New developments in the diagnosis and treatment of pelvic floor disorders have created a need for a concise synopsis for clinicians. Our aim is to critically review new diagnostic and treatment innovations rather than to comprehensively review this field.
Defecatory Disorders
It is now widely appreciated that there are 2 principal etiologies for symptoms of constipation: delayed transit through the colon and impaired evacuation of the rectum. Impaired rectal evacuation can result from mechanical obstruction (eg, from rectal cancer or intussusception of the mucosa), but the more commonly encountered causes are inadequate rectal propulsion owing to a failure to increase intrarectal pressure during evacuation, or paradoxical contraction or impaired ability to relax the pelvic floor muscles during defecation. “Disordered defecation” is an umbrella term meant to encompass the last 2 causes of dysfunctional evacuation.1
Etiology and Pathophysiology
Disordered defecation is often viewed as maladaptive learning of sphincter contraction motivated by avoidance of pain or trauma.2 However, alternative etiologies and pathophysiologic mechanisms have been suggested, including rectal hyposensitivity,3 perineal laxity manifested by excessive perineal descent,4 and delayed colonic transit.5,6Rectal hyposensitivity and delayed transit may be consequences rather than causes of obstructed defecation because they improve after successful biofeedback treatment.6
Clinical Evaluation
Symptoms of excessive straining, anal digitation, and a sense of anal blockage strongly suggest disordered defecation. Rectal examination findings of high anal canal resting pressure, reduced or increased perineal descent, and paradoxical contraction when instructed to strain to defecate are also suggestive, but lack specificity.
Diagnostic assessment
The Rome criteria for diagnosis of disordered defecation include symptoms of chronic constipation consistent with the diagnosis of functional constipation7 plus at least 2 of 3 physiologic signs:1 (1) inadequate intra-abdominal pressure during straining, (2) incomplete evacuation of the rectum, and/or (3) <20% relaxation of anal canal pressures or pelvic floor electromyographic (EMG) activity during straining. In most patients, anorectal manometry and a rectal balloon expulsion test suffice. When these tests are discrepant or differ from the clinical impression, defecography or pelvic floor imaging may be useful.
Management
Biofeedback—a learning-based treatment that relies on providing electronically augmented feedback to help patients learn how to relax or contract muscles at appropriate times to reduce symptoms—was proposed for the treatment of disordered defecation soon after the discovery of this type of constipation.8 Until recently, however, biofeedback was applied haphazardly and with inconsistent results, partly because of 2 widely held beliefs: (1) that disordered defecation and slow transit constipation frequently overlap with no clear distinction between them,9 and (2) that biofeedback is just as effective for slow transit constipation as it is for disordered defecation.10 A study by Chiarioni et al6 corrected these misperceptions. This team recruited 52 patients, all of whom had delayed transit on a Sitzmark test, and then used anorectal manometry and balloon defecation tests to identify a subgroup of 34 who also had disordered defecation. They treated all these patients with 5 sessions of biofeedback to teach relaxation of the pelvic floor muscles during defecation and showed that 71% of patients with dyssynergic defecation achieved adequate relief of constipation and 76% had ≥3 bowel movements per week after biofeedback training, compared with 8% of patients with slow transit only. They also showed that transit times improved and were within the normal range after biofeedback for 65% of patients with disordered defecation but just 8% of the slow transit only group. Thus, this study showed that (1) the only constipated patients who are likely to benefit from this type of biofeedback treatment are those who have disordered defecation as evidenced by failure to evacuate a 50-mL, water-filled balloon and/or failure to relax pelvic floor muscles when straining to defecate and (2) the confusion over whether biofeedback also benefits patients with slow transit constipation is likely due to the fact that disordered defecation may secondarily delay transit, with normalization after biofeedback to eliminate dyssynergia.
Three new randomized, controlled trials (RCTs) provide compelling evidence that biofeedback is an effective treatment for disordered defecation in adults. One study compared biofeedback with a laxative, polyethylene glycol11; a second compared biofeedback with sham feedback12; and the third compared it with a muscle relaxer, diazepam.13 Another RCT made the interesting observation that successful muscle retraining can be accomplished without electronic feedback provided the therapist substitutes verbal feedback on performance and praise for success based on ongoing digital rectal examination.14
These 4 RCTs resulted in standardization of the biofeedback training protocol15 and recognition that the skill and experience of the therapist and the patient’s motivation are critical factors. Successful protocols have employed 5–6 training sessions lasting 30–60 minutes and spaced 2 weeks apart. Training sessions should include (1) patient education about the normal physiology of defecation and what the patient is doing wrong; (2) straining training, which involves showing the patient how to increase intra-abdominal pressure appropriately; (3) using electronic feedback of pelvic floor EMG or anal canal pressures to show the patient how to relax pelvic floor muscles when straining; and (4) practice of simulated defecation, usually accomplished by having them defecate an air-filled balloon while the therapist assists by pulling on a catheter attached to the balloon. (5) Some centers also include sensory training to teach the patient how to recognize weaker sensations of rectal filling. Although this biofeedback training protocol has been successful in all recent RCTs in adults, it does not seem to be more effective than laxatives in children,16 possibly because children lack the sustained attention and motivation that is required for biofeedback training.
