Abstract
Pseudobranch function has long interested scientists, but its role has yet to be elucidated. Several studies have suggested that pseudobranchs serve respiratory, osmoregulatory, and sensory functions. This work investigated the immunolocalization of pseudobranch carbonic anhydrase (CA) in the teleost fish species rainbow trout (Oncorhynchus mykiss) to clarify its physiological function. CA was purified from rainbow trout gills O. mykiss and specific antibodies were raised. Immunoblotting between tissue homogenates of pseudobranch and gill CA antibodies showed specific immunostaining with only one band corresponding to CA in the pseudobranch homogenate. Results of immunohistochemical technique revealed that CA was distributed within pseudobranch cells and more precisely in the apical parts (anti-vascular) of cells. The basal (vascular) parts of cells, tubular system, blood capillaries, and pillar cells were not immunostained. Immunocytochemistry confirmed these results and showed that some CA enzyme was cytoplasmic and the remainder was linked to membranous structures. The results also showed that the lacunar tissue layers did not display immunoperoxidase activity. Our results indicated that pseudobranch CA may have a function related to the extracellular medium wherein CA intervenes with the mechanism of stimulation of afferent nerve fibers.
Keywords: Pseudobranch, Carbonic anhydrase, Rainbow trout, Immunohistochemistry, Immunocytochemistry, Physiology
1. Introduction
Carbonic anhydrases (CAs; EC 4.2.1.1) are Zn-metalloenzymes ubiquitous in prokaryotes and eukaryotes that are encoded by five evolutionarily unrelated gene families (Supuran, 2008; Sethi et al., 2011). Sixteen different CAs are found in mammals, and these CAs differ in their enzymatic properties, amino acid sequences, and expression sites (Esbaugh and Tufts, 2006; Ekinci et al., 2011). The most important function of CA is related to the respiration and transport of CO2/bicarbonate in various metabolizing tissues (Georgalis et al., 2006a; Gilmour and Perry, 2009). This enzyme is also involved in electrolyte secretion, CO2 and pH homeostasis, as well as biosynthetic reactions such as gluconeogenesis and ureagenesis (Esbaugh and Tufts, 2006; Imtaiyaz Hassan et al., 2012). Searches in genetic databases reveal more than 16 isoenzymes of CA reported and/or predicted in fish species (Georgalis et al., 2006b; Lin et al., 2008; Gilmour and Perry, 2009). Rahim et al. (1988) first purified two distinct branchial and blood CA isoenzymes in freshwater rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio), respectively. This finding has been confirmed by the cloning of rainbow trout blood and cytosolic CA isoenzymes (Esbaugh et al., 2005). Sender et al. (1999) found identical CA isoenzymes purified from blood and gills of flounder (Platichthys flesus). Moreover, Georgalis et al. (2006b) cloned an additional CA isoenzyme from rainbow trout O. mykiss kidney that most closely resembles the mammalian membrane-bound isoenzyme CA IV.
Broussonet (1785) considered the pseudobranch to be a small gill whose function is related to respiration. This hypothesis was rejected by Hyrtl (1838), who demonstrated that the pseudobranch perfused by arterial blood originates from the efferent arterial gill. The morphology of the pseudobranch has been studied in the review article of Laurent and Dunel-Erb (1984), and other studies have suggested its involvement in functions including vision (Dimberg, 1995; Bridges et al., 1998; Mölich et al., 2009), osmoregulation (Quinn et al., 2003), and secretion (Bridges et al., 1998). Laurent and Rouzeau (1972) and Laurent (1974) reported that the pseudobranch contains several types of chemoreceptors that are sensitive to hydrostatic pressure, oxygen partial pressure, pH, osmotic pressure, and increased sodium concentration. Chemoreceptors also reportedly support the oxygen concentrating mechanisms in the eyes of teleost fishes, and this mechanism thought to underlie the oxygen concentration mechanisms is the root effect (Bridges et al., 1998; Waser and Heisler, 2005; Berenbrink, 2007; Rummer and Brauner, 2011).
Large quantities of CA in pseudobranch tissue have been detected by both enzymatic activity assay (Maetz, 1956) and the cobalt histochemical technique (Hansson, 1967; Laurent et al., 1969). Laurent et al. (1969) showed that CA is localized in the vascular part of pseudobranchial cells and related to the tubular system in the basal part of the cells. They concluded that this enzyme plays an important role in stimulating nerve endings located in the extracellular spaces of the pseudobranch epithelium. The specificity of the histochemical technique used by Laurent et al. (1969) has been questioned on several occasions (Churg, 1973; Muther, 1977).
