Background: Fragmented hyaluronan (a major extracellular matrix component) and eicosanoids (potent lipid mediators) are associated with chronic inflammatory diseases and cancer.
Results: Fragmented hyaluronan stimulates lipid mediator production in human monocytes and macrophages and influences macrophage differentiation toward a distinct activation pattern.
Conclusion: These findings reveal a novel link between hyaluronan-mediated inflammation and lipid metabolism.
Significance: This link may provide new targets for disease therapeutics.
Keywords: Cyclooxygenase (COX) Pathway, Eicosanoid, Extracellular Matrix, Inflammation, Monocytes, Toll-like Receptors (TLR), Hyaluronan, Prostaglandin, cPLA2, Macrophage Polarization
Abstract
Hyaluronan (HA) is the major glycosaminoglycan in the extracellular matrix. During inflammation, there is an increased breakdown of HA, resulting in the accumulation of low molecular weight (LMW) HA and activation of monocytes and macrophages. Eicosanoids, derived from the cytosolic phospholipase A2 group IVA (cPLA2α) activation, are potent lipid mediators also attributed to acute and chronic inflammation. The aim of this study was to determine the effect of LMW HA on cPLA2α activation, arachidonic acid (AA) release, and subsequent eicosanoid production and to examine the receptors and downstream mechanisms involved in these processes in monocytes and differently polarized macrophages. LMW HA was a potent stimulant of AA release in a time- and dose-dependent manner, induced cPLA2α, ERK1/2, p38, and JNK phosphorylation, as well as activated COX2 expression and prostaglandin (PG) E2 production in primary human monocytes, murine RAW 264.7, and wild-type bone marrow-derived macrophages. Specific cPLA2α inhibitor blocked HA-induced AA release and PGE2 production in all of these cells. Using CD44, TLR4, TLR2, MYD88, RHAMM or STAB2 siRNA-transfected macrophages and monocytes, we found that AA release, cPLA2α, ERK1/2, p38, and JNK phosphorylation, COX2 expression, and PGE2 production were activated by LMW HA through a TLR4/MYD88 pathway. Likewise, PGE2 production and COX2 expression were blocked in Tlr4−/− and Myd88−/− mice, but not in Cd44−/− mice, after LMW HA stimulation. Moreover, we demonstrated that LMW HA activated the M1 macrophage phenotype with the unique cPLA2α/COX2high and COX1/ALOX15/ALOX5/LTA4Hlow gene and PGE2/PGD2/15-HETEhigh and LXA4low eicosanoid profile. These findings reveal a novel link between HA-mediated inflammation and lipid metabolism.
Introduction
Hyaluronan, which is a major component of extracellular matrix, is ubiquitously present in many tissues (1). Hyaluronan occurs as a high molecular weight (HMW)2 polymer, reaching >106–107 Da, but it also exists in much smaller forms (2). Under physiological conditions, stable levels of HA are regulated locally by the opposing activities of HA synthases and hyaluronidases, whereas its systemic metabolism is controlled by liver and kidneys (2). During acute and chronic inflammation or tissue injury, reactive oxygen species (3) and matrix metalloproteinases (4) can significantly alter the HA turnover, generating local (5, 6) and systemic (7, 8) accumulation of low molecular weight HA (LMW HA) fragments. Both high and low molecular weight fragments have opposing immunoregulatory effects. In general, high molecular weight HA has been shown to be anti-inflammatory, anti-angiogenic, and immunosuppressive (1, 2). In contrast, fragmented LMW HA stimulates expression of pro-inflammatory cytokines, chemokines, and growth factors (9, 10). It can also activate intracellular inflammasomes (11), trigger sterile inflammation (12, 13), and participate in cancer progression (14, 15). There has been significant progress in understanding the immunoregulatory and signaling role of HA in many clinical and experimental conditions; however, the mechanisms of HA actions in lipid mediator signaling have not been extensively studied.
Eicosanoids and other lipid mediators have an established role in the pathogenesis of many chronic inflammatory diseases (16), neurological disorders (17), and cancer (18). Selective and nonselective nonsteroidal anti-inflammatory drugs that inhibit production of eicosanoids are commonly used to treat a wide array of inflammatory diseases. These drugs are used as prophylactic agents in a number of malignancies (19). Because of the newly described pro-resolving lipid family (20) and the discovery of new functions of previously characterized eicosanoids (16), lipid mediators are becoming recognized as key factors in the orchestration of inflammation and its resolution (21) The rate-limiting enzyme in eicosanoid production is cytosolic phospholipase A2 group IVA (PLA2G4A or cPLA2α), which is involved in the liberation of arachidonic acid (AA) from cellular membranes (22). AA is the precursor of leukotrienes, prostaglandins, hydroxyeicosatetraenoic acids, thromboxanes, and lipoxins (22, 23). cPLA2α and its downstream metabolites can also directly modulate cellular function by altering intracellular transport (24) and regulating gene transcription (25, 26). In experimental models of asthma (27), pulmonary fibrosis (28), ARDS (29), multiple sclerosis (30), and rheumatoid arthritis (31), cPLA2α knock-out mice have reduced symptoms compared with wild-type mice (32). Moreover, cPLA2α itself (33) or COX2-dependent synthesis of PGE2 is known to participate in the development and progression of cancer (34). cPLA2α is activated by intracellular calcium elevation and phosphorylation at serine residues (22). Several pro-inflammatory mediators, such as IL-1β, TNFα, IFNγ, phosphatidylinositol phosphate, ceramide 1-phosphate, sphingosine 1-phosphate, and LPS, have been reported to activate cPLA2α in various cells through different pathways (35–40). However, to date there is no evidence of HA-dependent lipid mediator activation.
cPLA2α is a protein expressed constitutively in many cells involved in sterile inflammation, tissue injury, and cancer (26). Among these cells, monocytes and macrophages are the most abundant found in diverse tissues. They play a central role in the maintenance of tissue integrity, as well as initiation and resolution of innate and adaptive immunity partially by specialized lipid mediators (41). Tissue macrophages, in the steady state, are responsible for clearing and scavenging unnecessary metabolism products and the sequestration of antigens from the immune system (41). Therefore, monocytes and macrophages are constantly prone to interact with HA in its different size forms. There are at least five reported surface receptors that are able to bind HA to induce different signaling programs (1, 2). They are CD44, RHAMM, TLR2, TLR4, and STAB2. However, the mechanisms of HA actions through various receptors in terms of lipid signaling remain unresolved. According to recent reports, various tissue-resident macrophages are established prior to birth. In the adult steady state (42) or in the context of inflammation (43), they might be independent from monocyte replenishment. Nonetheless, under the influence of local environmental conditions, monocytes, monocyte-derived macrophages, or tissue-resident macrophages are activated to acquire specialized phenotypic characteristics with distinct functions (44, 45). M1 macrophages (previously known as classically activated) are induced by IFN-γ in conjunction with microbial stimuli (e.g. LPS) or cytokines (e.g. TNF or GM-CSF). They are characterized by IL-12high/IL-23high phenotypes and are efficient in the production of inflammatory cytokines (IL-1β, TNF, and IL-6) as well as having potent microbicidal effector activity (45). In contrast, M2 macrophages sharing IL-12low/IL-23low phenotypes (46) are currently subcategorized into at least three groups. M2a macrophages (previously known as alternatively activated), which are induced by IL-4 or IL-13 signaling, are important in wound healing, tissue remodeling, and inhibition of inflammation (44, 47, 48). M2b cells, simultaneously activated by LPS and Fcγ receptor signaling, lead to Th2 cell differentiation (45, 49, 50). M2c macrophages, which are the least responsive among macrophages, are generated in the presence of anti-inflammatory cytokines such as IL-10 or TGFβ. M2c cells retain their phagocytic functions (51). Macrophages, even after initial polarization, remain very plastic and are sensitive to changes in the local environment. They might serve as a potential target for therapeutic interventions (44). Therefore, it is important to characterize their responses to extracellular matrix components, especially to LMW HA. Moreover, apart from the extensive studies leading to functional characterization of gene, transcriptome, and microRNA profiles in differentially polarized macrophages, little is known in terms of lipid mediator signaling in these cells.
Because of the growing evidence of a regulatory role of extracellular matrix in various inflammatory and proliferative disorders, we investigated the effect of LMW HA on cPLA2α activation and subsequent eicosanoid production in human monocytes, mouse macrophages, and human differently polarized macrophages. Here, we report that LMW HA potently activates cPLA2α and cPLA2α-induced AA release, eicosanoid production, and COX2 (PTGS2) expression through TLR4 but not through CD44, RHAMM, STAB2, or TLR2. This pathway is MYD88-dependent and involves activation of ERK1/2, p38, and JNK MAPK kinases. Moreover, LMW HA is able to polarize human macrophages toward an M1 phenotype, characterized not only by pro-inflammatory cytokine production but also a distinct AA-derived eicosanoid profile. These findings suggest a novel mechanism of LMW HA signaling and provide a previously unforeseen link between HA, cPLA2α activation, lipid metabolism, and macrophage polarization.
EXPERIMENTAL PROCEDURES
Reagents
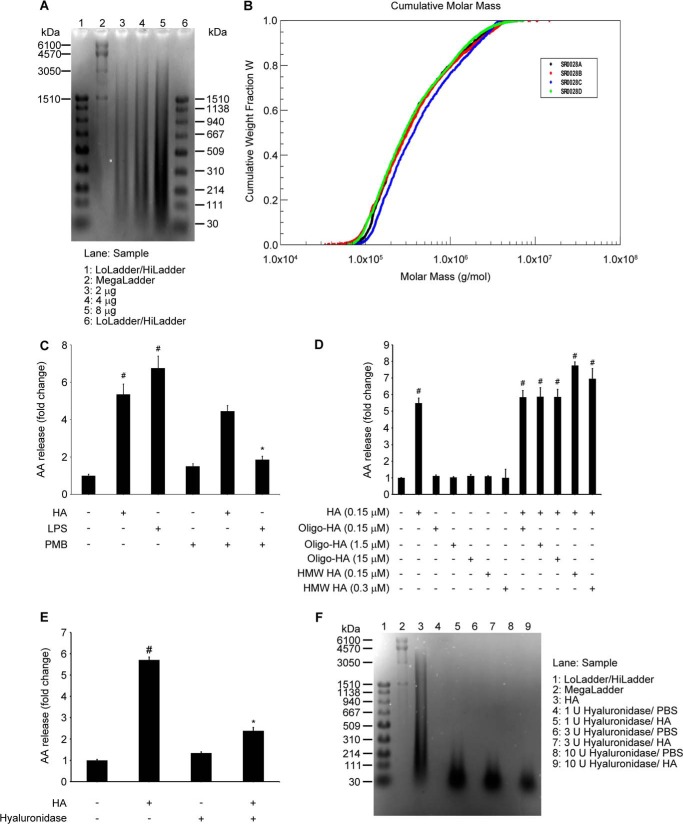
Purified HA was obtained from MP Biomedicals (Solon, OH). HA analysis was performed by Hyalose, LLC (Oklahoma City, OK). Analysis results indicated that 70% of HA fragments range between 50 and 600 kDa (Fig. 1A) and up to 80% of the fragments are lower than 1 × 106 Da (Fig. 1B). We confirmed key experiments using purified LMW HA purchased from Calbiochem. This source contained a mixture of fragments, with the average molecular mass of 200 kDa (52). Oligo-HA (4 kDa) was purchased from Sigma. HMW HA (2500 kDa) was purchased from Hyalose. To calculate the molar concentrations of each HA (Fig. 1D), we applied weight average molecular weight. We used the terms HA or LMW HA throughout the “Results” to refer to HA used in this study. Each experiment was performed in the presence of polymyxin B sulfate (Calbiochem) (10 μg/ml) to minimize the effect of LPS contamination in the HA (Fig. 1C). N-{(2S,4R)-4-(biphenyl-2-ylmethylisobutylamino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl] acrylamide, HCl (cPLA2α inhibitor), and SP600125 were purchased from Calbiochem. We also used U0126 (Cell Signaling Technology, Danvers, MA), SB202190 (Sigma), M-CSF (Invitrogen). IL-4 and IFN-γ were purchased from PeproTech (Rocky Hill, NJ). Antibodies against phospho-cPLA2α (Ser-505), total cPLA2α, phospho-p44/42 MAPK (ERK1/2) (Thr-202/Tyr-204), total p44/42 MAPK (ERK1/2), phospho-SAPK/JNK (Thr-183/Tyr-185) (98F2), total SAPK/JNK (56G8), phospho-p38 MAPK (Thr-180/Tyr-182), and total p38 MAPK were purchased from Cell Signaling Technology. Murine and human COX2 antibodies were obtained from Cayman (Ann Arbor, MI). Monoclonal horseradish peroxidase (HRP)-conjugated β-actin antibody was obtained from GenScript (Piscataway, NJ). HRP-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA).
FIGURE 1.