Other Approaches to Treatment
In children, disordered defecation is called functional fecal retention, and treatment recommendations include dietary changes, use of laxatives, and cognitive and behavioral interventions to decrease phobia of the toilet.17 When these conservative measures fail, investigational treatments have included botulinum toxin injection into the puborectalis muscle, partial division of the puborectalis, and myectomy. Botulinum produced better short-term outcomes than biofeedback in 1 study,18 and short-term improvements comparable with partial division of the puborectalis19 or myectomy20 in other studies. An uncontrolled study21 suggests that botulinum may also improve disordered defecation in adults. However, improvements with botulinum were short lived, limiting its usefulness for this chronic disorder. Partial division of the puborectalis and myectomy produced sustained improvements in constipation, but a few patients developed fecal incontinence after division of the puborectalis. The authors do not regard myectomy or partial division of the puborectalis as a viable alternative to behavioral or medical treatment of disordered defecation because it is believed to be a behavioral disorder2—there is no neurologic or structural lesion—and surgical treatments for behavioral disorders entail an unacceptable risk of morbidity. Small uncontrolled studies suggest that sacral nerve stimulation (SNS) may also improve symptoms in some patients with chronic constipation and disordered defecation.22
Chronic Proctalgia
Chronic proctalgia is defined by chronic or recurring bouts of rectal pain in which episodes last ≥20 minutes and in which no structural or inflammatory etiology can be identified.1 When posterior traction on the puborectalis muscle during digital examination produces tenderness, the more specific diagnosis is levator ani syndrome. This common and debilitating condition is frustrating to treat.
Diagnostic assessment
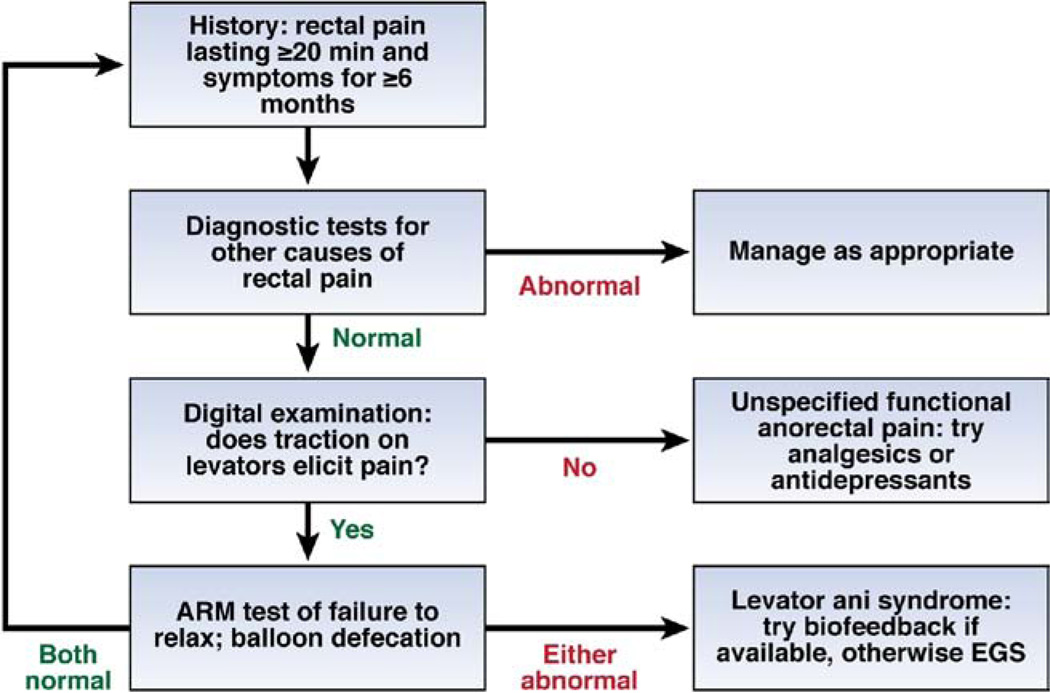
The diagnosis of chronic, idiopathic proctalgia is made primarily by exclusion of other diseases that could explain the symptom of chronic rectal pain, and the differential is large and poorly standardized. For example, in the recent study by Chiarioni et al,23 the diagnostic evaluation included digital rectal examination by a gastroenterologist, colonoscopy, pelvic ultrasound, and surgical consultation in all patients, plus referral to a gynecologist or urologist when indicated by clinical history or findings. The principal innovation in diagnostic assessment is that tenderness from palpation of the levator muscles is an excellent predictor of the likelihood of benefit from biofeedback (Figure 1).23
Figure 1.