In the present study, we used antiserum obtained from fish gill CA specific to pseudobranch CA. We found that the enzyme is localized in the apical part (anti-vascular) of pseudobranch cells, which differed from the finding of Laurent et al. (1969).
2. Materials and methods
2.1. Sample collection and preparation of gill CA
Fresh water rainbow trout (O. mykiss) (250–300 g) were obtained from a fish farm in Strasbourg, France, and kept in external laboratory aquaria containing tap water. All experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals (Rahim, 1988). Gill CA was purified as described by Rahim et al. (1988). In a typical procedure, gills were freed of blood and then filaments were excised and homogenized in phosphate buffered saline (PBS) containing 1% Triton X-100 (Sigma). The supernatant was contained both the soluble and bound forms of gill CAs, and purification was performed by affinity chromatography on sulfanilamide Sepharose gel (Filippi et al., 1978). The purity was tested using 1D polyacrylamide (10%) gel electrophoresis under denaturing conditions (Laemmli, 1970), and markers were used to determine the molecular weight of the purified enzyme (bovine CA 30 kDa, ovalbumine 43 kDa, albumin 67 kDa, and phosphprylase-b 94 kDa).
2.2. Preparation of antibodies against gill CA
Pure antigens purified from the gills of freshwater rainbow trout O. mykiss were used to produce antibodies in rabbits (Delaunoy, 1983). Three rabbits were immunized by the subcutaneous injection of 1 mg of CA emulsified in complete Freund’s adjuvant. After three weeks, the animals received a second subcutaneous injection of CA as a booster injection in incomplete Freund’s adjuvant. The animals were bled by heart puncture 50 d after the first injection. Antisera were fractionated and stored at −30 °C. The monospecificity of the antisera was examined by immunoblotting (Towbin et al., 1979) against homogenates of gill and pseudobranch samples. After transferring the proteins from sodium dodecyl sulphate (SDS)-gel electrophoresis onto a nitrocellulose membrane, they were incubated with gill CA antibodies (1/100 dilution) and then with sheep anti-rabbit IgG (1/500 dilution) peroxidase conjugate (Biosys-France) for 2 h. The nitrocellulose membranes were washed three times for 10 min in PBS. Antigen-antibody complexes were detected with 4-chloro-1-napthol. The reaction was carried out in darkness at laboratory temperature for 15 min (Rahim et al., 1988).
2.3. Immunohistochemical technique
The pseudobranch was excised and immediately immersed for 2–3 h in a cold fixative containing 4:69:5:22 (v/v) formaldehyde:ethyl alcohol:acetic acid: distilled water (Cammer and Tansey, 1987). Dehydration of the pseudobranch samples, paraffin embedding, and deparaffination of sections were performed according to the method of Kumpulainen (1981). All treatments were performed at 4 °C. Thick sections (7 μm) from pseudobranch samples were cut and mounted on a slide for indirect immunoperoxidase staining. Endogenous activity was inhibited by incubating the sections with 3% H2O2. The sections were then incubated with gill CA antibodies (1/200 dilution) for 1 h. After washing three times for 10 min in PBS, the sections were treated with sheep anti-rabbit serum IgG-peroxidase conjugate (1/150 dilution) for 1 h and washed three times for 10 min in PBS. The sections were then incubated for 10 min with 3,3'-diaminobenzidine for immunoreactivity detection. Control sections were treated as above except that non-immune rabbit serum replaced gill CA antibodies (Rahim et al., 1988).
2.4. Immunocytochemical technique
The pseudobranch was excised, fragmented (2–4 mm), and fixed for 2–3 h in a cold fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 mol/L PBS (pH 7.4). After fixation, the tissues were washed in PBS (3×10 min). Pseudobranch fragments were embedded in gelatin (25%) and cut (50 μm) using a vibratome (Campder Instruments Ltd., UK). The sections were washed with PBS for 1 h with gentle agitation at 4 °C, incubated with rabbit anti-gill CA serum (1/100 dilution) for 1 h, washed with PBS (4×15 min), incubated with sheep anti-rabbit serum IgG-peroxidase conjugate (1/100 dilution) for 1 h, washed again with PBS (3×15 min), and treated with 3,3'-diaminobenzidine for 10 min for immunoperoxidase detection (Graham and Karnovsky, 1966). The sections were rewashed with PBS for 1–2 h and post-fixed for electron microscopy observation with osmic acid (1%) for 1 h. After dehydration in ethyl alcohol, sections were embedded in araldite resin and ultrathin sections were prepared using an ultramicrotome. The ultrathin sections were then observed under an electron microscope (SIEMENS, Germany) without coloration. Control sections were prepared as above except that non-immune rabbit serum was used instead of gill CA antibodies.