HA quality control and size specificity. A and B, HA (MP Biomedicals) size analysis. A, agarose gel electrophoresis, performed loading different amounts of HA, showing that the majority of fragments are localized between 50 and 600 kDa. B, multiangle laser light scattering-size exclusion chromatography, cumulative molar mass graph, showing that molar mass of 80% of fragments is below 1.0 × 106 g/mol. Average results of quadruplicate analyses show that analyzed HA has Mn (number average molecular weight) of 250 kDa and Mw (weight average molecular weight) of 689 kDa and polydispersity (Mw/Mn) of 2.77. Analysis was performed by Hyalose, LCC (Oklahoma City, OK). C, polymyxin B blocks LPS-induced, but not HA-induced, AA release. RAW 264.7 (5 × 105) cells were treated with HA (100 μg/ml) or LPS (10 ng/ml), with/without polymyxin B (10 μg/ml), for 6 h. Thus, polymyxin B was used in all experiments in addition to HA or vehicle to ensure an endotoxin-free environment. Data are presented as the fold change compared with the untreated cells. Data represent the mean ± S.E. from three independent experiments, each performed in triplicate. #, p < 0.05 as compared with untreated cells; *, p < 0.05 as compared with LPS-treated cells. D, LMW-HA, but not oligo-HA or HMW-HA, induces AA release. RAW 264.7 (5 × 105) cells were treated with HA (100 μg/ml = 0.145 μm) and/or with indicated amounts of oligo-HA (4 kDa) or HMW HA (2500 kDa) for 6 h. Data are presented as the fold change compared with the vehicle-treated cells. Data represent the mean ± S.E. from three independent experiments, each performed in triplicate. #, p < 0.05 as compared with vehicle-treated cells. E, enzymatic digestion of HA blocks HA-induced AA release. RAW 264.7 (5 × 105) cells were treated with HA (100 μg/ml) or 100 μg/ml HA with hyaluronidase from S. hyalurolyticus (1 unit/0.3 mg of HA for 4 h) for 6 h. Data are presented as the fold change compared with the vehicle-treated cells. Data represent the mean ± S.E. from three independent experiments, each performed in triplicate. #, p < 0.05 as compared with vehicle-treated cells; *, p < 0.05 as compared with HA-only treated cells. F, agarose gel electrophoresis, showing the 4-h digestion efficiency with indicated doses of hyaluronidase. Loading, 5 μg.
Primary Cells, Mice, and Cell Lines
Human elutriated monocytes from healthy donors were obtained by an institutional review board-approved protocol from the National Institutes of Health Blood Bank (Bethesda, MD). Monocytes were resuspended in RPMI 1640 medium with 2 mm of l-glutamine and supplemented with 10% heat-inactivated FBS (Invitrogen) and used the same day for the experiments and transfection. 15 × 106 monocytes were seeded into T75 tissue culture flasks in 15 ml of Iscove's modified Dulbecco's medium (IMDM) (Invitrogen) with 10% FBS and 50 ng/ml M-CSF for 7 days; half of the medium was replaced every 2–3 days. 48 h before experiments were to be performed, macrophages were trypsinized (Reagent Pack, Lonza, Walkersville, MD), scraped, and plated into 12-well plates at a density of 0.25 × 106/well. The day after plating, polarization cytokines, 20 ng/ml IFN-γ with 100 ng/ml LPS (for M1) or 20 ng/ml IL-4 (for M2), were added for 18 h followed by treatment with/without HA for 6 h. Bone marrow cells were extracted from femurs of Myd88−/−, Cd44−/−, and Tlr4−/− mice, and their WT littermate controls (C57BL/6) (The Jackson Laboratory). Bone marrow-derived macrophages (BMDM) were grown in 100-mm culture dishes in 10 ml of RPMI 1640 medium with 2 mm l-glutamine and supplemented with 10% heat-inactivated FBS, 1% penicillin/streptomycin (Lonza), and 50 ng/ml M-CSF for 7 days before the experiments were to be performed, and half of the medium was replaced every 2–3 days. 24 h before the experiments began, the mouse macrophages were scraped and plated into 12-well plates in the density of 1 × 106/well. The mouse macrophage cell line RAW 264.7 was obtained from American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% heat-inactivated FBS and 5% penicillin/streptomycin. All experiments with RAW 264.7 were performed in DMEM with 10% heat-inactivated FBS without antibiotics.
HA Digestion Procedure
Hyaluronic acid digestion was performed as described previously by Maharjan et al. (53) with some modifications. Briefly, 0.3 mg of hyaluronic acid dissolved in sterile PBS (3 mg/ml) or PBS control (pH adjusted to 5.5 at 37 °C) was treated with 1–10 units (10–100 units/ml) of hyaluronidase from Streptomyces hyalurolyticus (Sigma) (dissolved in 20 mm sodium phosphate, 77 mm sodium chloride with 0.01% (w/v) albumin, pH 7) for 4 h followed by incubation at 100 °C for 10 min to inactivate the enzyme. After digestion, the samples were adjusted back to neutral pH and used for cell stimulation without further filtration or purification. pH adjustments were performed with sterile cell culture graded 1 n NaOH and HCl. Digested samples were evaluated by electrophoresis on a 0.9% agarose gel (25 V, 1 h followed by 35 V, 5 h), followed by overnight staining in 0.005% Stains-All (Sigma) in 50% ethanol/water and further destaining as described previously (54).
TLR2, RHAMM, CD44, TLR4, MYD88, and STAB2 Knockdown
ON-TARGETplus SMART pool small interfering RNA (siRNA) (Dharmacon, Thermo Scientific, Lafayette, CO) against mouse Tlr2 (L-062838-02), Hmmr (encoding RHAMM) (L-045234-01), Tlr4 (L-047487-00), CD44 (L-041132-01) together with ON-TARGETplus Control Nontargeting pool (D-001810-10) were used to perform knockdown experiments in RAW 264.7 cells. Silencer Select predesigned siRNA against mouse Myd88 (s70237) and negative control siRNA (Ambion, Invitrogen) was used for Myd88 knockdown in RAW 264.7 cells. RAW 264.7 cells were seeded in a density of 5 × 104 cells per well in 12-well plates for 24 h before transfection. siRNA constructs were transfected into RAW 264.7 cells at a final concentration of 50 nm using 2.5 μl of Dharmafect 4 transfection reagent per well (1 ml), according to the manufacturer's protocol (Dharmacon). Elutriated human monocytes (5 × 106) were transfected with 100 nm ON-TARGETplus SMART pool siRNA against human TLR4 (L-008088-01), CD44 (L-009999-00), or ON-TARGETplus Control nontargeting pool (D-001810-10) (Dharmacon) using a P3 Primary Cell 4D-Nucleofector X Kit L (Amaxa, Cologne, Germany). Primary human monocyte-derived macrophages were seeded at a density of 3 × 105 per well in 12-well plates for 24 h before transfection. ON-TARGETplus SMART pool siRNA against human STAB2 (L-015260-01) or ON-TARGETplus Control nontargeting pool (D-001810-10) siRNA constructs were transfected into these cells at a final concentration 100 nm using 2.5 μl of Dharmafect 1 transfection reagent per well (1 ml), according to the manufacturer's protocol (Dharmacon). The silencing of gene expression was confirmed by RT-PCR. All experiments on transfected cells were performed after 48 h.
Real Time PCR
Total RNA was extracted from cells using QIAshredder columns and RNeasy mini kit and treated with DNase (Qiagen, Valencia, CA). Reverse transcription was performed using an iScript cDNA Synthesis Kit (Bio-Rad). Gene expression was assessed using RT-PCR performed on an ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) using commercially available probe and primers sets (Applied Biosystems) as follows: human: CD44, Hs01075862_m1; TLR4, Hs00152939_m1; STAB2, Hs00213948_m1; GAPDH, Hs02758991_g1; IL-1β, Hs01555410_m1; IL-6, Hs00174131_m1; TNF, Hs99999043_m1; IL-23A, Hs00372324_m1; IL12A, Hs01073447_m1; IL-12B, Hs01011518_m1; CCL18, Hs00268113_m1; P2Y12, Hs00375457_m1; IL-10, Hs00961622_m1; cPLA2α (PLA2G4A), Hs00233352_m1; COX1 (PTGS1), Hs00924808_m1; COX2 (PTGS2), Hs00153133_m1; ALOX15, Hs00993765_g1; ALOX5, Hs01095330_m1; LTA4H, Hs01075871_m1; mouse: TLR2, Mm00442346_m1; TLR4, Mm00445274_m1; CD44, Mm01277163_m1; RHAMM, Mm00469183_m1; MYD88, Mm01351743_g1; STAB2, Mm00454684_m1 and GAPDH, Mm99999915_g1, and SSO Fast Probes Supermix with ROX (Bio-Rad). Gene expression was normalized to GAPDH transcripts and represented as a relative quantification (RQ) compared with control.
Western Blot
siRNA-transfected or untransfected cells were treated with HA (100 μg/ml) for the indicated time points. Cells were then washed with ice-cold PBS and harvested with lysis buffer (50 mm Tris-HCl, pH 7.5, 1 mm EDTA, 1% Triton X-100) supplemented with protease inhibitor mixture (Roche Applied Science) and phosphatase inhibitors set I and II (Calbiochem). Cell lysates were centrifuged, and equivalent amounts of lysate protein (from 1 μg to detect cPLA2α in RAW 264.7, up to 40 μg to detect phospho-p38 and phospho-JNK in primary monocytes and RAW 264.7 cells) were loaded onto 4–12% NuPage Tris-glycine gels (Invitrogen). After electrophoresis, the proteins were transferred to nitrocellulose membranes using the IBlot Dry Blotting System (Invitrogen). The membranes were blocked with 5% nonfat dry milk (Bio-Rad) in PBST (Sigma). The blots were then incubated with primary antibodies (1:1000) overnight at 4 °C and washed with PBST. The blots were incubated with HRP-conjugated secondary antibodies (1:10,000) for 1 h. After washing, the blots were developed with the ECL Advanced Western blotting chemiluminescence detection kit (GE Healthcare), and the signals were captured on Gel Logic 2200PRO (Carestream Molecular Imaging, Rochester, NY).
ELISA
siRNA-transfected and -untransfected monocytes, BMDM, RAW cells, and M1, M2, or unpolarized human primary macrophages were stimulated with HA (100 μg/ml) for 6–8 h, with or without cPLA2α inhibitor (2 μm, 30 min pretreatment). Supernatants were harvested and stored at −80 °C. PGE2, PGD2, 15(S)-HETE, LTB4, and LTC4 (Cayman Chemicals) or LXA4 (Oxford Biomedical Research, Rochester Hills, MI,) determination was performed with commercially available EIA kits. Detection limits of the EIA kits were as follows: 15 pg/ml for PGE2, 55 pg/ml for PGD2, 170 pg/ml for 15(S)-HETE, 13 pg/ml for LTB4, 10 pg/ml for LTC4, and 20 pg/ml for LXA4.
[3H]AA Release
Untransfected primary monocytes, RAW 264.7 cells, or cells transfected with siRNAs were incubated overnight in appropriate medium supplemented with 10% FBS and 0.5 μCi of [5,6,8,9,11,12,14,15-3H]arachidonic acid (150–230 Ci/mmol/ml; Amersham Biosciences). In some experiments, cells were treated with HA with/without pretreatment with U0126 (1 μm, 2 h), SB202190 (1 μm, 2 h), SP600125 (5 μm, 2 h), or cPLA2α inhibitor (2 μm, 30 min). Whole cell AA release assessment was performed as described by Pawliczak et al. (55). Briefly, after overnight incubation, the medium was removed, and the cells were washed three times with serum-free medium. Cells were then incubated in fresh medium with or without the various treatments as specified. At the time points indicated, the media were collected and centrifuged at 1000 × g for 5 min. Aliquots of cell-free media were transferred to scintillation vials, and 10 ml of BioSafe II scintillation liquid was added (Research International Products, Mount Prospect, IL). Radioactivity was measured in an LS6500 scintillation counter (Beckman, Fullerton, CA). Data are presented as the fold change compared with the vehicle-treated cells.
Statistical Analysis
Data were analyzed by one-way analysis of variance with Holm-Sidak post hoc test, analysis of variance on ranks with Tukey post hoc test, or unpaired Student's t test or Mann-Whitney U test with Bonferroni correction for multiple comparisons, as appropriate. Differences were considered significant when p < 0.05. The data are presented as the mean ± S.E. from the indicated number of independent experiments, each performed in triplicate.