Algorithm for the diagnosis and management of chronic proctalgia. Abbreviations: ARM, anorectal manometry; EGS, electrogalvanic stimulation.
Management
Although there is no consensus on the pathophysiology of chronic proctalgia, the pain is often assumed to be due to tense pelvic floor muscles, and the most frequently recommended treatments are biofeedback, electrogalvanic stimulation (EGS), and massage of the puborectalis muscles to relax these muscles. Inconsistent results have been reported for each of these treatments. In a large RCT comparing these treatments, Chiarioni et al23 randomized 157 patients with at least weekly rectal pain to 9 sessions of biofeedback, EGS, or massage. Psychological counseling was included in each treatment arm. Before randomization, patients were stratified based on whether they reported tenderness with traction on the pelvic floor. Among patients with tenderness, 87% reported adequate relief after biofeedback versus 45% for EGS, and 22% for massage. These differences in subjective outcomes were confirmed by greater reductions in pain days per month with biofeedback, and improvements were maintained at 12 months follow-up. However, patients with no tenderness on digital examination did not benefit from any of these treatments.
This study revealed that the pathophysiology of levator ani syndrome is remarkably similar to disordered defecation. Inability to relax the pelvic floor muscles during attempted defecation and/or inability to evacuate a 50-mL, water-filled balloon before treatment predicted response to biofeedback therapy; moreover, the biofeedback protocol developed for treatment of disordered defecation was the most effective treatment, and improvement depended on acquisition during treatment of the ability to relax pelvic floor muscles during defecation and to evacuate a balloon. Although constipation is not a hallmark of levator ani syndrome and the patients had stool frequencies within the normal range, stool frequency nevertheless increased significantly in patients who reported adequate relief of rectal pain after treatment. Thus, levator ani syndrome and defecatory disorders seem to represent different symptom manifestations of the same underlying pathophysiology. Possibly other factors, such as whole gut transit and/or pain sensitivity, determine symptom selection in patients with pelvic floor dyssynergia. An algorithm for diagnosis and management of chronic proctalgia based on these new findings is given in Figure 1.
Fecal Incontinence
Epidemiology
Fecal incontinence—recurrent uncontrolled passage of feces but not flatus alone—has a prevalence of 2.2%– 15.3% in noninstitutionalized adults, and can substantially impair quality of life.24–28 Risk factors include age, diarrhea, urgency to defecate, and a variety of medical conditions.28–31
Etiology and Pathophysiology
Diseases that affect bowel habits and/or pelvic floor continence mechanisms can cause fecal incontinence.29 Iatrogenic anal sphincter injury, radiation proctitis, and rectal evacuation disorders are common causes in men. Overt obstetric anal sphincter injury can cause postpartum fecal incontinence. However, among community women, the median age of onset of fecal incontinence is the 7th decade.26 The contribution of obstetric anal injury, often evident by imaging only, to delayed onset fecal incontinence is unclear. The contributions of aging, menopause, and chronic straining to fecal incontinence are incompletely understood.
There have been 4 significant contributions to our understanding of the pathophysiology of fecal incontinence in the past 5 years. (1) In addition to sphincter tears or scars, some women with fecal incontinence have atrophy of the external anal sphincter or puborectalis identified by magnetic resonance imaging (MRI); in a controlled study 16% of women with fecal incontinence but only 5% of age-matched controls had puborectalis atrophy, which was associated with impaired functions in fecal incontinence32 (Supplementary Table 1). (2) Rectal urgency is now recognized to be an important risk factor for fecal incontinence that is independent of diarrhea.31 (3) Rectal hypersensitivity, a stiffer rectum, and reduced rectal capacity, which are frequently associated with the symptom of urgency, are risk factors for fecal incontinence.32–36 Anal weakness and rectal hyposensitivity were already identified as risk factors in a subset of patients, especially those with diabetic neuropathy.37 (4) New data suggest that dyssynergic defecation may result in incomplete rectal emptying and predispose to fecal incontinence.38
Clinical Evaluation
The history often reveals important clues (eg, an association between postcholecystectomy diarrhea and fecal incontinence) to the etiology of fecal incontinence, which can guide therapy.29 Bowel habits are most effectively characterized by pictorial scales.39 Severity and impact on quality of life can be rated by instruments.26,27,40 A careful digital rectal examination is very useful for gauging anal resting and squeeze pressures and puborectalis function. Examination in the seated position may be more accurate for assessing rectal prolapse, pouch of Douglas hernia, or excessive perineal descent.