3. Results
3.1. Gill CA purification
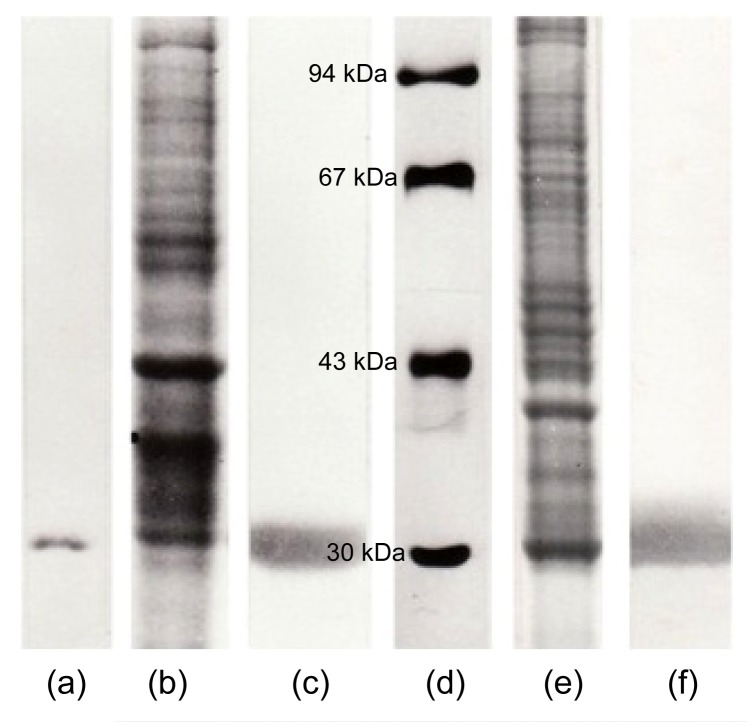
Gill CA was purified by affinity chromatography on sulfanilamide Sepharose gel. The active fraction of CA was eluted as a single peak with CNO−. The purity of the isolated CA fractions was analyzed by SDS-gel electrophoresis, and gill CA showed one single band at 30 kDa (Fig. 1a).
Fig. 1.
Molecular and immunological characterization of trout CA by commassie brilliant blue stained SDS-gel electrophoresis (10%) (a, b, d, e) and immunostained nitrocellulose membrane (c, f)
(a) Purified gill CA (25 μg). Only a single stained band of proteins (30 kDa) confirms the enzyme purity. (b) Gill homogenate (50 μg). (c) Nitrocellulose membrane from the corresponding SDS-gel electrophoresis (b) immunostained with gill CA antiserum (1/100 dilution). Only a single band confirms the antiserum specificity for gill CA. (d) Molecular weight markers: bovine CA, 30 kDa; ovalbumine, 43 kDa; albumin, 67 kDa; phosphprylase b, 94 kDa. (e) Pseudobranch tissue homogenate (50 μg). Proteins are stained with commassie brilliant blue. (f) Nitrocellulose membrane from the corresponding SDS-gel electrophoresis (e) immunostained with gill CA antiserum (1/100 dilution). Only a single band confirms the antiserum specificity for pseudobranch CA
3.2. Production of gill CA antiserum
The immunization of rabbits with isolated gill CA produced a specific antiserum, as confirmed by an immunoblotting experiment (Figs. 1b and 1c). Gill CA antiserum stained only one band corresponding to gill CA in the rainbow trout gill homogenate (Fig. 1c).
3.3. Immunoblotting of pseudobranch CA
The localization of pseudobranch CA was preceded by an immunoblotting experiment between the pseudobranch homogenate and gill CA antiserum (Figs. 1e and 1f). Gill CA antiserum showed a positive reaction with only one band corresponding to the enzyme CA in the pseudobranch tissue homogenate (Fig. 1f).