RESULTS
Low Molecular Weight Hyaluronic Acid (LMW HA) Activates cPLA2α-induced AA Release
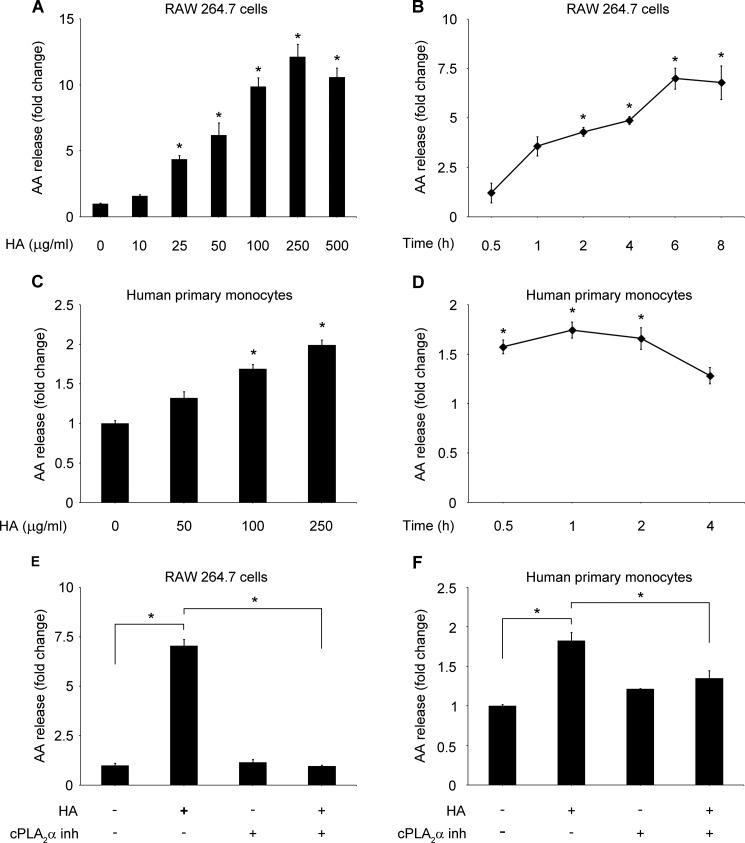
The majority of fragments of HA used in our study was between 50 and 600 kDa (Fig. 1A), and the molar mass of 80% of fragments was below 1.0 × 106 g/mol (Fig. 1B). Therefore, we refer to HA used in this study as LMW HA. Polymyxin B blocked LPS-induced but not HA-induced AA release (Fig. 1C). Thus, polymyxin B was used in all experiments in addition to HA or vehicle to ensure an endotoxin-free environment. To analyze if the signal was specific for the LMW HA, we used very small oligomers of HA (4 kDa), as well as high molecular weight HA fragments (2500 kDa). Neither oligomers of HA nor HMW HA induced AA release. Moreover, they did not block the LMW HA-induced signal (Fig. 1D). Digestion of HA with HA-specific hyaluronidase from Streptomyces hyalurolyticus significantly reduced the induction of AA release, confirming the specificity of the signal (Fig. 1, E and F). HA, in a dose-dependent manner, increased AA release in RAW 264.7 cells. A significant increase was noted at a concentration of 25 μg/ml, reaching a maximum effect at 250 μg/ml (Fig. 2A). Time course analysis revealed that HA (100 μg/ml) increased AA release, beginning at 30 min up to 8 h after stimulation, with the maximum effect at the 6-h time point in RAW 264.7 (Fig. 2B). In primary human monocytes, HA also significantly increased AA release starting from the dose of 100 μg/ml (Fig. 2C) and from the 30-min time point (Fig. 2D). We used a cPLA2α inhibitor to evaluate whether the observed AA release is cPLA2α-dependent. The cPLA2α inhibitor (2 μm) completely blocked AA release in both RAW 264.7 macrophages (Fig. 2E) and human primary monocytes (Fig. 2F). These results suggest that LMW HA, but not smaller or larger HA fragments, activates cPLA2α and cPLA2α-induced AA release in monocytes and macrophages.
FIGURE 2.
LMW HA induces cPLA2α-dependent arachidonic acid release. RAW 264.7 cells (5 × 105) were stimulated with the indicated concentrations of HA for 6 h (A) or with 100 μg/ml of HA at the indicated times (B). Primary human monocytes (1 × 106) were stimulated with the indicated concentrations of HA for 1 h (C) or with 100 μg/ml of HA at the indicated times (D). RAW 264.7 (5 × 105) (E) or primary human monocytes (1 × 106) (F) were pretreated with cPLA2 inhibitor (inh) (N-(2S,4R)-4-(biphenyl-2-ylmethylisobutylamino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl] acrylamide, HCl, 2 μm) or vehicle for 30 min and then stimulated with 100 μg/ml of HA for 6 h (E) or 1 h (F). Data are presented as the fold change compared with the vehicle-treated cells. Data represent the mean ± S.E. from three experiments in RAW 264.7 cells (A, B, and E) or in elutriated monocytes from three healthy donors (C, D, and F), each performed independently in triplicate. *, p < 0.05.
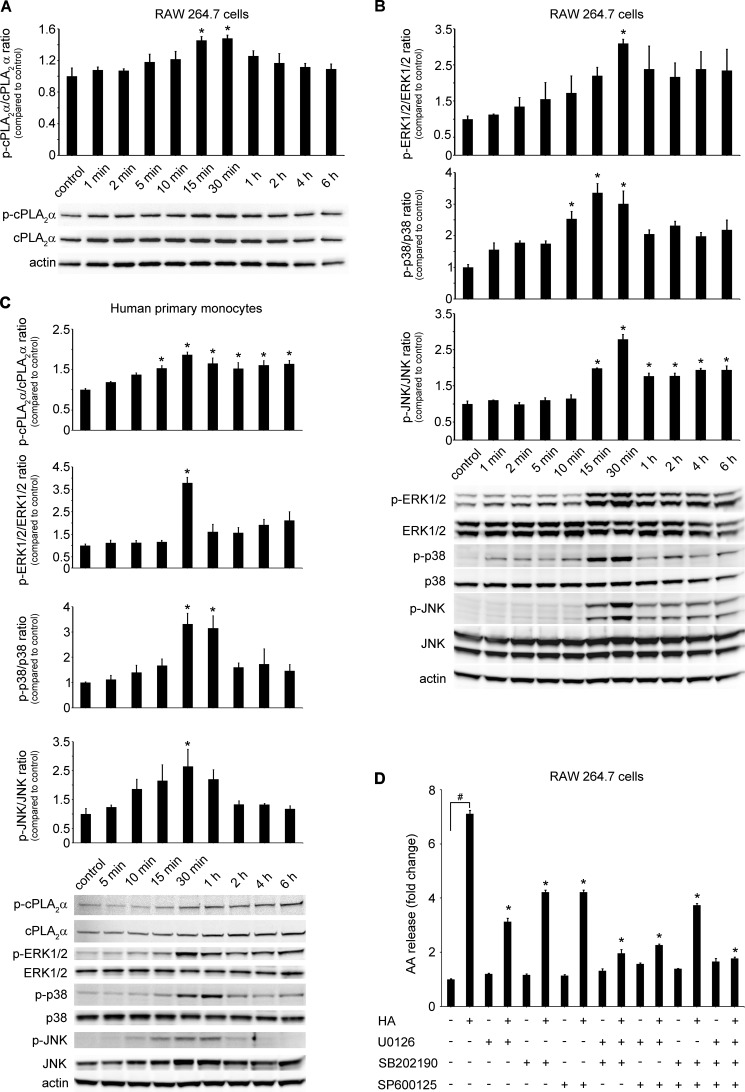
LMW HA Induces cPLA2α and MAPK Phosphorylation and HA-induced AA Release Is ERK1/2-, p38-, and JNK-dependent
Phosphorylation is the most common pathway that activates cPLA2α (56). Therefore, we examined the cPLA2α phosphorylation pattern, beginning at 1 min up to 6 h after HA stimulation. RAW 264.7 cells had a baseline level of phosphorylated cPLA2α that was further increased at 15 and 30 min after HA treatment (100 μg/ml) with a subsequent return to baseline levels at 6 h (Fig. 3A). cPLA2α phosphorylation increased in primary human monocytes over time after HA (100 μg/ml) treatment, starting at 15 min up to 6 h (Fig. 3C). Interestingly, we also found an increase in total cPLA2α protein expression in human primary monocytes, starting at 30 min after HA treatment and lasting up to 6 h (Fig. 3C). Various MAPKs were previously reported to take part in cPLA2α phosphorylation (57–59). Therefore, we analyzed the phosphorylation patterns of ERK1/2, p38, JNK, and PI3K-Akt kinases after HA (100 μg/ml) treatment over time. Akt was not phosphorylated by HA treatment (data not shown). In contrast, HA increased ERK1/2 phosphorylation with the maximum occurring at 30 min. ERK1/2 tended to remain phosphorylated up to 6 h in RAW 264.7 cells (Fig. 3B) and in human monocytes (Fig. 3C). p38 and JNK kinases were also phosphorylated after HA treatment with the maximum occurring at 15 and 30 min in RAW 264.7 cells (Fig. 3B) and 30 min and 1 h in human monocytes (Fig. 3C). The increased phosphorylation lasted up to 6 h after treatment in RAW 264.7 cells. To elucidate whether inhibition of these kinases would have an impact on HA-induced AA release, we used their chemical inhibitors U0126 (ERK1/2 inhibitor), SB202190 (p38 inhibitor), SP600125 (JNK inhibitor), and wortmannin (Akt inhibitor). Wortmanin had no impact on HA-induced AA release (data not shown), which is in line with the lack of Akt phosphorylation after HA treatment. The ERK1/2, p38, and JNK inhibitors significantly decreased HA-induced AA release with the efficacy as follows: U0126 > SB202190 > SP600125, and the combination of all three inhibitors completely blocked HA-induced AA release (Fig. 3D). Our results indicate that HA activates cPLA2α, through its phosphorylation by ERK1/2, p38, and JNK kinases pathways but not the Akt pathway.
FIGURE 3.
LMW HA induces cPLA2α and ERK1/2, p38, and JNK phosphorylation. cPLA2α activation is ERK1/2-, p38-, and JNK-dependent. RAW 264.7 (5 × 105) cells (A and B) or primary human monocytes (1 × 106) (C) were stimulated with 100 μg/ml of HA at the indicated time. Control represents cells treated with the vehicle, which did not change across different time points. The immunoblot is representative of three independent experiments in RAW 264.7 cells (A and B) or in elutriated monocytes from three healthy donors (C), each showing similar results. The bar graph shows the densitometry results, presented as the ratio of phosphorylated to total protein, as compared with the same ratio in the vehicle-treated cells (control). Data represent the mean ± S.E. from three independent experiments. *, p < 0.05 as compared with control. D, RAW 264.7 cells (5 × 105) were treated with ERK1/2 inhibitor (U0126; 1 μm), p38 inhibitor (SB202190, 1 μm), and JNK inhibitor (SP00125, 5 μm) separately or in combination or with the vehicle for 2 h and then stimulated with 100 μg/ml HA for 6 h. Data are presented as the fold change compared with the vehicle-treated cells. Data represent the mean ± S.E. from three experiments, each performed independently in triplicate. #, p < 0.05 as indicated, *, p < 0.05 as compared with HA-only treated cells.
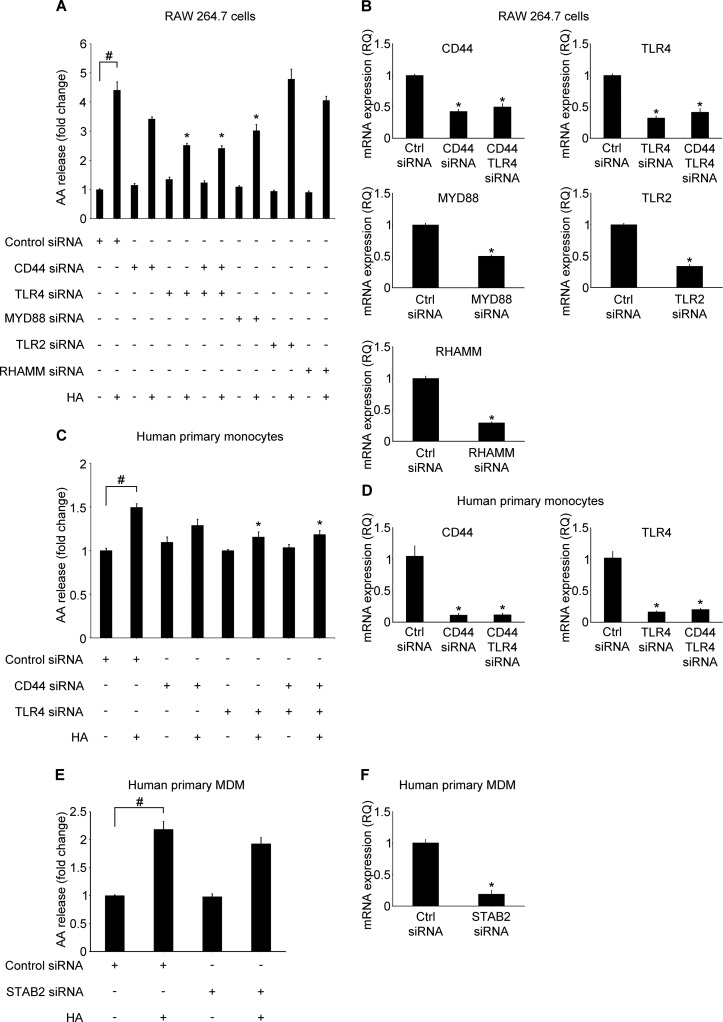
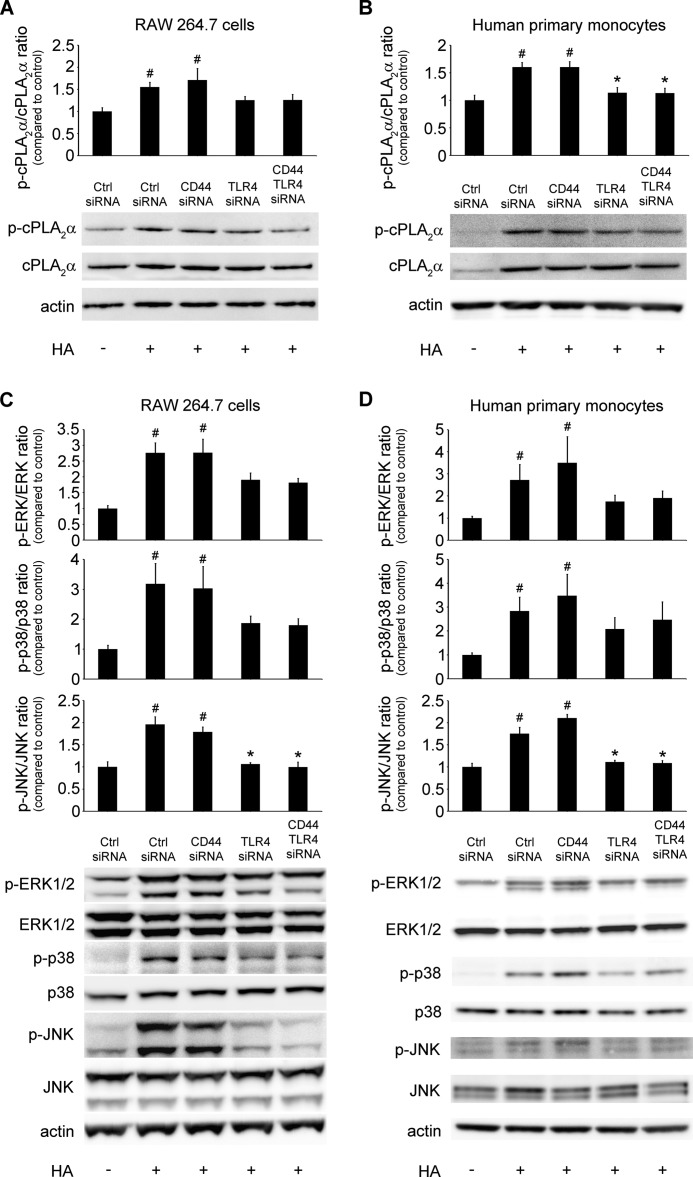
LMW HA Activates cPLA2α through TLR4 and MYD88 but Not through CD44, RHAMM, TLR2, or STAB2
A variety of receptors has been reported to bind HA in different cell types (2). The most abundant receptors on the surface of monocytes and macrophages include CD44, RHAMM, STAB2, and two toll-like receptors, TLR2 and TLR4. To address the hypothesis that any of these proteins could play a role in HA-induced cPLA2α activation and AA release, we used an siRNA pooling approach, as described under “Experimental Procedures.” We knocked down expression of RHAMM, TLR2, CD44, and TLR4 by ∼71, 66, 57, and 68%, respectively, in RAW 264.7 cells (Fig. 4B), as assessed by RT-PCR. CD44 and TLR4 siRNA decreased CD44 and TLR4 mRNA expression by 94 and 86% in human primary monocytes, respectively (Fig. 4D). STAB2 is weakly expressed by human monocytes and macrophages but not by RAW 264.7 cells. Because of its proven function in macrophage phagocytosis, we studied this receptor in human primary macrophages. We knocked down expression of STAB2 by ∼81%, as assessed by RT-PCR (Fig. 4F). To further explore the TLR2/4 signaling pathway, we knocked down MYD88 expression up to 50% (Fig. 4B). We found that TLR4 knockdown caused a significant decrease in HA-induced AA release in RAW 264.7 cells (Fig. 4A). However, despite earlier reports suggesting cooperative signaling (11), either single CD44 or simultaneous knockdown of CD44 and TLR4 did not further inhibit HA-induced AA release (Fig. 4A). RHAMM or TLR2 knockdown did not affect HA-induced AA release (Fig. 4A). Knockdown of MYD88 expression led to significant decrease in AA release in RAW 264.7 cells (Fig. 4A). Knockdown of TLR4 or simultaneous knockdown of TLR4 and CD44 significantly decreased HA-induced AA release from human primary monocytes (Fig. 4C). STAB2 knockdown did not change the HA-induced AA release from human primary monocyte-derived macrophages (Fig. 4E). Examination of the phosphorylation pattern of cPLA2α, ERK1/2, p38, and JNK, both in RAW 264.7 cells (Fig. 5, A and C) and primary human monocytes (Fig. 5, B and D), revealed a trend to decrease or significantly decrease the phosphorylation signal in each of these proteins in cells transfected with TLR4 or TLR4 and CD44 siRNA after HA treatment, confirming the AA release results. These results imply that the TLR4/MYD88 but not CD44, RHAMM, TLR2, or STAB2 are primarily involved in HA-activated cPLA2α signaling in human monocytes or mouse and human macrophages.