Diagnostic Testing
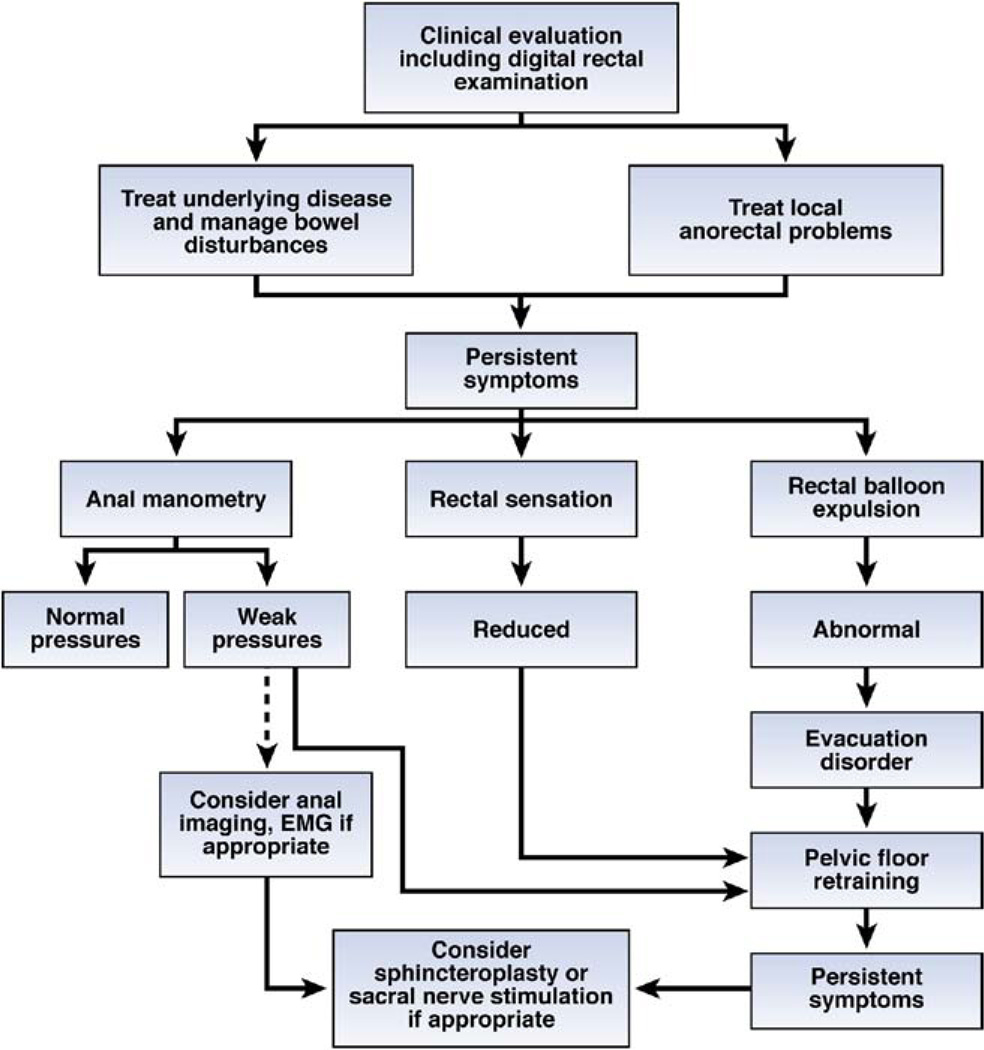
Endoscopy with biopsies if necessary should be considered. A rigorous trial of conservative measures is justified before diagnostic testing, particularly in older patients, those with mild symptoms, and those with bowel disturbances. Anal manometry, rectal sensation, and a rectal balloon expulsion test are useful initial tests, proceeding to anal imaging when anal pressures are reduced (Figure 2).41,42 Anal sphincter EMG is required infrequently, to confirm neurogenic injury, particularly a spinal cord or sacral root process. Pudendal nerve latencies are not accurate for identifying neurogenic injury in fecal incontinence.43 Evacuation proctography is useful to assess perineal descent, pelvic organ prolapse, rectoceles, defecation, and puborectalis contraction.43 With rapid MRI sequences, MRI can visualize both anal sphincter anatomy and global pelvic floor motion in real time without radiation exposure.4,32,44 Anal MRI and endoscopic ultrasound have reminded us that the puborectalis contributes to fecal continence in the proximal anal canal.45 In contrast with ultrasound examination, MRI also demonstrates atrophy of the external sphincter and puborectalis in some women with fecal incontinence.32,46 MRI is comparable to ultrasound for visualizing internal sphincter abnormalities.47 Although ultrasound is routinely performed with an endoanal probe, a transperineal probe may also visualize anal sphincter defects.48
Figure 2.
Algorithm for the diagnosis and management of fecal incontinence.
Consideration should be given to referring independently living patients with moderate-to-severe fecal incontinence to specialist centers for further assessment under the following circumstances: (1) When symptoms cannot be explained by routine diagnostic tests, for example, when anal sphincter weakness is mild and/or cannot be attributed to sphincter disturbances documented by ultrasound. (2) Before considering repair of external sphincter defects in older women. Because surgical repair is not always successful, careful consideration of other factors contributing to incontinence, perhaps supplemented by pelvic MRI to identify external sphincter atrophy may be useful. (4) For patients who have fecal and urinary incontinence, sacral nerve stimulation (SNS) is an US Food and Drug Administration (FDA)–approved procedure for urge urinary incontinence; bowel symptoms may also respond to SNS (see below).