3.4. Light microscopic immunohistochemistry
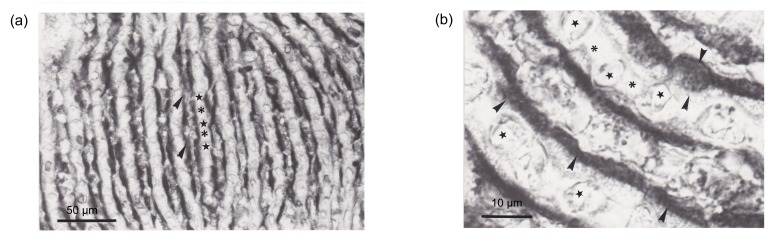
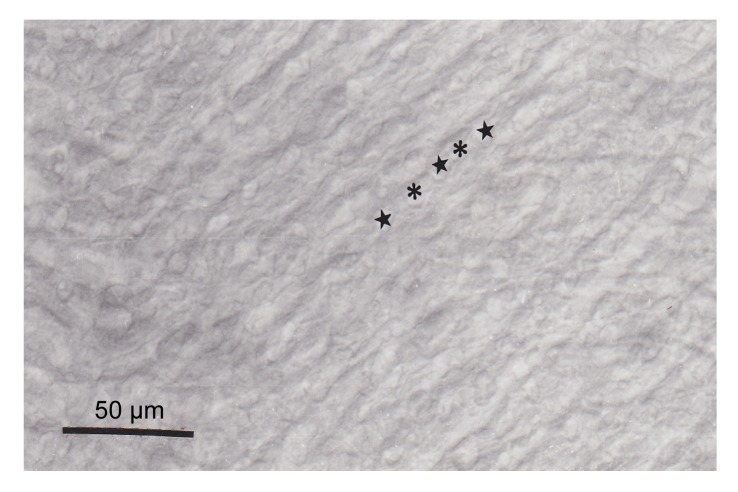
The paraffin sections showed that CA was localized in the apical part (anti-vascular) of pseudobranch cells (Fig. 2). CA was found to be distributed within pseudobranch cells particularly in the part adjacent to lacunar tissues (Fig. 2). The basal (vascular) part of cells, tubular system, endothelium of blood capillaries, and pillar cells were not stained (Fig. 2). Reaction specificity was confirmed by the complete absence of immunostaining in the control sections (Fig. 3) incubated with non-immune rabbit serum.
Fig. 2.
Light micrographs of paraffin sections revealing the presence of trout CA in pseudobranch tissue
(a) Note the high immunoreactivity in the anti-vascular zone (◂). The capillary endothelium (★) and pillar cells (*) are not immunostained. (b) Higher magnification image shows that the pseudobranch cells displaying CA immunoreactivity are in the apical region (◂). The basal region with capillary endothelium (★) and pillar cells (*) are not immunostained
Fig. 3.
Control section for reaction specificity
The control section showed a total absence of immunoreactivity. ★ and * show the emplacement of capillaries and pillar cells, respectively
3.5. Electron microscopic immunocytochemistry
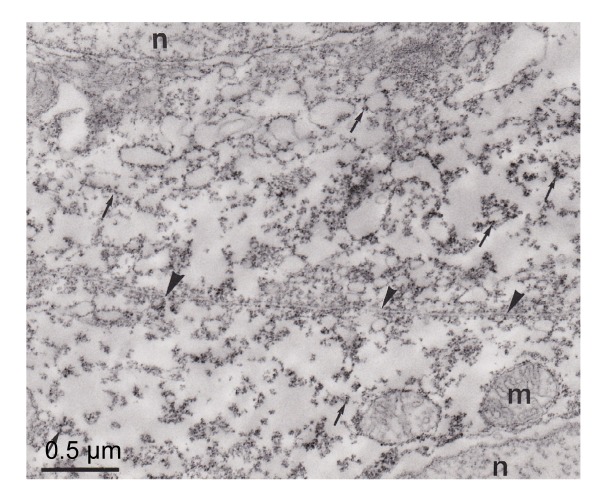
CA was distributed in pseudobranchial cells, mainly in the apical (anti-vascular) region of cells (Figs. 4–6). The tubular system in the basal (vascular) part of the cells and mitochondria were not marked (Figs. 5 and 6). These immunocytochemical results showed that some CA molecules were cytoplasmic and others were connected with membranous structures, particularly the plasma membrane of cells (Fig. 5). The lacunar tissue layers that separated the pseudobranch lamellae from the endothelium of capillaries did not display immunoreactivity using the immunocytochemical technique (Fig. 6). The control sections with non-immune rabbit serum instead of immune serum at an identical dilution did not show immunostaining (Fig. 7).
Fig. 4.
Ultrathin section of pseudobranch cells
The immunoreactivity distributed in the cytoplasm and linked with membranous structures (arrows). m: mitochondria; n: nuclei
Fig. 6.