FIGURE 4.
LMW HA activates arachidonic acid release through a TLR4/MYD88 pathway. RAW 264.7 cells (5 × 104) (A and B), primary human monocytes (5 × 106) (C and D), and primary human monocyte-derived macrophages (MDM) (3 × 105) (E and F) were transfected with the indicated siRNAs (50, 100, and 100 nm, respectively). 48 h after transfection, 5 × 104 cells were treated with 100 μg/ml HA for 6 h (A), 1 × 106 cells for 1 h (C), or 3 × 105 for 2 h (E). Data are presented as the fold change of AA release compared with the vehicle-treated, control siRNA-transfected cells. Data represent the mean ± S.E. from three experiments in RAW 264.7 cells (A), in elutriated monocytes from three healthy donors (C), or in monocyte-derived macrophage from three healthy donors (E), each performed independently in triplicate. #, p < 0.05 as indicated; *, p < 0.05 as compared with HA-only treated cells. B, D, and F, siRNA transfection efficiency control. RAW 264.7 (5 × 104) (B), primary human monocytes (5 × 106) (D), or primary human monocyte-derived macrophages (3 × 105) (F) were transfected with the indicated siRNAs. 48 h after transfection cells were lysed, and total RNA was extracted. Gene expression was assessed using RT-PCR. Gene expression was normalized to GAPDH transcripts and represented as a relative quantification (RQ) compared with control. Data represent the mean ± S.E. from three independent experiments, each performed in triplicate. *, p < 0.05 as compared with control siRNA (Ctrl siRNA).
FIGURE 5.
LMW HA induces cPLA2α, ERK1/2, p38, and JNK phosphorylation through TLR4. RAW 264.7 cells (5 × 104) (A and C) and primary human monocytes (5 × 106) (B and D) were transfected with the indicated siRNAs (50 and 100 nm, respectively). 48 h after transfection, cells were treated with 100 μg/ml of HA for 30 min. Vehicle-treated siRNA-transfected cells are represented by control siRNA. The immunoblot is representative of three independent experiments in RAW 264.7 cells (A and C) or in elutriated monocytes from three healthy donors (B and D), each showing similar results. The bar graph shows the densitometry results, presented as the ratio of phosphorylated to total protein, as compared with the same ratio in the vehicle-treated, control siRNA-transfected cells. Data represent the mean ± S.E. from three independent experiments. #, p < 0.05 as compared with the vehicle-treated, control siRNA-transfected cells; *, p < 0.05 as compared with HA-treated, control siRNA-transfected cells.
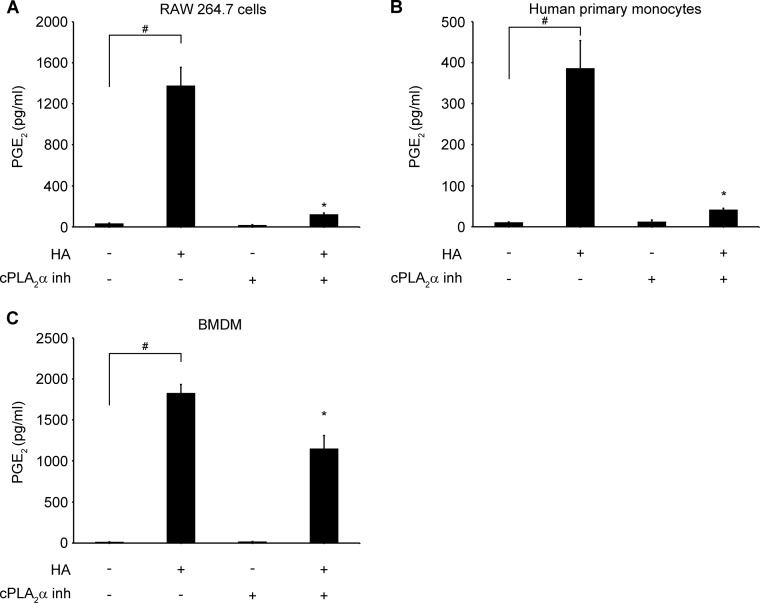
LMW HA-induced PGE2 Production Is cPLA2α-dependent
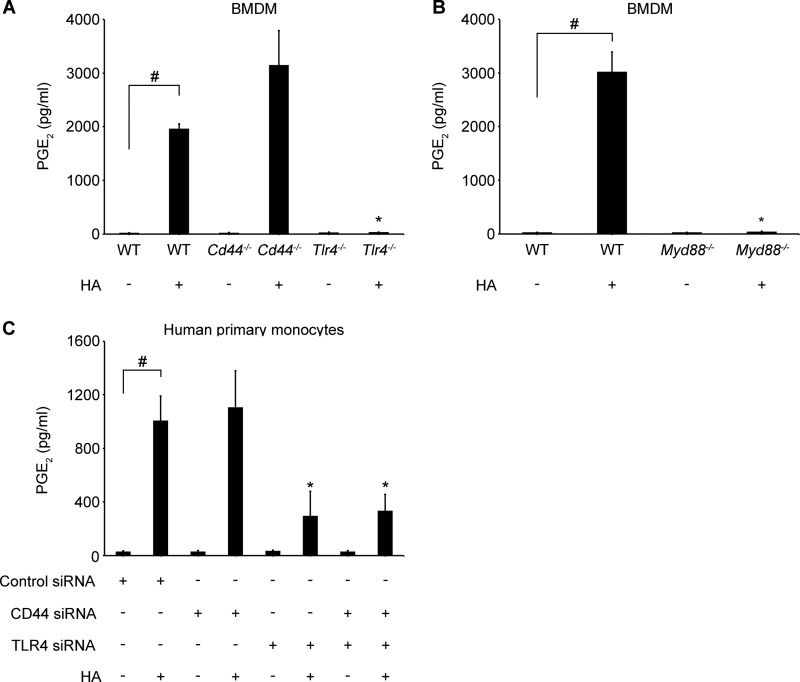
PGE2 is one of the specific downstream eicosanoids, produced by cyclooxygenases 1 (COX1 or PTGS1) and 2 (COX2 or PTGS2), from the release of AA from membrane phospholipids (60). To evaluate if PGE2 production could be stimulated by HA and to address the question of cPLA2α dependence, we treated different cell types with HA (100 μg/ml) in the presence or absence of cPLA2α inhibitor (2 μm). We found that HA significantly increased PGE2 secretion in RAW 264.7 cells (Fig. 6A), human monocytes (Fig. 6B), and WT BMDM (Fig. 6C). HA-induced PGE2 production was blocked by cPLA2α inhibitor in RAW 264.7 cells and human monocytes and significantly decreased in WT BMDM. Additionally, PGE2 production was absent in Tlr4−/− and Myd88−/− BMDM after HA stimulation in contrast to WT and Cd44−/− BMDM (Fig. 7, A and B). Also, TLR4 or CD44 and TLR4 simultaneous knockdown in human monocytes led to a significant decrease of PGE2 production (Fig. 7C). These observations suggest that HA through its direct effect on cPLA2α activation and AA release could also increase the downstream products of AA metabolism.
FIGURE 6.
LMW HA induces cPLA2α-dependent PGE2 production. RAW 264.7 cells (5 × 105) (A), primary human monocytes (1 × 106) (B), or WT bone marrow-derived macrophages (BMDM) (C57BL/6J) (1 × 106) (C) were treated with cPLA2 inhibitor (inh) (N-(2S,4R)-4-(biphenyl-2-ylmethylisobutylamino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl] acrylamide, HCl, 2 μm) or vehicle for 30 min and then stimulated with 100 μg/ml of HA for 8 h. All experiments were performed in 12-well plates with the indicated number of cells in 1 ml of medium per well. Data represent the mean ± S.E. from three experiments in RAW 264.7 cells (A), in elutriated monocytes from three healthy donors (B), or in BMDM from three animals, each performed independently in triplicate. #, p < 0.05 as indicated; *, p < 0.05 as compared with HA-only treated cells.
FIGURE 7.
LMW HA stimulates PGE2 production through a TLR4/MYD88 pathway. Cd44−/−, Tlr4−/−, and their WT control (A) or Myd88−/− and their WT control BMDM (1 × 106) (B) were stimulated with 100 μg/ml of HA for 8 h. Primary human monocytes (5 × 106) were transfected with the indicated siRNAs (100 nm) (C). 48 h after transfection, cells were treated with 100 μg/ml of HA for 8 h. All experiments were performed in 12-well plates with 1 × 106 of cells in 1 ml of medium per well. Data represent the mean ± S.E. from independent experiments in BMDM from two animals (A and B) or in elutriated monocytes from three healthy donors (C), each performed in triplicate (B). #, p < 0.05 as indicated; *, p < 0.05 as compared with HA-only treated cells.
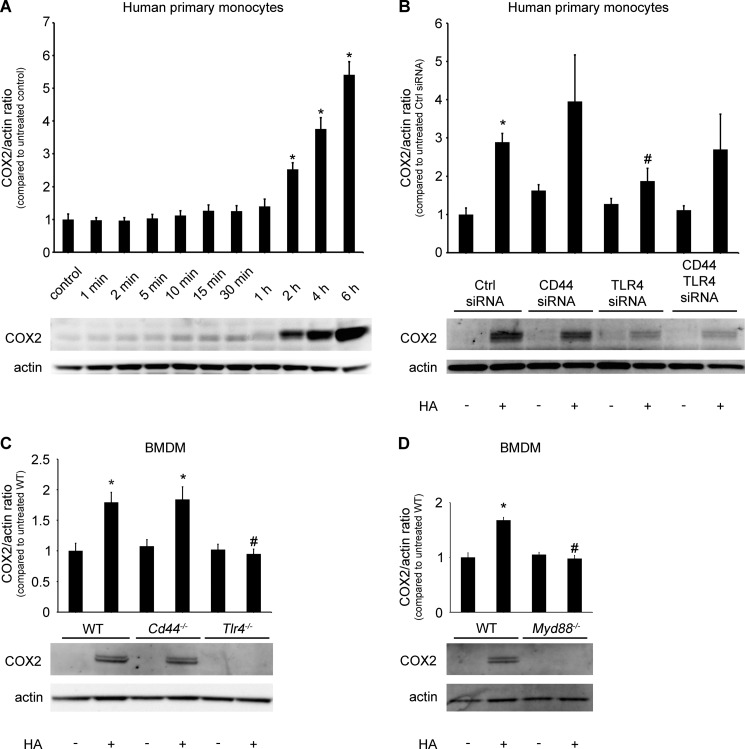
LMW HA Induces COX2 Expression through the TLR4 and MYD88 Pathway
We analyzed COX2 (PTGS2) expression to determine whether HA-induced PGE2 production is solely a derivative of increased AA levels or if other mechanisms are involved in this process. We found that stimulation with HA (100 μg/ml) augmented COX2 expression in human monocytes (Fig. 8, A and B) and WT BMDM (Fig. 8, C and D). In human monocytes, the increase started at 2 h and achieved the maximum at 6 h (Fig. 8A). This process is also TLR4/MYD88-dependent, as shown in TLR4 knockdown monocytes (Fig. 8B) and Tlr4−/− or Myd88−/− but not Cd44−/− BMDM (Fig. 8, C and D).