Management
Management is tailored to clinical manifestations and includes treatment of underlying diseases (Supplementary Table 2). Management of bowel disturbances with simple approaches (eg, anti-diarrheals taken before social occasions or meals) is critical and often therapeutic. Loperamide (2–4 mg, 30 minutes before meals, titrated to reduce diarrhea but avoid constipation, up to 16 mg/d) reduced diarrhea and fecal incontinence and increased anal tone.49 Diphenoxylate, alosetron (for refractory diarrhea), and cholestyramine (especially for postcholecystectomy diarrhea) are other options. Regular evacuation programs, incorporating timed evacuation by digital stimulation and/or bisacodyl/glycerol suppositories, fiber supplementation, and selective use of oral laxatives as detailed elsewhere are useful for constipation.50 Per-anal phenylephrine, which is an α1-adrenergic agonist, increased anal resting pressures but did not improve fecal incontinence.51,52
Controlled trials reinforce the role of conservative measures (eg, diet and skin care, bowel medications, urge suppression techniques), and when these measures are ineffective, biofeedback therapy for fecal incontinence.53 Using a rectal balloon with anal manometry or a surface electromyography device, patients are taught to contract the external anal sphincter when they perceive balloon distention; perception may be reinforced by visual tracings of balloon volume and anal pressure, and the procedure is repeated with progressively smaller volumes. In an RCT of 171 incontinent patients, effects on symptoms (ie, improved in 55% and resolved in 5%) and anal pressures were comparable in 4 groups: standard medical/nursing care (advice only), advice plus verbal instruction on sphincter exercises, hospital- based computer-assisted sphincter pressure biofeedback, or hospital biofeedback plus use of a home EMG biofeedback device.54 This improvement was sustained at 1 year after therapy. In another RCT of 108 patients, 22% responded to conservative therapy for 4 weeks. Among the remainder, response rates were better in those who received 6 biweekly sessions with EMG-assisted biofeedback and pelvic floor exercises (77% reported adequate relief and 66% were completely continent) than pelvic floor exercises alone (41% reported adequate relief and 48% were completely continent).53 A key question is whether instrumented biofeedback is comparable to teaching pelvic floor exercises by digital examination with verbal feedback.
Available surgical options include (1) anal sphincteroplasty for women with postpartum fecal incontinence and anal sphincter defects not responding to conservative management and (2) for women with truly medically refractory fecal incontinence—a colostomy, artificial anal sphincter, SNS, or dynamic graciloplasty, the hardware for which is not approved for use in the United States. The role of sphincteroplasty in older women with fecal incontinence and anal sphincter defects is limited because short-term symptom improvement is not sustained; for example, only 21% were continent at 40 months in 1 study.55 Although the intent-to-treat response rates for artificial sphincter and graciloplasty are 50%–60%, significant morbidity, including infections and device problems sometimes necessitating reoperation or explanation, are common.56,57
SNS is approved for treating fecal incontinence in Europe and is FDA-approved for treating urinary but not fecal incontinence in the United States. This is a staged procedure; when symptoms respond to temporary stimulation for 3 weeks, the device is implanted subcutaneously. The procedure is technically straightforward, complications are infrequent, and symptoms improve substantially. In 1 crossover study of 34 patients,58 symptoms improved by 90% during stimulation versus 76% without stimulation; the order of stimulation was randomized. In another controlled study, SNS improved symptoms and quality of life to a greater extent than “optimal medical management” (ie, bulking agents, pelvic floor exercises, and dietary management); use of anti-diarrheal agents was not specified.59 A North American multicenter study observed that 120 of 133 patients (90%) proceeded from test to chronic SNS at 12 months, 83% of subjects (95% CI; 74–90%) achieved therapeutic success, defined by ≥50% reduction in incontinence episodes.60 Limited data suggest that the SNS is also effective in patients with sphincter defects.61 SNS is approved for treating fecal incontinence by the National Institute for Clinical Excellence in the United Kingdom. The discrepancy between symptom improvement and inconclusive effects on anal pressures, rectal compliance, and rectal sensation is puzzling.62–64 Anal electrical stimulation is not beneficial and measures to bulk the anal sphincter with silicon and carbon beads are not ready for prime time.65–67 Anal sphincteric injection of autologous myoblasts derived from a pectoralis muscle biopsy was well tolerated and improved symptoms in 10 women with fecal incontinence, but there was no clinically significant improvement in anal resting and squeeze pressures.68 Attempts to bioengineer sphincteric rings from human internal anal sphincter smooth muscle cells are in progress.69
Summary
Recent studies strengthen substantially the evidence that biofeedback is the preferred treatment for disordered defecation and levator ani syndrome, and identify patient characteristics that predict successful outcomes. Biofeedback does not benefit patients with constipation due primarily to slow transit, but is effective in patients with either inability to evacuate a balloon or impaired relaxation of pelvic floor muscles during straining. For chronic proctalgia, the same 2 signs plus tenderness on palpation of the pelvic floor predict success. Conservative measures, including careful characterization and management of bowel disturbances, is key to managing fecal incontinence. A new RCT carried out in patients who failed conservative management demonstrated that biofeedback provided additional benefit for fecal incontinence and was superior to pelvic floor exercises. However, other studies suggest that when patients are taught how to perform pelvic floor exercises with verbal guidance from a therapist during digital rectal examination, this may be as effective as biofeedback provided by machines. Limitations of biofeedback are the paucity of well-trained therapists and limited efficacy in children. RCTs also support the efficacy of SNS for fecal incontinence, but this is not yet approved for use in the United States. New diagnostic techniques including pelvic floor MRI have increased our understanding of the risk factors and pathophysiology of anorectal disorders. Pending approval by the FDA, sacral nerve stimulation is a new option for patients with fecal incontinence who have failed conservative therapy.
