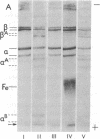
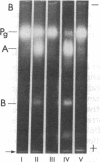
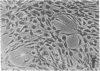
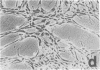
Abstract
We have observed that treatment of rabbit synovial fibroblasts with proteolytic enzymes can induce secretion of collagenase (EC 3.4.24.7) and plasminogen activator (EC 3.4.21.-). Cells treated for 2-24 hr with plasmin, trypsin, chymotrypsin, pancreatic elastase, papain, bromelain, thermolysin, or α-protease but not with thrombin or neuraminidase secreted detectable amounts of collagenase within 16-48 hr. Treatment of fibroblasts with trypsin also induced secretion of plasminogen activator. Proteases initiated secretion of collagenase (up to 20 units per 106 cells per 24 hr) only when treatment produced decreased cell adhesion. Collagenase production did not depend on continued presence of proteolytic activity or on subsequent cell adhesion, spreading, or proliferation. Routine subculturing with crude trypsin also induced collagenase secretion by cells. Secretion of collagenase was prevented and normal spreading was obtained if the trypsinized cells were placed into medium containing fetal calf serum. Soybean trypsin inhibitor, α1-antitrypsin, bovine serum albumin, collagen, and fibronectin did not inhibit collagenase production. Although proteases that induced collagenase secretion also removed surface glycoprotein, the kinetics of induction of cell protease secretion were different from those for removal of fibronectin. Physiological inducers of secretion of collagenase and plasminogen activator by cells have not been identified. These results suggest that extracellular proteases in conjunction with plasma proteins may govern protease secretion by cells.
Keywords: plasmin, morphology, cell surface glycoprotein, adhesion, serum inhibition
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Cobb C. M., Taylor R. E., Fullmer H. M. Synthesis and release of procollagenase by cultured fibroblasts. J Biol Chem. 1976 May 25;251(10):3162–3168. [PubMed] [Google Scholar]
- Bornstein P., Ash J. F. Cell surface-associated structural proteins in connective tissue cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2480–2484. doi: 10.1073/pnas.74.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Kiehn D. Protease effects on specific growth properties of normal and transformed baby hamster kidney cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2874–2878. doi: 10.1073/pnas.74.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Initiation of check cell division by trypsin action at the cell surface. Nature. 1977 Aug 18;268(5621):602–606. doi: 10.1038/268602a0. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Graham R., Russell G., Krane S. M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science. 1977 Jan 14;195(4274):181–183. doi: 10.1126/science.188134. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fisher H. W., Puck T. T., Sato G. MOLECULAR GROWTH REQUIREMENTS OF SINGLE MAMMALIAN CELLS: THE ACTION OF FETUIN IN PROMOTING CELL ATTACHMENT TO GLASS. Proc Natl Acad Sci U S A. 1958 Jan;44(1):4–10. doi: 10.1073/pnas.44.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLIMCHER M. J., FRANCOIS C. J., RICHARDS L., KRANE S. M. THE PRESENCE OF ORGANIC PHOSPHORUS IN COLLAGENS AND GELATINS. Biochim Biophys Acta. 1964 Dec 9;93:585–602. doi: 10.1016/0304-4165(64)90342-3. [DOI] [PubMed] [Google Scholar]
- Gordon S., Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976 Mar;73(3):872–876. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Reynolds J. J., Werb Z. Cytochalasin B increases collagenase production by cells in vitro. Nature. 1975 Sep 18;257(5523):243–244. doi: 10.1038/257243a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBERMAN I., LAMY F., OVE P. Nonidentity of fetuin and protein growth (flattening) factor. Science. 1959 Jan 2;129(3340):43–44. doi: 10.1126/science.129.3340.43. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Pohjanpelto P. Proteases stimulate proliferation of human fibroblasts. J Cell Physiol. 1977 Jun;91(3):387–392. doi: 10.1002/jcp.1040910308. [DOI] [PubMed] [Google Scholar]
- Rees D. A., Lloyd C. W., Thom D. Control of grip and stick in cell adhesion through lateral relationships of membrane glycoproteins. Nature. 1977 May 12;267(5607):124–128. doi: 10.1038/267124a0. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Bo Chen L. The role of surface proteins in cell proliferation as studied with thrombin and other proteases. Proc Natl Acad Sci U S A. 1975 Feb;72(2):413–417. doi: 10.1073/pnas.72.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Vassalli J. D., Reich E. Macrophage plasminogen activator: induction by products of activated lymphoid cells. J Exp Med. 1977 Feb 1;145(2):429–437. doi: 10.1084/jem.145.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C. A specific collagenase from rabbit fibroblasts in monolayer culture. Biochem J. 1974 Feb;137(2):373–385. doi: 10.1042/bj1370373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C., Barrett A. J., Starkey P. M. The interaction of alpha2-macroglobulin with proteinases. Binding and inhibition of mammalian collagenases and other metal proteinases. Biochem J. 1974 May;139(2):359–368. doi: 10.1042/bj1390359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Werb Z., Reynolds J. J. Stimulation by endocytosis of the secretion of collagenase and neutral proteinase from rabbit synovial fibroblasts. J Exp Med. 1974 Dec 1;140(6):1482–1497. doi: 10.1084/jem.140.6.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter B. R., Chen L. B., Buchanan J. M. Binding and internalization of thrombin by normal and transformed chick cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):596–600. doi: 10.1073/pnas.74.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter B. R., Chen L. B., Buchanan J. M. Effects of protease treatment on growth, morphology, adhesion, and cell surface proteins of secondary chick embryo fibroblasts. Cell. 1976 Mar;7(3):407–412. doi: 10.1016/0092-8674(76)90170-7. [DOI] [PubMed] [Google Scholar]