FIGURE 8.
LMW HA induces COX2 (PTGS2) expression through TLR4/MYD88 pathway. A, primary human monocytes (1 × 106) were stimulated with 100 μg/ml of HA at the indicated time. B, primary human monocytes (5 × 106) were transfected with the indicated siRNAs (100 nm). 48 h after transfection, 1 × 106 cells were treated with 100 μg/ml of HA for 6 h. Cd44−/−, Tlr4−/−, and their WT control (C) or Myd88−/− and their WT control BMDM (1 × 106) (D) were stimulated with 100 μg/ml HA for 6 h. The immunoblot is representative of independent experiments in elutriated monocytes from three healthy donors (A and B) or three experiments in BMDM from two animals (C and D), each showing similar results. The bar graph shows the densitometry results, presented as the ratio of COX2 to actin, as compared with the same ratio in the appropriate vehicle-treated control cells. Data represent the mean ± S.E. from three independent experiments. *, p < 0.05 as compared with the vehicle-treated cells; #, p < 0.05 as compared with HA-treated cells.
LMW HA Is a Potent Activator of M1 Macrophage Phenotype, Increases Pro-Inflammatory Gene Expression and PGE2, PGD2, and 15-HETE Release in Human Primary Monocyte-derived Macrophages
We used an in vitro model of human monocyte-derived macrophages to investigate if LMW HA has the potency to induce AA-derived eicosanoid production and eicosanoid metabolism gene expression not only in human monocytes and murine macrophages but also in primary human macrophages. Macrophages have been reported to change their phenotype and function, depending on the polarization stage; therefore, we studied their responses in M0, M1, and M2 (specifically in M2a) phenotypes.
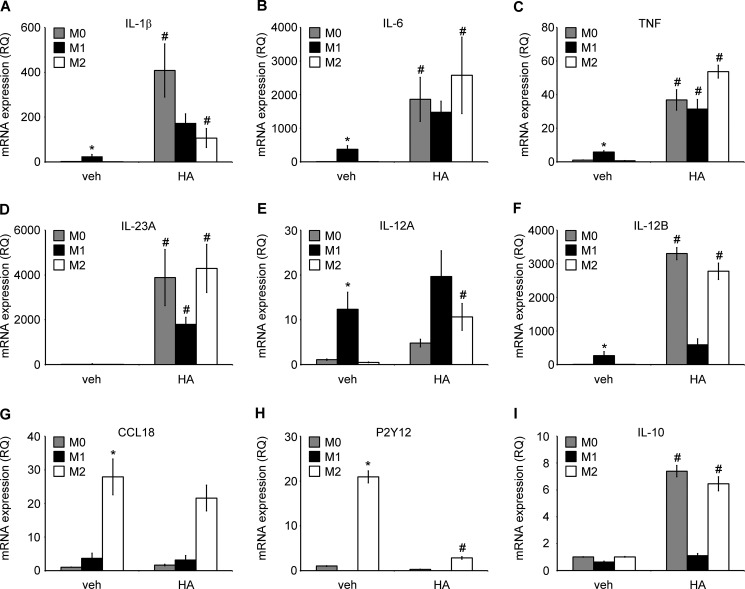
We stimulated M0 macrophages to polarize into M1 or M2 phenotype, as assessed by expression of previously reported genes by using 100 ng/ml LPS and 20 ng/ml IFN-γ or 20 ng/ml IL-4, respectively, for 24 h (61). M1 macrophages were characterized by an increased expression of IL-1β, IL-6, TNF, IL-12A, and IL-12B and an undetectable expression of P2Y12, whereas M2 macrophages were characterized by increased expression of CCL18 and P2Y12 (Fig. 9, A–H) as compared with M0 macrophages. There were no changes in IL-10 gene expression in any of the initial polarization stages (Fig. 9I). Interestingly, we found that HA (100 μg/ml) treatment for 6 h significantly changed the phenotype of M0 and M2 macrophages into M1 phenotype, with higher expression of M1-like genes than in M1-like macrophages without HA treatment (Fig. 9, A–H). HA treatment further augmented the LPS and IFN-γ effects in case of M1 genes. The only exception was IL-10 gene expression, which was induced by HA treatment in M0 and in M2 macrophages (Fig. 9I). This could possibly suggest that slightly different phenotypes of LMW HA-induced M1 macrophages are associated with additional regulatory properties.
FIGURE 9.
LMW HA induces polarization of human macrophages toward M1 phenotype. A–I, mRNA expression of cytokines, chemokines, and receptors involved in macrophage polarization process and function, with/without HA treatment. Primary human monocyte-derived macrophages (0.25 × 106) were polarized into M1 phenotype by adding 20 ng/ml IFN-γ with 100 ng/ml LPS into the M2 phenotype by adding 20 ng/ml IL-4 or were left unstimulated for the initial 18 h. Subsequently, cells were treated with/without HA for 6 h, together with polarizing cytokines. The cells were then lysed, and total RNA was extracted. Gene expression was assessed using RT-PCR. Gene expression was normalized to GAPDH transcripts and represented as a relative quantification (RQ) compared with M0-unstimulated cells. Data represent the mean ± S.E. from independent experiments in monocyte-derived macrophages from three healthy donors, each performed in triplicate. *, p < 0.05 as compared with unstimulated M0 macrophages in cells without HA treatment; #, p < 0.05 as compared with vehicle (veh)-treated, same initially polarized phenotype.
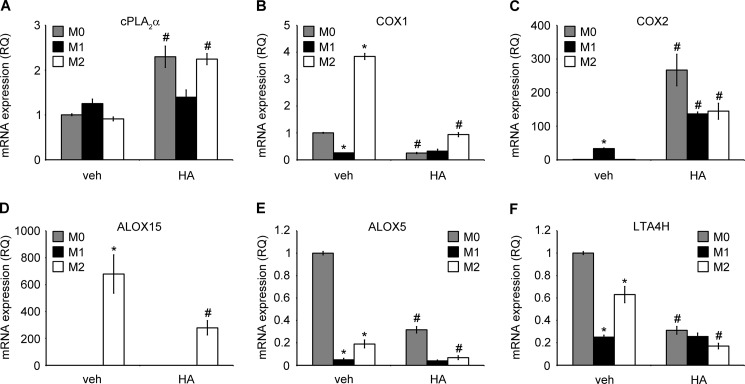
Furthermore, we studied the effect of LMW HA on the main metabolic pathways of AA-derived eicosanoids. Specifically we looked at the gene expression of enzymes and production of downstream lipids. We found that as with our results in human monocytes, HA in a concentration of 100 μg/ml induced cPLA2α expression in M0 and in M2 macrophages (Fig. 10A). COX1 (PTGS1) gene expression was decreased in M1 macrophages and increased in M2 macrophages. Furthermore, HA treatment potently decreased COX1 expression in M0 and M2 cells (Fig. 10B). COX2 (PTGS2) gene expression was increased in M1 macrophages, whereas HA treatment significantly increased its expression in each polarization stage (Fig. 10C). ALOX15 gene expression was highly elevated in M2 macrophages, although it was low or not detectable in M0 or M1 macrophages, respectively. HA treatment decreased ALOX15 gene expression in M2 cells (Fig 10D). Finally, ALOX5 and LTA4H gene expression was significantly lower in both M1 and M2 macrophages as compared with M0 cells and was further decreased by HA in M0 and M2 cells (Fig 10, E and F).
FIGURE 10.
LMW HA induces a cPLA2α/COX2high and COX1/ALOX15/ALOX5/LTA4Hlow gene expression profile in human macrophages. A–F, mRNA expression of enzymes involved in arachidonic acid and eicosanoid metabolism, with/without HA treatment. Primary human monocyte-derived macrophages (0.25 × 106) were polarized into M1 phenotype by adding 20 ng/ml IFN-γ with 100 ng/ml LPS into M2 phenotype by adding 20 ng/ml IL-4 or were left unstimulated for the initial 18 h. Subsequently, cells were treated with/without HA for 6 h, together with polarizing cytokines. The cells were then lysed, and total RNA was extracted. Gene expression was assessed using RT-PCR. Gene expression was normalized to GAPDH transcripts and represented as a relative quantification (RQ) compared with M0-unstimulated cells. Data represent the mean ± S.E. from independent experiments in monocyte-derived macrophages from three healthy donors, each performed in triplicate. *, p < 0.05 as compared with unstimulated M0 macrophages in cells without HA treatment; #, p < 0.05 as compared with vehicle (veh)-treated, same initially polarized phenotype.
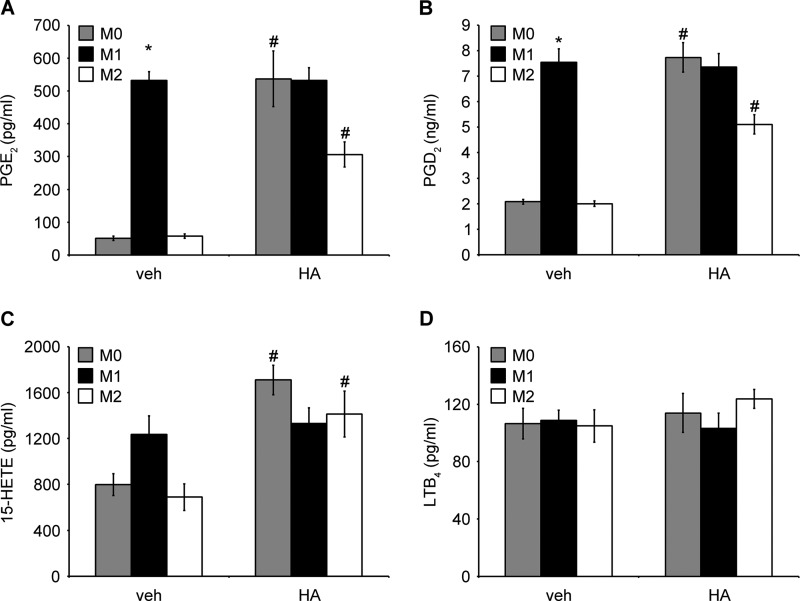
In terms of the lipid profile in differently polarized macrophages, PGE2 and PGD2 release was highly increased in M1 macrophages. HA treatment potently stimulated PGE2 and PGD2 release by M0 and M2 macrophages. Their levels were still elevated but unchanged in M1 macrophages (Fig. 11, A and B). These results correlated with the observed COX2 and COX1 gene expression. No changes were observed in 15-HETE levels between differently polarized macrophages; however, HA-stimulated 15-HETE secretion was detected in M0 and M2 macrophages (Fig. 11C). We also measured LXA4 secretion, which is dependent on ALOX15 enzymatic activity. We were able to detect small amounts of LXA4 (32.6 ± 9.8 pg/ml; mean ± S.E. n = 3) only in M2 macrophages that were not treated with HA. LXA4 was below the detection level in the other cells. This might suggest that LXA4 secretion correlates with ALOX15 gene expression, reflecting anti-inflammatory profile of M2-like macrophages. 15-HETE production might depend on increased HA-induced AA availability and reflect the pro-inflammatory HA-dependent macrophage profile. LTB4 levels were unchanged by HA treatment in all polarization stages, even though LTA4H expression was decreased in M1 and M2 macrophages after HA treatment (Fig. 11D). Finally, LTC4 was below the detection level under all conditions tested. In summary, these observations suggest that LMW HA is able to potently polarize human macrophages toward the M1 phenotype, characterized not only by “typical” pro-inflammatory cytokine profile but also by a specific AA-derived eicosanoid profile.
FIGURE 11.
LMW HA induces a PGE2/PGD2/15-HETEhigh and LXA4low eicosanoid profile in human macrophages. PGE2 (A), PGD2 (B), 15-HETE (C), and LTB4 (D) secretion by differently polarized macrophages, with/without HA treatment. Primary human monocyte-derived macrophages (0.25 × 106) were polarized into M1 phenotype by adding 20 ng/ml IFN-γ with 100 ng/ml LPS into M2 phenotype by adding 20 ng/ml IL-4 or were left unstimulated for the initial 18 h. Subsequently, cells were treated with/without HA for 6 h, together with polarizing cytokines. Cell supernatant was harvested, and the concentration of indicated eicosanoids was measured by enzyme immunoassay. All experiments were performed in 12-well plates with the indicated number of cells in 1 ml of medium per well. Data represent the mean ± S.E. from independent experiments in monocyte-derived macrophages from three healthy donors, each performed in triplicate. *, p < 0.05 as compared with unstimulated M0 macrophages in cells without HA treatment; #, p < 0.05 as compared with vehicle (veh)-treated, same initially polarized phenotype. Small amounts of LXA4 (32.6 ± 9.8 pg/ml; mean ± S.E. from three experiments) were detected only in M2 macrophages, without HA treatment. In the other cells, LXA4 was below the detection level.