Supplementary Material
Acknowledgments
Funding
Supported in part by USPHS NIH Grants R01 DK78924 and R24 DK067674.
Footnotes
Supplementary Material
The first 5 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of the article. To access the remaining references, as well as additional online-only data, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10.1053/j.gastro.2010.02.036.
Conflicts of interest
Dr Adil Bharucha is a consultant for American Medical Systems.
References
- 1.Wald A, Bharucha AE, Enck P, et al. Functional anorectal disorders. In: Drossman DA, Corazziari E, Delvaux M, et al., editors. Rome III: The Functional Gastrointestinal Disorders. 3rd ed. McLean, Virginia: Degnon Associates; 2006. pp. 639–685. [Google Scholar]
- 2.Whitehead WE, Di Lorenzo C, Leroi AM, et al. Conservative and behavioral management of constipation. Neurogastroenterol Motil. 2009;21(Suppl 2):55–61. doi: 10.1111/j.1365-2982.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 3.Gladman MA, Lunniss PJ, Scott SM, et al. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2009;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
References (Online Only)
- 6.Chiarioni G, Salandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005;129:86–97. doi: 10.1053/j.gastro.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. In: Drossman DA, Corazziari E, Delvaux M, et al., editors. Rome III: The Functional Gastrointestinal Disorders. 3rd ed. McLean, VA: Degnon Associates; 2006. pp. 487–555. [Google Scholar]
- 8.Bleijenberg G, Kuijpers HC. Treatment of the spastic pelvic floor syndrome with biofeedback. Dis Colon Rectum. 1987;30:108–111. doi: 10.1007/BF02554946. [DOI] [PubMed] [Google Scholar]
- 9.Duthie GS, Bartolo DC. Anismus: the cause of constipation? Results of investigation and treatment. World J Surg. 1992;16:831–835. doi: 10.1007/BF02066978. [DOI] [PubMed] [Google Scholar]
- 10.Chiotakakou-Faliakou E, Kamm MA, Roy AJ, et al. Biofeedback provides long-term benefit for patients with intractable, slow and normal transit constipation. Gut. 1998;42:517–521. doi: 10.1136/gut.42.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006;130:657–664. doi: 10.1053/j.gastro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007;5:331–338. doi: 10.1016/j.cgh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum. 2007;50:428–441. doi: 10.1007/s10350-006-0814-9. [DOI] [PubMed] [Google Scholar]
- 14.Koutsomanis D, Lennard-Jones JE, Roy AJ, et al. Controlled randomised trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut. 1995;37:95–99. doi: 10.1136/gut.37.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiarioni G, Whitehead WE. Biofeedback therapy for constipation. In: Parkman HP, Rao SSC, McCallum R, editors. Gastrointestinal Motility Testing: Laboratory and Office Handbook. Thorofare, NJ: Slack Inc; 2009. [Google Scholar]
- 16.van der Plas RN, Benninga MA, Buller HA, et al. Biofeedback training in treatment of childhood constipation: a randomised controlled study. Lancet. 1996;348:776–780. doi: 10.1016/s0140-6736(96)03206-0. [DOI] [PubMed] [Google Scholar]
- 17.Di LC, Benninga MA. Pathophysiology of pediatric fecal incontinence. Gastroenterology. 2004;126:S33–S40. doi: 10.1053/j.gastro.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Farid M, El Monem HA, Omar W, et al. Comparative study between biofeedback retraining and botulinum neurotoxin in the treatment of anismus patients. Int J Colorectal Dis. 2009;24:115–120. doi: 10.1007/s00384-008-0567-0. [DOI] [PubMed] [Google Scholar]
- 19.Farid M, Youssef T, Mahdy T, et al. Comparative study between botulinum toxin injection and partial division of puborectalis for treating anismus. Int J Colorectal Dis. 2009;24:327–334. doi: 10.1007/s00384-008-0609-7. [DOI] [PubMed] [Google Scholar]
- 20.Keshtgar AS, Ward HC, Sanei A, et al. Botulinum toxin, a new treatment modality for chronic idiopathic constipation in children: long-term follow-up of a double-blind randomized trial. J Pediatr Surg. 2007;42:672–680. doi: 10.1016/j.jpedsurg.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Maria G, Cadeddu F, Brandara F, et al. Experience with type A botulinum toxin for treatment of outlet-type constipation. Am J Gastroenterol. 2006;101:2570–2575. doi: 10.1111/j.1572-0241.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamm M, Dudding T, Melenhorst J, et al. Sacral nerve stimulation for constipation: an international multi-centre study. Gastroenterology. 2007;132(Suppl 2):A40(198). [Abstract] [Google Scholar]