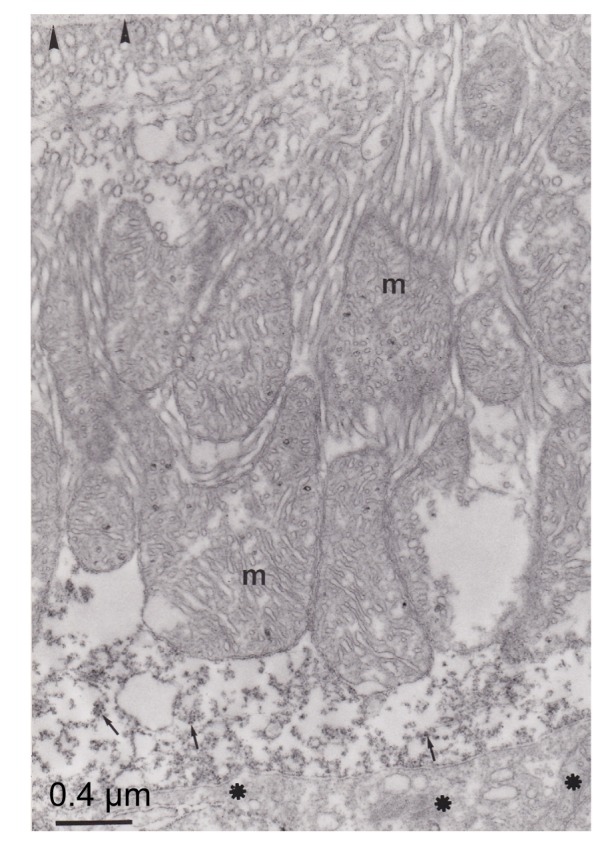
Electron micrograph of pseudobranch cells
Note that the immunoreactivity was distributed in the apical part of the cell (arrow). Lacunars tissue (asterisks) is not marked. Basement membrane (arrowhead) was not immunostained. Mitochondria (m) and tubular system are not marked
Fig. 5.
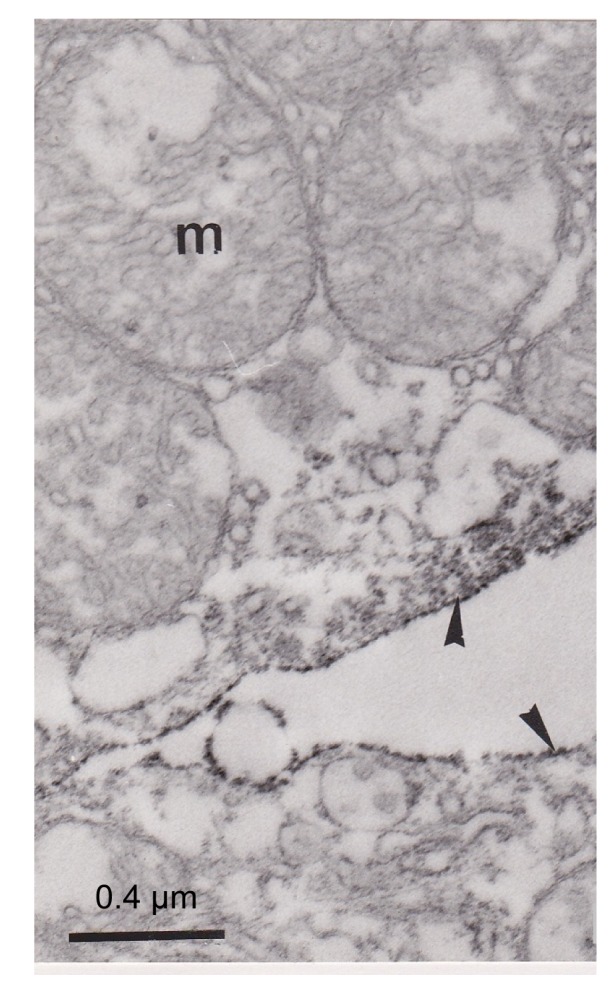
Ultrathin section showing apical parts (arrowheads) of two separated pseudobranch cells
Note that the immunoreactivity was distributed principally in the apical part of the cell and the plasma membrane of these two cells was highly marked (arrowhead). m: mitochondria
Fig. 7.