DISCUSSION
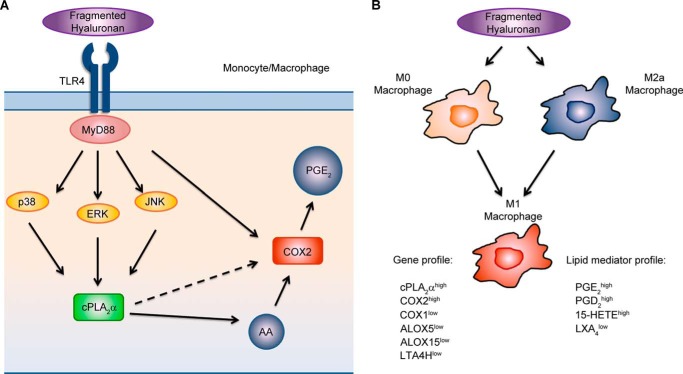
Increased HA levels and HA degradation products are found in bronchoalveolar lavage and in lung tissue of patients with asthma, allergic alveolitis, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, ARDS, and other acute and chronic lung diseases (6, 62). LMW HA increases uptake of oxidized low density lipoprotein into monocytes (63), and HA overproduction promotes atherosclerosis development in the aorta of ApoE-deficient mice (64). Serum HA levels correlate with poor blood glucose control and diabetic angiopathy (65) and are increased in patients with chronic renal failure (66), advanced liver cirrhosis, and in critically ill patients (67). Overproduction of HA is associated with different types of tumors and correlates with malignancy and poor prognosis (68, 69). A role for various macrophage- and epithelial cell-derived eicosanoids and activation of AA-releasing cPLA2α has been also established in many inflammatory and proliferative diseases (26, 56). cPLA2α knock-out mice used in models of asthma (27), pulmonary fibrosis (28), ARDS (29), multiple sclerosis (30), and rheumatoid arthritis (31) present with reduced symptoms when compared with wild-type mice (32). cPLA2α (70), COX2 (34), or PGE2 receptor EP2 (71) deficiency decreases the size and number of intestinal polyps in the experimental model of colon cancer, whereas cPLA2α depletion in bone marrow-derived macrophages protects against lung cancer progression and metastasis (33). Our current findings indicate a novel, direct link of ubiquitously present HA with cPLA2α and downstream lipid mediators both in murine macrophages and human primary monocytes and differentially polarized macrophages. We have shown here that lower molecular weight fragments of HA are potent stimulants of cPLA2α phosphorylation, AA, and PGE2 release and COX2 expression mainly through the TLR4 receptor in murine macrophages and in human primary monocytes. We also have demonstrated that LMW HA is a potent activator of PGE2, PGD2, 15-HETE production, and M1 polarization phenotype in human monocyte-derived macrophages (Fig. 12).
FIGURE 12.
Proposed model of HA-induced cPLA2α activation and eicosanoid production in monocytes and macrophages: impact of HA on macrophage polarization and acquiring eicosanoid-specific profile. A, fragmented HA stimulates cPLA2α activation, AA release, and downstream eicosanoid production through the TLR4/MYD88/ERK1/2, p38, and JNK pathway, independently of CD44, TLR2, RHAMM, or STAB2. B, fragmented HA potently polarizes M0 and M2a macrophages into the M1 macrophage phenotype with the unique PGE2/PGD2/15-HETEhigh and LXA4low eicosanoid profile. This profile is, at least partially, acquired through the pathway depicted in A. Please refer to the text for further details.
Liang et al. (6) showed that fibroblasts from asthmatic lungs produced more lower molecular weight hyaluronan either at baseline or after pro-inflammatory stimulation. They also found that asthmatic alveolar macrophages secreted significantly more IL-8 after HA treatment than macrophages from healthy controls (6). Giannattasio et al. (72) found that cPLA2α is the major enzyme, responsible for lipid mediator production in human lung macrophages. Our findings are in line with the above studies and add additional insight into pro-inflammatory potential of HA. First, we showed that in murine macrophages and primary human monocytes, LMW HA potently stimulates AA release (Fig. 2, A–D). Second, we demonstrated that this stimulation is entirely cPLA2α-dependent in both cell types (Fig. 2, E and F). Recent metabolomic approaches toward understanding asthma pathogenesis revealed that many AA-derived eicosanoids are significantly increased at baseline in bronchoalveolar lavage of asthmatics and are further elevated after allergen provocation (73). Among them, products of all three pathways of AA metabolism, leukotrienes, prostanoids, as well as a number of cytochrome P450 metabolites, followed that trend (73). In our previous work, we have also described increased cPLA2α expression in peripheral blood mononuclear cells in severe asthmatic patients (74). Likewise, cPLA2α expression is markedly increased in polyps of ApcΔ716-knock-out mice, a model of human familial adenomatous polyposis (70, 75). COX2-dependent synthesis of PGE2 is involved in the development of colorectal cancer (34). Here, we showed that after HA stimulation PGE2 is significantly increased, and this effect is entirely cPLA2α-dependent (Fig. 6, A–C). These findings suggest that elevated HA levels in bronchoalveolar lavage and lung tissue in asthma or its local overproduction by tumor environment might stimulate monocyte-derived or tissue-resident macrophages to release AA and AA-derived eicosanoids through activation of cPLA2α. HA has been reported to increase PGE2 production in human umbilical vein endothelial cells and in vivo in HA-treated mice, which was associated with simultaneous elevation of COX2 expression (76, 77). Here, we showed that, even though COX2 expression was significantly increased in human monocytes and BMDM (Fig. 8, A–D), PGE2 secretion was blocked in the presence of cPLA2α inhibitor (Fig. 6, A and B), indicating that cPLA2α is the rate-limiting enzyme in this cascade. HA-induced COX2 expression was TLR4/MYD88-dependent in our model (Fig. 8, B–D), which raises the possibility of its cPLA2α dependence, as we have shown previously in A549 cells (25). Currently, a preclinical evaluation study of a cPLA2α inhibitor in asthma treatment has been published, showing promising results using various in vitro and in vivo approaches (78). COX2 inhibitors are well known drugs used in prevention of various tumors (79). Our current results might consequently serve as an additional point toward consideration of cPLA2α blockade in chronic inflammatory and proliferative disorders.
There are at least five candidate receptors for HA on the surface of monocytes/macrophages that could hypothetically lead to cPLA2α activation and AA release (2). TLR2 and TLR4 have been previously shown to sense different fragments of HA (80, 81). However, differing effects have been demonstrated, and involvement of lipid mediators has not been studied. In Tlr2/Tlr4 double knockouts, bleomycin treatment causes exaggerated lung injury with impaired pro-inflammatory cell recruitment and decreased pro-inflammatory cell responses to low molecular weight HA (82). In Tlr4 knockouts, intratracheal installation of LMW HA augmented the lung injury score and also caused increased pro-inflammatory cell influx and production of IL-1β, TNFα, IL-6, and MIP-2 (83). We have determined here that knockdown of TLR4 significantly diminished HA-induced AA release (Fig. 4, A and C), cPLA2α, p38, ERK1/2, and JNK phosphorylation (Fig. 5, A–D), PGE2 production (Fig. 7, A and C), and COX2 expression (Fig. 8, B and C) in murine and human cells. CD44, described sometimes as a primary HA receptor, is a broadly expressed membrane glycoprotein. It has been shown to play a role in a wide range of processes, from signal transduction to controlling cell growth, differentiation, and survival (84). CD44 and TLR4 cooperation in response to HA has been previously demonstrated in the model of sterile injury (12). Taylor et al. (12) proposed the interesting concept of TLR4 association with CD44 and the co-receptor molecule MD2 leading to MYD88 and NF-κB activation. We have confirmed TLR4/MYD88 involvement in these processes using MYD88 siRNA-transfected RAW 264.7 cells and Myd88−/− BMDM (Figs. 4A, 7B, and 8D). However, we did not find any evidence of CD44 involvement in any of the analyzed processes, either in murine or in human cells. In CD44 siRNA-transfected cells and Cd44−/− BMDM, HA-induced effects were similar to control cells (Figs. 4, A and C, 5, A–D, 7, A and C, and 8, B and C). Moreover, simultaneous knockdown of TLR4 and CD44 did not have more of an effect than TLR4 knockdown alone (Figs. 4, A and C, 5, A–D, 7C, and 8B). As mentioned above, TLR2 has been also described as a potent HA receptor, with subsequent activation of the MYD88/NF-κB pathway (80, 82). However, we have not observed any changes after knockdown of TLR2 (Fig. 4A). The receptor for hyaluronic acid-mediated motility (RHAMM) is an HA-binding protein that is localized to the cell surface, cytoplasm, mitochondria, and nucleus (85, 86). Overexpression of RHAMM has been identified in many types of cancers and has been associated with a poor prognosis and metastasis (87). Expression of RHAMM is also highly increased in lung macrophages in noninfectious lung injury (88). Here, however, we have excluded involvement of this receptor in HA-induced lipid mediator activation (Fig. 4A). Stabilin-2 (STAB2, also called HARE for hyaluronan receptor for endocytosis) is weakly expressed by human primary monocyte-derived macrophages but not by RAW 246.7 cells (89). It is mainly involved in macrophage phagocytosis (89). 40–400-kDa HA was shown to act through this receptor to activate NF-κB signaling and ERK1/2 activation (90). In our hands however, this receptor was not involved in the HA-mediated AA release (Fig. 4E). Taken together, this suggests that indeed the activation of TLR4/MYD88 and downstream MAPKs is the primary mechanism of HA-induced cPLA2α activation and eicosanoid production in human primary monocytes and mouse macrophages.
We have determined that AA release was specifically induced by the mix of HA fragments falling into the category of LMW HA. Smaller or larger HA fragments did not induce AA release nor did they inhibit LMW-HA-induced AA release (Fig. 1D). Oligo-HA has been reported to act through TLR4 and activate specific signaling events (91) or through TLR3 and inhibit TLR4-mediated signals (92). In some studies, HMW-HA was shown to inhibit LMW HA-mediated TLR2 signaling but not TLR4 mediated signaling (80). In others, it was capable of reducing LPS-mediated events (93), mainly in a CD44-dependent manner (94). However, the exact mechanisms of these processes and discrepancies are not known. Taking into consideration that in our study HMW HA showed a trend to increase LMW HA-induced AA release, it might suggest that not only the “core” size of HA, but the ratios of different sizes might have an impact on specific signaling events. Usually, such a mix is present at the site of inflammation. There are also different cells responding to these fragments. Therefore, further studies are needed to clarify this problem.
We and others have shown in monocytes and macrophages that cPLA2α phosphorylation might be caused by various MAPKs (39, 95). We found here that HA-induced cPLA2α activation is associated with ERK1/2, p38, and JNK activation (Fig. 3, B and C). Interestingly, the time course of ERK1/2 phosphorylation was different from other studies showing TLR4 involvement (39, 96). Even though the peak phosphorylation of all three MAPKs was at 30 min, ERK1/2 phosphorylation tended to be sustained up to 6 h, both in murine macrophages and in human monocytes (Fig. 3, B and C), which points out the HA-specific mode of TLR4 activation, different from classic TLR4 stimuli such as LPS (39, 96). Independent inhibition of all these kinases significantly decreased HA-induced AA release, with the most prominent effect of ERK1/2 inhibitor (Fig. 3D). Yet only the simultaneous inhibition of three of these MAPKs resulted in complete blockade of HA-induced AA release, suggesting redundancy among them (Fig. 3D). HA-induced p38 phosphorylation has been previously described as an effect of HA signaling through CD44 (97). HA-induced ERK1/2 phosphorylation has been also previously observed, mainly as an effect driven through RHAMM (98) and/or RHAMM and EGF receptor cooperation (99), via CD44 and EGF receptor interaction (100) or STAB2 (90). Here, we showed that after TLR4 but not CD44 knockdown, ERK1/2, p38, and JNK phosphorylation was decreased in response to HA treatment (Fig. 5, C and D). This suggests that in human primary monocytes and mouse macrophages, MAPK phosphorylation and downstream effects on cPLA2α and cPLA2α-induced effects are mainly TLR4-dependent.
Recent studies have brought significant progress in understanding macrophage biology from re-defining their diverse origins, description of transcriptional complexity, as well as the ability of phenotypic switching in response to environmental stimuli in maintaining homeostasis and in the disease pathogenesis (41). Having demonstrated the mechanism of LMW HA stimulation on cPLA2α and cPLA2α-derived lipid signaling in mouse macrophages and human primary monocytes, we decided to further study LMW HA effects in human primary macrophages. We focused on unexplored lipid metabolism gene expression and eicosanoid production. At baseline levels, in accordance with the pioneer findings in human polarized macrophages (61), we observed opposing gene expression of the main enzymes involved in eicosanoid metabolism in M1 and M2 macrophages (Fig. 10, A–F), complementing these results by characterization of enzyme-specific eicosanoid release (Fig. 11, A–D). Increase of PGE2 production in M1 macrophages, apart from COX2 induction, might also be related to up-regulation of mPGES in macrophages (101). Next, we noted the potency of LMW HA to switch the phenotype of macrophages toward the M1 phenotype, starting from untreated M0 cells, increasing “typical” gene expression in initially M1-polarized cells, as well as from M2 phenotype (Fig. 9, A–H). The same was observed in terms of eicosanoid enzymes (Fig. 10, A–F) and lipid profiles (Fig 11, A–D). The increased presence of LMW HA at the site of tissue injury or initial inflammatory response might be therefore one of the evolutionary mechanisms activating infiltrating monocytes as well as tissue-resident macrophages to actively fight the insult. Induction of IL-10 by LMW HA in the same macrophages (Fig. 9I) might, under these conditions, serve as self-limiting loop. IL-10 could possibly begin quieting the initial response and can be secondary to the increase in PGE2 (102), followed by a rise in cAMP signaling leading to development of a pro-resolution macrophage phenotype (103). However, in the scenario of prolonged inflammation, the potency of overproduced LMW HA to promote the M1 macrophage phenotype, increase of PGE2, PGD2, and 15-HETE, and a decrease of anti-inflammatory and pro-resolving lipoxin A4 (LXA4), might be an additional factor potentiating the chronicity of the inflammatory response. Interestingly, it was shown that short (18 h) stimulation of human monocytes with 50–200-kDa HA fragments elicited their potent activation, while prolonged (6 days) culture in these conditions induced suppressive M2 macrophage phenotype (104). Kuang et al. (104) presented these results as one of the potential mechanisms of solid tumors to inhibit inflammatory macrophage responses and activation of M2-like tumor-associated macrophages. The clear difference in our findings might come from the modification of culture conditions (6 h versus 6 days), which in fact can suggest a time-dependent effect of LMW HA in terms of cancer biology. Pro-inflammatory M1 macrophages, which might come from temporary but recurrent increases in LMW HA, have been shown to play a role in tumor initiation and promotion (105). Once the tumor is established and LMW HA is chronically overproduced, it can promote immunosuppressive M2-like tumor-associated macrophage development (104), potentially through PGE2 and IL-10 signaling as observed in our model. It has also been shown that HA of different sulfation states (106) or covalently linked with the heavy chain 1 of inter-α-trypsin inhibitor (HC-HA) promotes M2 macrophage polarization (107) and acts in an anti-inflammatory manner.
