapeutic applications. Prog. Neurobiol. 92, 293–315 [DOI] [PubMed] [Google Scholar]
- 32. Griffin W. S., Sheng J. G., Royston M. C., Gentleman S. M., McKenzie J. E., Graham D. I., Roberts G. W., Mrak R. E. (1998) Glial-neuronal interactions in Alzheimer's disease. The potential role of a “cytokine cycle” in disease progression. Brain Pathol. 8, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]