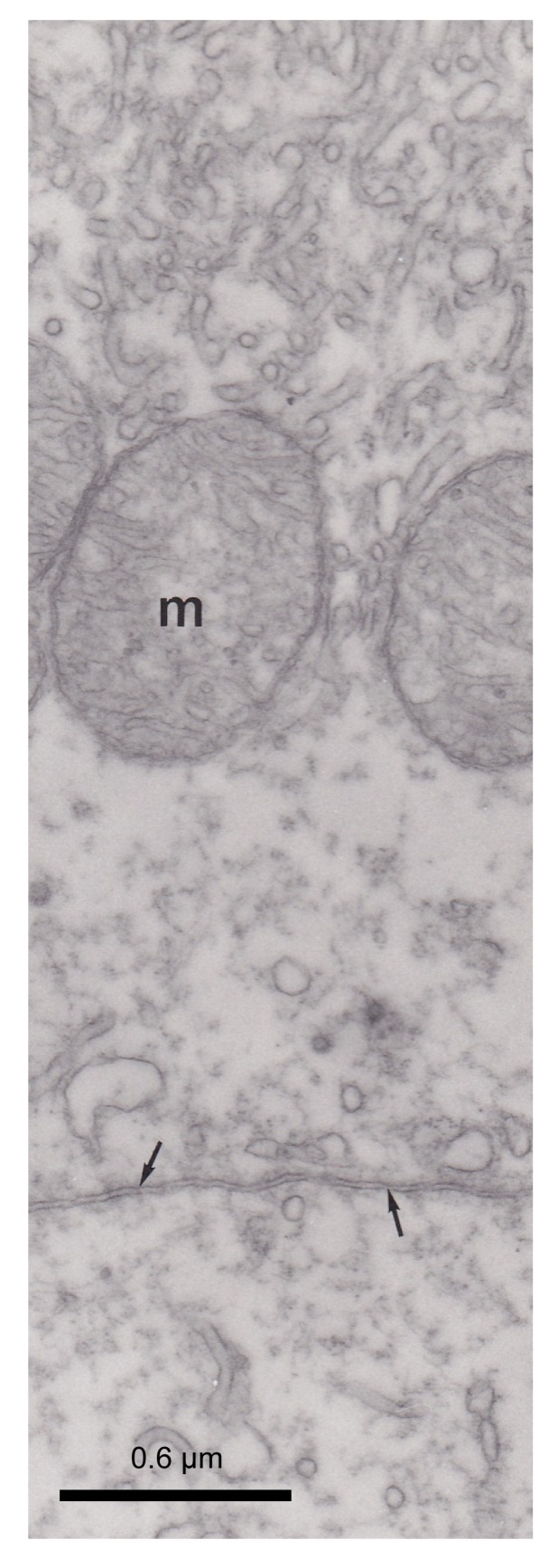
Control section with non-immune rabbit serum which did not revealed any immunoreactivity
Arrows indicates apical parts of two adjacent pseudobranch cells. m: mitochondria
4. Discussion
CA localization in the pseudobranch tissue observed under light and electron microscopes confirmed the presence and localization of CA within pseudobranch cells. Some CA molecules were distributed in the cytoplasm of the apical (anti-vascular) area of pseudobranch cells, and some were connected with membranous structures, particularly with the apical membrane of cells. The results also showed that the lacunar tissues contiguous to the apical part of pseudobranch cells did not display immunostaining. The pillar cells and capillary endothelium were also not immunostained. These results did not agree with those obtained by a previous histochemical technique (Laurent et al., 1969), in which the histochemical reaction products were found to be distributed in the basal part of pseudobranch cells. Our immunohistochemical results that localized the enzyme per se did not agree with the results of a cobalt histochemical technique. This technique localized the reaction products catalyzed by CA but suggests that the product cobalt carbonate may accumulate in the invaginations of the basolateral membrane. On the other hand, the immunocytochemical localization of pseudobranch CA is similar to that of CA in the gill epithelium of rainbow trout (Rahim et al., 1988), and the enzyme is distributed in the apical part of gill epithelium. This finding suggested that the function of CA is related to the external environment. Regarding the pseudobranch, our results suggested that CA can function in the same manner but in relation to the extracellular medium and not to the respired water.
CA in the apical part of pseudobranch cells is involved in the stimulation of afferent nerve fibers (Laurent, 1967; Laurent et al., 1969). The stimulation of afferent fibers occurs not through electrical or chemical synapses but through a liquid phase in which changes in the extracellular ionic composition may act on nerve endings. For example, stimulation of afferent nerve fibers can be the consequence of a direct action of K+ ions released by pseudobranch cells when the H+ ion concentration increases, particularly under the conditions of hypoxia and hypercapnia. CA may be able to speed up the response time to these stimuli.
In conclusion, our results demonstrated that pseudobranch CA was immunolocalized in the apical (anti-vascular) part of cells, different from the finding of the histochemical method of Hansson (1967). However, further physiological studies are needed to elucidate the functions of the pseudobranch and CA.