In conclusion, we have shown here that LMW HA stimulates cPLA2α activation, AA release, and downstream eicosanoid production in murine macrophages and in human primary monocytes through the TLR4/MYD88/ERK1/2, p38, and JNK pathway, independently of CD44, TLR2, RHAMM, or STAB2. Moreover, we demonstrated that LMW HA potently activated an M1 macrophage phenotype with the unique PGE2/PGD2/15-HETEhigh and LXA4low eicosanoid profile (Fig. 12). We have performed a thorough, translational in vitro approach to address our hypothesis simultaneously in a murine and human system. These findings, in the context of recent discoveries on the role of extracellular matrix in inflammatory and proliferative diseases, provide novel insight into potential mechanisms of lipid mediators and their precursors in the pathogenesis of these diseases.
Acknowledgment
We thank Yee Chan-Li for help with animal tissue processing.
This work was supported, in whole or in part, by National Institutes of Health intramural program.
This article was selected as a Paper of the Week.
- HMW
- high molecular weight
- 15-HETE
- 15-hydroxyeicosatetraenoic acid
- AA
- arachidonic acid
- BMDM
- bone marrow-derived macrophage
- COX
- cyclooxygenase
- cPLA2α
- cytosolic phospholipase A2 group IVA
- HA
- hyaluronan
- LMW
- low molecular weight
- LTB4 and C4
- leukotriene B4 and C4
- LXA4
- lipoxin A4
- MDM
- monocyte-derived macrophage
- PGE2 and D2
- prostaglandin E2 and D2
- RHAMM
- receptor for hyaluronan-mediated motility
- PG
- prostaglandin
- ARDS
- acute respiratory distress syndrome.
REFERENCES
- 1. Jiang D., Liang J., Noble P. W. (2011) Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erickson M., Stern R. (2012) Chain gangs: new aspects of hyaluronan metabolism. Biochem. Res. Int. 2012, 893947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tammi M. I., Day A. J., Turley E. A. (2002) Hyaluronan and homeostasis: a balancing act. J. Biol. Chem. 277, 4581–4584 [DOI] [PubMed] [Google Scholar]
- 4. Isnard N., Legeais J. M., Renard G., Robert L. (2001) Effect of hyaluronan on MMP expression and activation. Cell Biol. Int. 25, 735–739 [DOI] [PubMed] [Google Scholar]
- 5. Saari H., Konttinen Y. T. (1989) Determination of synovial-fluid hyaluronate concentration and polymerization by high performance liquid-chromatography. Ann. Rheum. Dis. 48, 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liang J., Jiang D., Jung Y., Xie T., Ingram J., Church T., Degan S., Leonard M., Kraft M., Noble P. W. (2011) Role of hyaluronan and hyaluronan-binding proteins in human asthma. J. Allergy Clin. Immunol. 128, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chajara A., Raoudi M., Delpech B., Leroy M., Basuyau J. P., Levesque H. (2000) Increased hyaluronan and hyaluronidase production and hyaluronan degradation in injured aorta of insulin-resistant rats. Arterioscler Thromb. Vasc. Biol. 20, 1480–1487 [DOI] [PubMed] [Google Scholar]
- 8. George J., Stern R. (2004) Serum hyaluronan and hyaluronidase: very early markers of toxic liver injury. Clin. Chim. Acta 348, 189–197 [DOI] [PubMed] [Google Scholar]
- 9. Horton M. R., McKee C. M., Bao C., Liao F., Farber J. M., Hodge-DuFour J., Puré E., Oliver B. L., Wright T. M., Noble P. W. (1998) Hyaluronan fragments synergize with interferon-γ to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J. Biol. Chem. 273, 35088–35094 [DOI] [PubMed] [Google Scholar]
- 10. Powell J. D., Horton M. R. (2005) Threat matrix: low molecular-weight hyaluronan (HA) as a danger signal. Immunol. Res. 31, 207–218 [DOI] [PubMed] [Google Scholar]
- 11. Yamasaki K., Muto J., Taylor K. R., Cogen A. L., Audish D., Bertin J., Grant E. P., Coyle A. J., Misaghi A., Hoffman H. M., Gallo R. L. (2009) NLRP3/cryopyrin is necessary for interleukin-1β (IL-1β) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 284, 12762–12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 13. Frey H., Schroeder N., Manon-Jensen T., Iozzo R. V., Schaefer L. (2013) Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 280, 2165–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourguignon L. Y., Singleton P. A., Zhu H., Diedrich F. (2003) Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J. Biol. Chem. 278, 29420–29434 [DOI] [PubMed] [Google Scholar]
- 15. Kouvidi K., Berdiaki A., Nikitovic D., Katonis P., Afratis N., Hascall V. C., Karamanos N. K., Tzanakakis G. N. (2011) Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J. Biol. Chem. 286, 38509–38520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyce J. A. (2008) Eicosanoids in asthma, allergic inflammation, and host defense. Curr. Mol. Med. 8, 335–349 [DOI] [PubMed] [Google Scholar]
- 17. Lima I. V., Bastos L. F., Limborco M., Fiebich B. L., de Oliveira A. C. (2012) Role of prostaglandins in neuroinflammatory and neurodegenerative diseases. Mediat. Inflamm. 10.1155/2012/946813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D., Dubois R. N. (2010) Eicosanoids and cancer. Nat. Rev. Cancer 10, 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menter D. G., Schilsky R. L., DuBois R. N. (2010) Cyclooxygenase-2 and cancer treatment: Understanding the risk should be worth the reward. Clin. Cancer Res. 16, 1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serhan C. N., Petasis N. A. (2011) Resolvins and protectins in inflammation resolution. Chem Rev. 111, 5922–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buckley C. D., Gilroy D. W., Serhan C. N., Stockinger B., Tak P. P. (2013) The resolution of inflammation. Nat. Rev. Immunol. 13, 59–66 [DOI] [PubMed] [Google Scholar]
- 22. Dennis E. A., Cao J., Hsu Y. H., Magrioti V., Kokotos G. (2011) Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111, 6130–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 24. Yang J. S., Valente C., Polishchuk R. S., Turacchio G., Layre E., Moody D. B., Leslie C. C., Gelb M. H., Brown W. J., Corda D., Luini A., Hsu V. W. (2011) COPI acts in both vesicular and tubular transport. Nat. Cell Biol. 13, 996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawliczak R., Logun C., Madara P., Lawrence M., Woszczek G., Ptasinska A., Kowalski M. L., Wu T., Shelhamer J. H. (2004) Cytosolic phospholipase A2 Group IVα but not secreted phospholipase A2 Group IIA, V, or X induces interleukin-8 and cyclooxygenase-2 gene and protein expression through peroxisome proliferator-activated receptors γ 1 and 2 in human lung cells. J. Biol. Chem. 279, 48550–48561 [DOI] [PubMed] [Google Scholar]
- 26. Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. (2011) Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog. Lipid Res. 50, 152–192 [DOI] [PubMed] [Google Scholar]
- 27. Uozumi N., Kume K., Nagase T., Nakatani N., Ishii S., Tashiro F., Komagata Y., Maki K., Ikuta K., Ouchi Y., Miyazaki J., Shimizu T. (1997) Role of cytosolic phospholipase A2 in allergic response and parturition. Nature 390, 618–622 [DOI] [PubMed] [Google Scholar]
- 28. Nagase T., Uozumi N., Ishii S., Kita Y., Yamamoto H., Ohga E., Ouchi Y., Shimizu T. (2002) A pivotal role of cytosolic phospholipase A(2) in bleomycin-induced pulmonary fibrosis. Nat. Med. 8, 480–484 [DOI] [PubMed] [Google Scholar]
- 29. Nagase T., Uozumi N., Ishii S., Kume K., Izumi T., Ouchi Y., Shimizu T. (2000) Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat. Immunol. 1, 42–46 [DOI] [PubMed] [Google Scholar]
- 30. Marusic S., Leach M. W., Pelker J. W., Azoitei M. L., Uozumi N., Cui J., Shen M. W., DeClercq C. M., Miyashiro J. S., Carito B. A., Thakker P., Simmons D. L., Leonard J. P., Shimizu T., Clark J. D. (2005) Cytosolic phospholipase A2 α-deficient mice are resistant to experimental autoimmune encephalomyelitis. J. Exp. Med. 202, 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hegen M., Sun L., Uozumi N., Kume K., Goad M. E., Nickerson-Nutter C. L., Shimizu T., Clark J. D. (2003) Cytosolic phospholipase A2α-deficient mice are resistant to collagen-induced arthritis. J. Exp. Med. 197, 1297–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sapirstein A., Bonventre J. V. (2000) Specific physiological roles of cytosolic phospholipase A(2) as defined by gene knockouts. Biochim. Biophys. Acta 1488, 139–148 [DOI] [PubMed] [Google Scholar]
- 33. Weiser-Evans M. C., Wang X. Q., Amin J., Van Putten V., Choudhary R., Winn R. A., Scheinman R., Simpson P., Geraci M. W., Nemenoff R. A. (2009) Depletion of cytosolic phospholipase A(2) in bone marrow-derived macrophages protects against lung cancer progression and metastasis. Cancer Res. 69, 1733–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oshima M., Dinchuk J. E., Kargman S. L., Oshima H., Hancock B., Kwong E., Trzaskos J. M., Evans J. F., Taketo M. M. (1996) Suppression of intestinal polyposis in Apc(Δ716) knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87, 803–809 [DOI] [PubMed] [Google Scholar]
- 35. Jiang Y. J., Lu B., Choy P. C., Hatch G. M. (2003) Regulation of cytosolic phospholipase A2, cyclooxygenase-1 and -2 expression by PMA, TNFα, LPS, and M-CSF in human monocytes and macrophages. Mol. Cell. Biochem. 246, 31–38 [PubMed] [Google Scholar]
- 36. Wu T., Levine S. J., Lawrence M. G., Logun C., Angus C. W., Shelhamer J. H. (1994) Interferon-γ induces the synthesis and activation of cytosolic phospholipase A2. J. Clin. Invest. 93, 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casas J., Gijón M. A., Vigo A. G., Crespo M. S., Balsinde J., Balboa M. A. (2006) Phosphatidylinositol 4,5-bisphosphate anchors cytosolic group IVA phospholipase A2 to perinuclear membranes and decreases its calcium requirement for translocation in live cells. Mol. Biol. Cell 17, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pettus B. J., Bielawska A., Subramanian P., Wijesinghe D. S., Maceyka M., Leslie C. C., Evans J. H., Freiberg J., Roddy P., Hannun Y. A., Chalfant C. E. (2004) Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 279, 11320–11326 [DOI] [PubMed] [Google Scholar]
- 39. Qi H. Y., Shelhamer J. H. (2005) Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 280, 38969–38975 [DOI] [PubMed] [Google Scholar]
- 40. Chen L. Y., Woszczek G., Nagineni S., Logun C., Shelhamer J. H. (2008) Cytosolic phospholipase A2α activation induced by S1P is mediated by the S1P3 receptor in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L326–L335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wynn T. A., Chawla A., Pollard J. W. (2013) Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yona S., Kim K. W., Wolf Y., Mildner A., Varol D., Breker M., Strauss-Ayali D., Viukov S., Guilliams M., Misharin A., Hume D. A., Perlman H., Malissen B., Zelzer E., Jung S. (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jenkins S. J., Ruckerl D., Cook P. C., Jones L. H., Finkelman F. D., van Rooijen N., MacDonald A. S., Allen J. E. (2011) Local macrophage proliferation, rather than recruitment from the blood, is a signature of T(H)2 inflammation. Science 332, 1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mantovani A., Biswas S. K., Galdiero M. R., Sica A., Locati M. (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229, 176–185 [DOI] [PubMed] [Google Scholar]
- 45. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 47. Sica A., Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gerber J. S., Mosser D. M. (2001) Reversing lipopolysaccharide toxicity by ligating the macrophage Fc γ receptors. J. Immunol. 166, 6861–6868 [DOI] [PubMed] [Google Scholar]
- 50. Anderson C. F., Mosser D. M. (2002) A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukocyte Biol. 72, 101–106 [PubMed] [Google Scholar]
- 51. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 52. Collins S. L., Black K. E., Chan-Li Y., Ahn Y. H., Cole P. A., Powell J. D., Horton M. R. (2011) Hyaluronan fragments promote inflammation by down-regulating the anti-inflammatory A2a receptor. Am. J. Respir. Cell Mol. Biol. 45, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maharjan A. S., Pilling D., Gomer R. H. (2011) High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One 6, e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cowman M. K., Chen C. C., Pandya M., Yuan H., Ramkishun D., LoBello J., Bhilocha S., Russell-Puleri S., Skendaj E., Mijovic J., Jing W. (2011) Improved agarose gel electrophoresis method and molecular mass calculation for high molecular mass hyaluronan. Anal. Biochem. 417, 50–56 [DOI] [PubMed] [Google Scholar]