- 23.Chiarioni G, Nardo A, Vantini I, et al. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. 2009 doi: 10.1053/j.gastro.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–484. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson RL. Epidemiology of fecal incontinence. Gastroenterology. 2004;126:S3–S7. doi: 10.1053/j.gastro.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Bharucha AE, Zinsmeister AR, Locke GR, et al. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004–1009. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512–517. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharucha AE. Fecal incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 30.Quander CR, Morris MC, Melson J, et al. Prevalence of and factors associated with fecal incontinence in a large community study of older individuals. Am J Gastroenterol. 2005;100:905–909. doi: 10.1111/j.1572-0241.2005.30511.x. [DOI] [PubMed] [Google Scholar]
- 31.Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101:1305–1312. doi: 10.1111/j.1572-0241.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 32.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ’idiopathic’ faecal incontinence. Gut. 1992;33:807–813. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siproudhis L, El AM, El AM, et al. Low rectal volumes in patients suffering from fecal incontinence: what does it mean? Aliment Pharmacol Ther. 2005;22:989–996. doi: 10.1111/j.1365-2036.2005.02675.x. [DOI] [PubMed] [Google Scholar]
- 35.Andrews C, Bharucha AE, Seide B, et al. Rectal sensorimotor dysfunction in women with fecal incontinence. Am J Physiol Gastrointest Liver Physiol. 2007;292:G282–G289. doi: 10.1152/ajpgi.00176.2006. [DOI] [PubMed] [Google Scholar]
- 36.Deutekom M, Dobben AC, Terra MP, et al. Clinical presentation of fecal incontinence and anorectal function: what is the relationship? Am J Gastroenterol. 2007;102:351–361. doi: 10.1111/j.1572-0241.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 37.Wald A, Tunuguntla AK. Anorectal sensorimotor dysfunction in fecal incontinence and diabetes mellitus. Modification with biofeedback therapy. N Engl J Med. 1984;310:1282–1287. doi: 10.1056/NEJM198405173102003. [DOI] [PubMed] [Google Scholar]
- 38.Parellada CM, Miller AS, Williamson ME, et al. Paradoxical high anal resting pressures in men with idiopathic fecal seepage. Dis Colon Rectum. 1998;41:593–597. doi: 10.1007/BF02235265. [DOI] [PubMed] [Google Scholar]
- 39.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 40.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–1532. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 41.Bharucha AE. Update of tests of colon and rectal structure and function. J Clin Gastroenterol. 2006;40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 42.Bharucha AE, Fletcher JG. Recent advances in assessing anorectal structure and functions. Gastroenterology. 2007;133:1069–1074. doi: 10.1053/j.gastro.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 43.Diamant NE, Kamm MA, Wald A, et al. AGA technical review on anorectal testing techniques. Gastroenterology. 1999;116:735–760. doi: 10.1016/s0016-5085(99)70195-2. [DOI] [PubMed] [Google Scholar]
- 44.El Sayed RF, El MS, Farag A, et al. Pelvic floor dysfunction: assessment with combined analysis of static and dynamic MR imaging findings. Radiology. 2008;248:518–530. doi: 10.1148/radiol.2482070974. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Guaderrama N, Nager CW, et al. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol. 2006;101:1092–1097. doi: 10.1111/j.1572-0241.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 46.Terra MP, Deutekom M, Beets-Tan RG, et al. Relationship between external anal sphincter atrophy at endoanal magnetic resonance imaging and clinical, functional, and anatomic characteristics in patients with fecal incontinence. Dis Colon Rectum. 2006;49:668–678. doi: 10.1007/s10350-006-0507-4. [DOI] [PubMed] [Google Scholar]