Footnotes
Compliance with ethics guidelines: S. M. RAHIM, A. G. MAZLAN, K. D. SIMON, J. P. DELAUNOY, and P. LAURENT declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Berenbrink M. Historical reconstructions of evolving physiological complexity: O2 secretion in the eye and swim bladder of fishes. J Exp Biol. 2007;210(9):1641–1652. doi: 10.1242/jeb.003319. [DOI] [PubMed] [Google Scholar]
- 2.Bridges CR, Berenbrink M, Müller R, et al. Physiology and biochemistry of the pseudobranch: an unanswered question? Comp Biochem Physiol Part A Mol & Int Physiol. 1998;119(1):67–77. doi: 10.1016/S1095-6433(97). [DOI] [PubMed] [Google Scholar]
- 3.Broussonet PMA. Mémoire pour servir à l′histoire de la respiration des Poissons. Mem Acad R Sci Paris. 1785:174–196. (in French) [Google Scholar]
- 4.Cammer W, Tansey FA. Immunocytochemical localization of carbonic anhydrase in myelinated fiber in peripheral nerves of rat and mouse. J Histochem Cytochem. 1987;35(8):865–870. doi: 10.1177/35.8.3110266. [DOI] [PubMed] [Google Scholar]
- 5.Churg A. Carbonic anhydrase histochemistry: evidence for non-enzymatic reaction and artifact production. Histochimie. 1973;36(4):293–302. doi: 10.1007/BF00305708. [DOI] [PubMed] [Google Scholar]
- 6.Delaunoy JP. Etude du I' Anhydrase Carbonique (EC4.2.II) Dans le System Nerveux Central du Rat. Strasbourg, France: University of Louis-Pasteur; 1983. (in French) PhD Thesis. [Google Scholar]
- 7.Dimberg K. Investigation of pseudobranch organ in rainbow trout (Oncorhyncus mykiss): endogenous substrates and activities of carbonic anhydrase, lactate dehydrogenase and 3-hydroxy-acyl CoA dehydrogenase. Fish Physiol Biochem. 1995;14(4):323–327. doi: 10.1007/BF00004070. [DOI] [PubMed] [Google Scholar]
- 8.Ekinci D, Ceyhun SB, Sentürk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax . Bioorg Med Chem. 2011;19(2):744–748. doi: 10.1016/j.bmc.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isoenzymes in the respiratory system of vertebrates. Respir Physiol Neurobiol. 2006;154(1):185–198. doi: 10.1016/j.resp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Esbaugh AJ, Perry SF, Bayaa M, et al. Cytoplasmic carbonic anhydrase isozymes in rainbow trout Oncorhynchus mykiss: comparative physiology and molecular evolution. J Exp Biol. 2005;208(10):1951–1961. doi: 10.1242/jeb.01551. [DOI] [PubMed] [Google Scholar]
- 11.Filippi D, Sciaky M, Limozin N, et al. Anhydrase carbonique de system nerveux central du rat. Isolement et proprieties. Biochimie. 1978;60:99–102. doi: 10.1016/s0300-9084(78)80206-5. (in French) [DOI] [PubMed] [Google Scholar]
- 12.Georgalis T, Perry SF, Gilmour KM. The role of branchial carbonic anhydrase in acid-base regulation in rainbow trout (Oncorhynchus mykiss) J Exp Biol. 2006;209(3):518–530. doi: 10.1242/jeb.02018. [DOI] [PubMed] [Google Scholar]
- 13.Georgalis T, Gilmour KM, Yorston J, et al. Roles of cytosolic and membrane-bound carbonic anhydrase in renal control of acid-base balance in rainbow trout (Oncorhynchus mykiss) Am J Physiol Renal Physiol. 2006;291(2):F407–F421. doi: 10.1152/ajprenal.00328.2005. [DOI] [PubMed] [Google Scholar]
- 14.Gilmour KM, Perry SF. Carbonic anhydrase and acid-base regulation in fish. J Exp Biol. 2009;212(11):1647–1661. doi: 10.1242/jeb.029181. [DOI] [PubMed] [Google Scholar]
- 15.Graham R, Karnovsky MJ. The early stage of adsorption of injected horse radish peroxidase in the proximal tubules of mouse kidney: ultrstructurale cytochemistry by a new technique. J Histochem Cytochem. 1966;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- 16.Hansson HPJ. Histochemical demonstration of carbonic anhydrase activity. Histochimie. 1967;11(2):112–128. doi: 10.1007/BF00571716. [DOI] [PubMed] [Google Scholar]
- 17.Hassan I, Shajee B, Waheed A, et al. Structure, function and application of carbonic anhydrase isozymes. Bioorg Med Chem. 2012;21(6):1570–1582. doi: 10.1016/j.bmc.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Hyrtl J. Beobachtungen aus dem gebiete der vergleichenden gefasslehere. II. Uber den bau der kiemen der fische. Med Jahrb. 1838;15:232–248. (in German) [Google Scholar]