- 55. Pawliczak R., Huang X. L., Nanavaty U. B., Lawrence M., Madara P., Shelhamer J. H. (2002) Oxidative stress induces arachidonate release from human lung cells through the epithelial growth factor receptor pathway. Am. J. Respir. Cell Mol. Biol. 27, 722–731 [DOI] [PubMed] [Google Scholar]
- 56. Ghosh M., Tucker D. E., Burchett S. A., Leslie C. C. (2006) Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 45, 487–510 [DOI] [PubMed] [Google Scholar]
- 57. Letsiou E., Kitsiouli E., Nakos G., Lekka M. E. (2011) Mild stretch activates cPLA2 in alveolar type II epithelial cells independently through the MEK/ERK and PI3K pathways. Biochim. Biophys. Acta 1811, 370–376 [DOI] [PubMed] [Google Scholar]
- 58. Suram S., Gangelhoff T. A., Taylor P. R., Rosas M., Brown G. D., Bonventre J. V., Akira S., Uematsu S., Williams D. L., Murphy R. C., Leslie C. C. (2010) Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J. Biol. Chem. 285, 30676–30685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Walters J. N., Bickford J. S., Beachy D. E., Newsom K. J., Herlihy J. D., Peck M. V., Qiu X., Nick H. S. (2011) cPLA(2) α gene activation by IL-1β is dependent on an upstream kinase pathway, enzymatic activation and downstream 15-lipoxygenase activity: a positive feedback loop. Cell. Signal. 23, 1944–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mancini A. D., Di Battista J. A. (2011) The cardinal role of the phospholipase A(2)/cyclooxygenase-2/prostaglandin E synthase/prostaglandin E(2) (PCPP) axis in inflammostasis. Inflamm. Res. 60, 1083–1092 [DOI] [PubMed] [Google Scholar]
- 61. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 62. Lennon F. E., Singleton P. A. (2011) Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am. J. Physiol. Lung Cell. Mol. Physiol. 301, L137–L147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tabata T., Mine S., Okada Y., Tanaka Y. (2007) Low molecular weight hyaluronan increases the uptaking of oxidized LDL into monocytes. Endocr. J. 54, 685–693 [DOI] [PubMed] [Google Scholar]
- 64. Chai S., Chai Q., Danielsen C. C., Hjorth P., Nyengaard J. R., Ledet T., Yamaguchi Y., Rasmussen L. M., Wogensen L. (2005) Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ. Res. 96, 583–591 [DOI] [PubMed] [Google Scholar]
- 65. Mine S., Okada Y., Kawahara C., Tabata T., Tanaka Y. (2006) Serum hyaluronan concentration as a marker of angiopathy in patients with diabetes mellitus. Endocr. J. 53, 761–766 [DOI] [PubMed] [Google Scholar]
- 66. Woodrow G., Turney J. H., Davison A. M., Cooper E. H. (1996) Serum hyaluronan concentrations predict survival in patients with chronic renal failure on maintenance haemodialysis. Nephrol. Dial. Transplant. 11, 98–100 [PubMed] [Google Scholar]
- 67. Yagmur E., Koch A., Haumann M., Kramann R., Trautwein C., Tacke F. (2012) Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clin. Biochem. 45, 82–87 [DOI] [PubMed] [Google Scholar]
- 68. Toole B. P. (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528–539 [DOI] [PubMed] [Google Scholar]
- 69. Anttila M. A., Tammi R. H., Tammi M. I., Syrjänen K. J., Saarikoski S. V., Kosma V. M. (2000) High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 60, 150–155 [PubMed] [Google Scholar]
- 70. Hong K. H., Bonventre J. C., O'Leary E., Bonventre J. V., Lander E. S. (2001) Deletion of cytosolic phospholipase A(2) suppresses Apc(Min)-induced tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 3935–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sonoshita M., Takaku K., Sasaki N., Sugimoto Y., Ushikubi F., Narumiya S., Oshima M., Taketo M. M. (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc(Δ716) knockout mice. Nat. Med. 7, 1048–1051 [DOI] [PubMed] [Google Scholar]
- 72. Giannattasio G., Lai Y., Granata F., Mounier C. M., Nallan L., Oslund R., Leslie C. C., Marone G., Lambeau G., Gelb M. H., Triggiani M. (2009) Expression of phospholipases A2 in primary human lung macrophages: role of cytosolic phospholipase A2-α in arachidonic acid release and platelet activating factor synthesis. Biochim. Biophys. Acta 1791, 92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lundström S. L., Yang J., Källberg H. J., Thunberg S., Gafvelin G., Haeggström J. Z., Grönneberg R., Grunewald J., van Hage M., Hammock B. D., Eklund A., Wheelock Å. M., Wheelock C. E. (2012) Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS One 7, e33780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sokolowska M., Stefanska J., Wodz-Naskiewicz K., Cieslak M., Pawliczak R. (2010) Cytosolic phospholipase A2 group IVA is overexpressed in patients with persistent asthma and regulated by the promoter microsatellites. J. Allergy Clin. Immunol. 125, 1393–1395 [DOI] [PubMed] [Google Scholar]
- 75. Takaku K., Sonoshita M., Sasaki N., Uozumi N., Doi Y., Shimizu T., Taketo M. M. (2000) Suppression of intestinal polyposis in Apc(Δ716) knockout mice by an additional mutation in the cytosolic phospholipase A(2) gene. J. Biol. Chem. 275, 34013–34016 [DOI] [PubMed] [Google Scholar]
- 76. Murphy J. F., Lennon F., Steele C., Kelleher D., Fitzgerald D., Long A. C. (2005) Engagement of CD44 modulates cyclooxygenase induction, VEGF generation, and proliferation in human vascular endothelial cells. FASEB J. 19, 446–448 [DOI] [PubMed] [Google Scholar]
- 77. Riehl T. E., Foster L., Stenson W. F. (2012) Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G309–G316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hewson C. A., Patel S., Calzetta L., Campwala H., Havard S., Luscombe E., Clarke P. A., Peachell P. T., Matera M. G., Cazzola M., Page C., Abraham W. M., Williams C. M., Clark J. D., Liu W. L., Clarke N. P., Yeadon M. (2012) Preclinical evaluation of an inhibitor of cytosolic phospholipase A2α for the treatment of asthma. J. Pharmacol. Exp. Ther. 340, 656–665 [DOI] [PubMed] [Google Scholar]
- 79. Harris R. E., Beebe-Donk J., Doss H., Burr Doss D. (2005) Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: A critical review of non-selective COX-2 blockade (review). Oncol. Rep. 13, 559–583 [PubMed] [Google Scholar]
- 80. Scheibner K. A., Lutz M. A., Boodoo S., Fenton M. J., Powell J. D., Horton M. R. (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 177, 1272–1281 [DOI] [PubMed] [Google Scholar]
- 81. Taylor K. R., Trowbridge J. M., Rudisill J. A., Termeer C. C., Simon J. C., Gallo R. L. (2004) Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J. Biol. Chem. 279, 17079–17084 [DOI] [PubMed] [Google Scholar]
- 82. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 83. Zhao H., Leu S. W., Shi L., Dedaj R., Zhao G., Garg H. G., Shen L., Lien E., Fitzgerald K. A., Shiedlin A., Shen H., Quinn D. A., Hales C. A. (2010) TLR4 is a negative regulator in noninfectious lung inflammation. J. Immunol. 184, 5308–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ponta H., Sherman L., Herrlich P. A. (2003) CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 4, 33–45 [DOI] [PubMed] [Google Scholar]
- 85. Joukov V., Groen A. C., Prokhorova T., Gerson R., White E., Rodriguez A., Walter J. C., Livingston D. M. (2006) The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell 127, 539–552 [DOI] [PubMed] [Google Scholar]
- 86. Turley E. A., Noble P. W., Bourguignon L. Y. (2002) Signaling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 [DOI] [PubMed] [Google Scholar]
- 87. Zlobec I., Terracciano L., Tornillo L., Günthert U., Vuong T., Jass J. R., Lugli A. (2008) Role of RHAMM within the hierarchy of well established prognostic factors in colorectal cancer. Gut 57, 1413–1419 [DOI] [PubMed] [Google Scholar]
- 88. Zaman A., Cui Z., Foley J. P., Zhao H., Grimm P. C., Delisser H. M., Savani R. C. (2005) Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am. J. Respir. Cell Mol. Biol. 33, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Park S. Y., Jung M. Y., Kim H. J., Lee S. J., Kim S. Y., Lee B. H., Kwon T. H., Park R. W., Kim I. S. (2008) Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 15, 192–201 [DOI] [PubMed] [Google Scholar]
- 90. Pandey M. S., Baggenstoss B. A., Washburn J., Harris E. N., Weigel P. H. (2013) The hyaluronan receptor for endocytosis (HARE) activates NF-κB-mediated gene expression in response to 40–400-kDa, but not smaller or larger, hyaluronans. J. Biol. Chem. 288, 14068–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., Simon J. C. (2002) Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim M. Y., Muto J., Gallo R. L. (2013) Hyaluronic acid oligosaccharides suppress TLR3-dependent cytokine expression in a TLR4-dependent manner. PLoS One 8, e72421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Austin J. W., Gilchrist C., Fehlings M. G. (2012) High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. J. Neurochem. 122, 344–355 [DOI] [PubMed] [Google Scholar]
- 94. Liang J., Jiang D., Griffith J., Yu S., Fan J., Zhao X., Bucala R., Noble P. W. (2007) CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J. Immunol. 178, 2469–2475 [DOI] [PubMed] [Google Scholar]
- 95. Lee C. W., Lee I. T., Lin C. C., Lee H. C., Lin W. N., Yang C. M. (2010) Activation and induction of cytosolic phospholipase A2 by IL-1β in human tracheal smooth muscle cells: role of MAPKs/p300 and NF-κB. J. Cell. Biochem. 109, 1045–1056 [DOI] [PubMed] [Google Scholar]
- 96. Bhattacharyya S., Brown D. E., Brewer J. A., Vogt S. K., Muglia L. J. (2007) Macrophage glucocorticoid receptors regulate Toll-like receptor 4-mediated inflammatory responses by selective inhibition of p38 MAP kinase. Blood 109, 4313–4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pivetta E., Scapolan M., Wassermann B., Steffan A., Colombatti A., Spessotto P. (2011) Blood-derived human osteoclast resorption activity is impaired by hyaluronan-CD44 engagement via a p38-dependent mechanism. J. Cell. Physiol. 226, 769–779 [DOI] [PubMed] [Google Scholar]
- 98. Zhang S., Chang M. C., Zylka D., Turley S., Harrison R., Turley E. A. (1998) The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J. Biol. Chem. 273, 11342–11348 [DOI] [PubMed] [Google Scholar]
- 99. Entwistle J., Hall C. L., Turley E. A. (1996) HA receptors: regulators of signalling to the cytoskeleton. J. Cell. Biochem. 61, 569–577 [DOI] [PubMed] [Google Scholar]
- 100. Meran S., Luo D. D., Simpson R., Martin J., Wells A., Steadman R., Phillips A. O. (2011) Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J. Biol. Chem. 286, 17618–17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mosca M., Polentarutti N., Mangano G., Apicella C., Doni A., Mancini F., De Bortoli M., Coletta I., Polenzani L., Santoni G., Sironi M., Vecchi A., Mantovani A. (2007) Regulation of the microsomal prostaglandin E synthase-1 in polarized mononuclear phagocytes and its constitutive expression in neutrophils. J. Leukocyte Biol. 82, 320–326 [DOI] [PubMed] [Google Scholar]
- 102. MacKenzie K. F., Clark K., Naqvi S., McGuire V. A., Nöehren G., Kristariyanto Y., van den Bosch M., Mudaliar M., McCarthy P. C., Pattison M. J., Pedrioli P. G., Barton G. J., Toth R., Prescott A., Arthur J. S. (2013) PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J. Immunol. 190, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bystrom J., Evans I., Newson J., Stables M., Toor I., van Rooijen N., Crawford M., Colville-Nash P., Farrow S., Gilroy D. W. (2008) Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood 112, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuang D. M., Wu Y., Chen N., Cheng J., Zhuang S. M., Zheng L. (2007) Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 110, 587–595 [DOI] [PubMed] [Google Scholar]
- 105. Balkwill F. R., Mantovani A. (2012) Cancer-related inflammation: common themes and therapeutic opportunities. Semin. Cancer Biol. 22, 33–40 [DOI] [PubMed] [Google Scholar]
- 106. Franz S., Allenstein F., Kajahn J., Forstreuter I., Hintze V., Möller S., Simon J. C. (2013) Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan promote phenotypic and functional modulation of human pro-inflammatory M1 macrophages. Acta Biomater. 9, 5621–5629 [DOI] [PubMed] [Google Scholar]
- 107. He H., Zhang S., Tighe S., Son J., Tseng S. C. (2013) Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J. Biol. Chem. 288, 25792–25803 [DOI] [PMC free article] [PubMed] [Google Scholar]