- 47.Stoker J. Magnetic resonance imaging in fecal incontinence. Semin Ultrasound CT MR. 2008;29:409–413. doi: 10.1053/j.sult.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein MM, Pretorius DH, Jung SA, et al. Transperineal three-dimensional ultrasound imaging for detection of anatomic defects in the anal sphincter complex muscles. Clin Gastroenterol Hepatol. 2009;7:205–211. doi: 10.1016/j.cgh.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Read M, Read NW, Barber DC, et al. Effects of loperamide on anal sphincter function in patients complaining of chronic diarrhea with fecal incontinence and urgency. Dig Dis Sci. 1982;27:807–814. doi: 10.1007/BF01391374. [DOI] [PubMed] [Google Scholar]
- 50.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 51.Carapeti EA, Kamm MA, Phillips RK. Randomized controlled trial of topical phenylephrine in the treatment of faecal incontinence. Br J Surg. 2000;87:38–42. doi: 10.1046/j.1365-2168.2000.01306.x. [DOI] [PubMed] [Google Scholar]
- 52.Cheetham MJ, Kamm MA, Phillips RK. Topical phenylephrine increases anal canal resting pressure in patients with faecal incontinence. Gut. 2001;48:356–359. doi: 10.1136/gut.48.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52:1730–1737. doi: 10.1007/DCR.0b013e3181b55455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norton C, Hosker G, Brazzelli M. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD002111. CD002111. [DOI] [PubMed] [Google Scholar]
- 55.Cheung O, Wald A. Review article: the management of pelvic floor disorders. Aliment Pharmacol Ther. 2004;19:481–495. doi: 10.1111/j.1365-2036.2004.01886.x. [DOI] [PubMed] [Google Scholar]
- 56.Chapman AE, Geerdes B, Hewett P, et al. Systematic review of dynamic graciloplasty in the treatment of faecal incontinence. Br J Surg. 2002;89:138–153. doi: 10.1046/j.0007-1323.2001.02018.x. [DOI] [PubMed] [Google Scholar]
- 57.Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artifical bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45:1139–1153. doi: 10.1007/s10350-004-6381-z. [DOI] [PubMed] [Google Scholar]
- 58.Mowatt G, Glazener C, Jarrett M. Sacral nerve stimulation for fecal incontinence and constipation in adults: a short version Cochrane review. Neurourol Urodyn. 2008;27:155–161. doi: 10.1002/nau.20565. [DOI] [PubMed] [Google Scholar]
- 59.Tjandra JJ, Chan MK, Yeh CH, et al. Sacral Nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51:494–502. doi: 10.1007/s10350-007-9103-5. [DOI] [PubMed] [Google Scholar]
- 60.Wexner SD, Coller JA, Devroede G, et al. Sacral nerve stimulation for fecal incontinence: results of a 120-patient prospective multicenter study. Annals of Surgery. 2010;251:441–449. doi: 10.1097/SLA.0b013e3181cf8ed0. [DOI] [PubMed] [Google Scholar]
- 61.Boyle DJ, Knowles CH, Lunniss PJ, et al. Efficacy of sacral nerve stimulation for fecal incontinence in patients with anal sphincter defects. Dis Colon Rectum. 2009;52:1234–1239. doi: 10.1007/DCR.0b013e31819f7400. [DOI] [PubMed] [Google Scholar]
- 62.Ganio E, Ratto C, Masin A, et al. Neuromodulation for fecal incontinence: outcome in 16 patients with definitive implant. The initial Italian Sacral Neurostimulation Group (GINS) experience. Dis Colon Rectum. 2001;44:965–970. doi: 10.1007/BF02235484. [DOI] [PubMed] [Google Scholar]
- 63.Rosen HR, Urbarz C, Holzer B, et al. Sacral nerve stimulation as a treatment for fecal incontinence. Gastroenterology. 2001;121:536–541. doi: 10.1053/gast.2001.27120. [DOI] [PubMed] [Google Scholar]
- 64.Roman S, Tatagiba T, Damon H, et al. Sacral nerve stimulation and rectal function: results of a prospective study in faecal incontinence. Neurogastroenterol Motil. 2008;20:1127–1131. doi: 10.1111/j.1365-2982.2008.01154.x. [DOI] [PubMed] [Google Scholar]
- 65.Andrews CN, Bharucha AE. The etiology, assessment, and treatment of fecal incontinence. Nat Clin Pract Gastroenterol Hepatol. 2005;2:516–525. doi: 10.1038/ncpgasthep0315. [DOI] [PubMed] [Google Scholar]
- 66.Norton C, Gibbs A, Kamm MA. Randomized, controlled trial of anal electrical stimulation for fecal incontinence. Dis Colon Rectum. 2006;49:190–196. doi: 10.1007/s10350-005-0251-1. [DOI] [PubMed] [Google Scholar]
- 67.Maeda Y, Vaizey CJ, Kamm MA. Long-term results of perianal silicone injection for faecal incontinence. Colorectal Dis. 2007;9:357–361. doi: 10.1111/j.1463-1318.2006.01164.x. [DOI] [PubMed] [Google Scholar]
- 68.Frudinger A, Kolle D, Schwaiger W, et al. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. GUT. 2010;59:55–61. doi: 10.1136/gut.2009.181347. [DOI] [PubMed] [Google Scholar]
- 69.Somara S, Gilmont RR, Dennis RG, et al. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137:53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.