- 19.Kumpulainen T. Human carbonic anhydrase isozyme C. Histochemistry. 1981;72(3):425–431. doi: 10.1007/BF00501784. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Laurent P. La pseudobranchie des téléostéens: preuves electrophsiologiques de ses fonctions chémoréceptrice et baroréceptrice. CR Hebd Séances Acad Sci. 1967;264:1879–1882. (in French) [PubMed] [Google Scholar]
- 22.Laurent P. Pseudobranchial receptors in teleosts. In: Fessard A, editor. Electroreceptors and Other Specialized Receptors in Lower Vertrebrates. Springer Verlag; 1974. pp. 279–296. [DOI] [Google Scholar]
- 23.Laurent P, Rouzeau J. Afferent neural activity from pseudobranch of teleost. Effect of P O2, pH, osmotic pressure and Na+ ions. Respir Physiol. 1972;14(3):307–331. doi: 10.1016/0034-5687(72)90037-0. [DOI] [PubMed] [Google Scholar]
- 24.Laurent P, Dunel-Erb S. The pseudobranch: morphology and function. In: Hoar WS, Randall DJ, editors. Fish Physiology. Vol. X. New York: Academic Press; 1984. pp. 285–325. [Google Scholar]
- 25.Laurent P, Dunel S, Barets A. Localisation histochimique de l’anhydrase carbonique au niveau de chémorécepteurs artériels de mammifères, des batraciens et des poisons. Histochimie. 1969;17(2):99–107. doi: 10.1007/BF00277775. (in French) [DOI] [PubMed] [Google Scholar]
- 26.Lin TY, Liao BK, Horng JL, et al. Carbonic anhydrase 2-like a and 15a are involved in acid-base regulation and Na+ uptake in zebrafish H+-ATPase-rich cells. Am J Physiol Cell Physiol. 2008;294(5):C1250–C1260. doi: 10.1152/ajpcell.00021.2008. [DOI] [PubMed] [Google Scholar]
- 27.Maetz J. Le rôle biologique de l'anhydrase carbonique chez quelques téléostéens. Bull Biol France-Belgique Suppl. 1956;XL:1–129. (in French) [Google Scholar]
- 28.Mölich A, Waser W, Heisler N. The teleost pseudobranch: a role for preconditioning of ocular blood supply? Fish Physiol Biochem. 2009;35(2):273–286. doi: 10.1007/s10695-008-9207-4. [DOI] [PubMed] [Google Scholar]
- 29.Muther TF. On the lack of specificity of the cobalt bicarbonate method for carbonic anhydrase. J Histochem Cytochem. 1977;25(9):1043–1050. doi: 10.1177/25.9.71324. [DOI] [PubMed] [Google Scholar]
- 30.Quinn MCJ, Veillette PA, Young G. Pseudobranch and gill Na+, K+-ATPase activity in juvenile Chinook salmon, Oncorhynchus tshaytscha: developmental changes and effect of growth hormone, cortisol and seawater transfer. Comp Biochem Physiol Part A Mol Int Physiol. 2003;135(2):249–262. doi: 10.1016/S1095-6433(03). [DOI] [PubMed] [Google Scholar]
- 31.Rahim SM. Contribution à l' Étude de l' Anhydrase Carbonique des Poissons: Mise en Evidence de Deux Isoenzymes Érythrocytaire et Branchiale. Strasbourg, France: University Louis Pasteur; 1988. (in French) PhD Thesis. [Google Scholar]
- 32.Rahim SM, Delaunoy JP, Laurent P. Identification and immunocytochemical localization of two carbonic anhydrase isoenzymes in teleostean fish erythrocytes and gill epithelia. Histochemistry. 1988;89(5):451–459. doi: 10.1007/BF00492602. [DOI] [PubMed] [Google Scholar]
- 33.Rummer JL, Brauner CJ. Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: in vitro evidence in rainbow trout, Oncorhynchus mykiss . J Exp Biol. 2011;214(14):2319–2328. doi: 10.1242/jeb.054049. [DOI] [PubMed] [Google Scholar]
- 34.Sethi KK, Verma SM, Kumar PM, et al. Carbonic anhydrase I and II inhibition with natural products: Leucas cephalotes . Pharmacogn Commun. 2011;1(2):41–46. doi: 10.5530/pc.2011.2.8. [DOI] [Google Scholar]
- 35.Supuran CT. Carbonic anhydrase—an overview. Curr Pharm Des. 2008;14(7):603–614. doi: 10.2174/138161208783877884. [DOI] [PubMed] [Google Scholar]
- 36.Towbin H, Staekelin T, Gordon T. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. PNAS. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waser W, Heisler N. Oxygen delivery to the fish eye: root effect as crucial factor for elevated retinal P O2 . J Exp Biol. 2005;208(21):4035–4047. doi: 10.1242/jeb.01874. [DOI] [PubMed] [Google Scholar]