Abstract
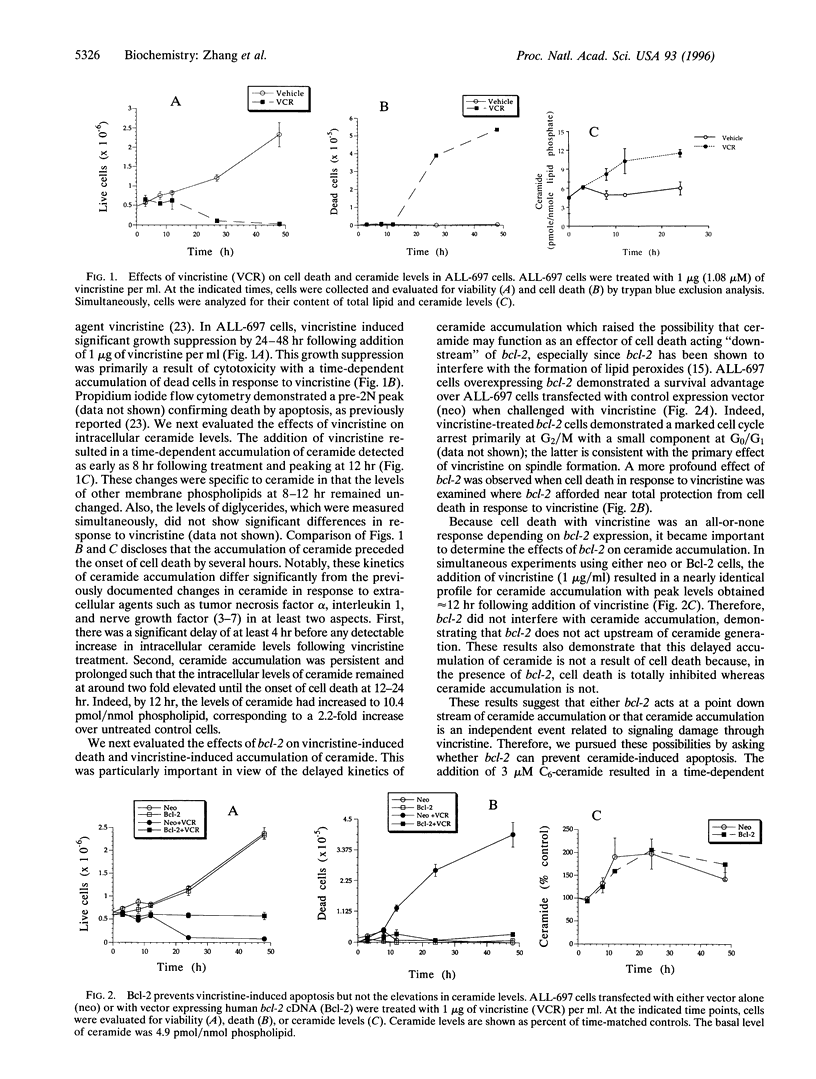
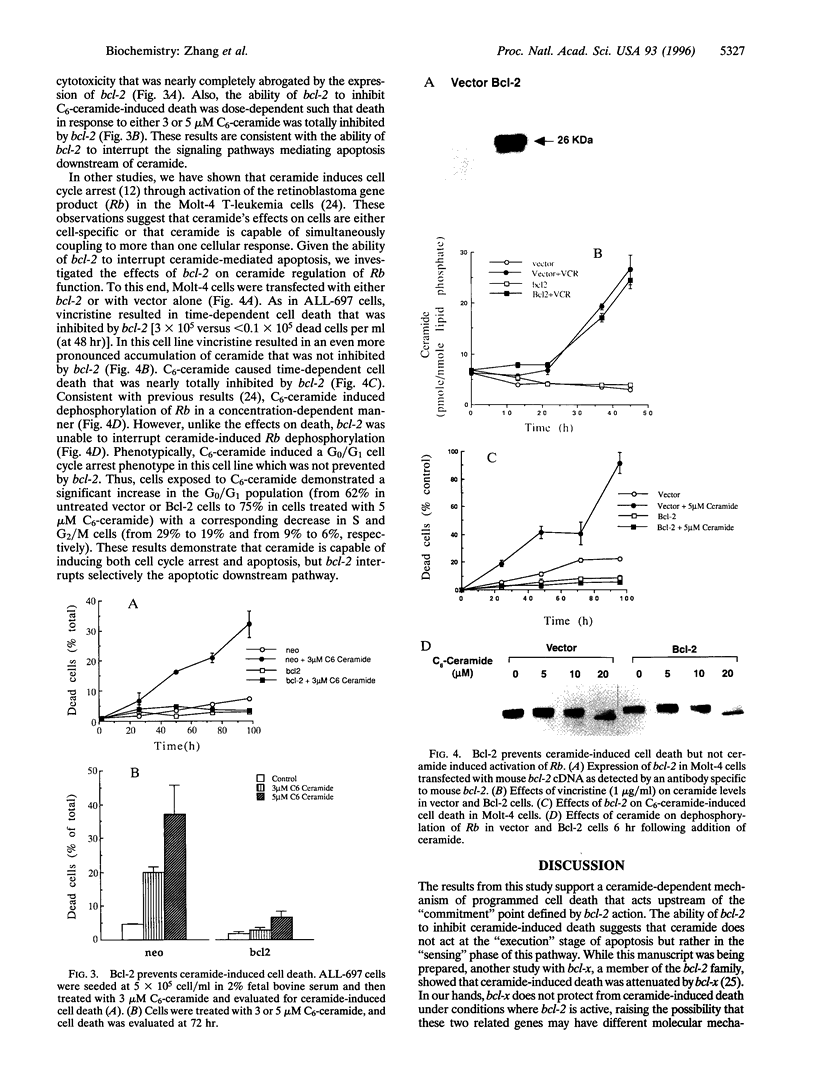
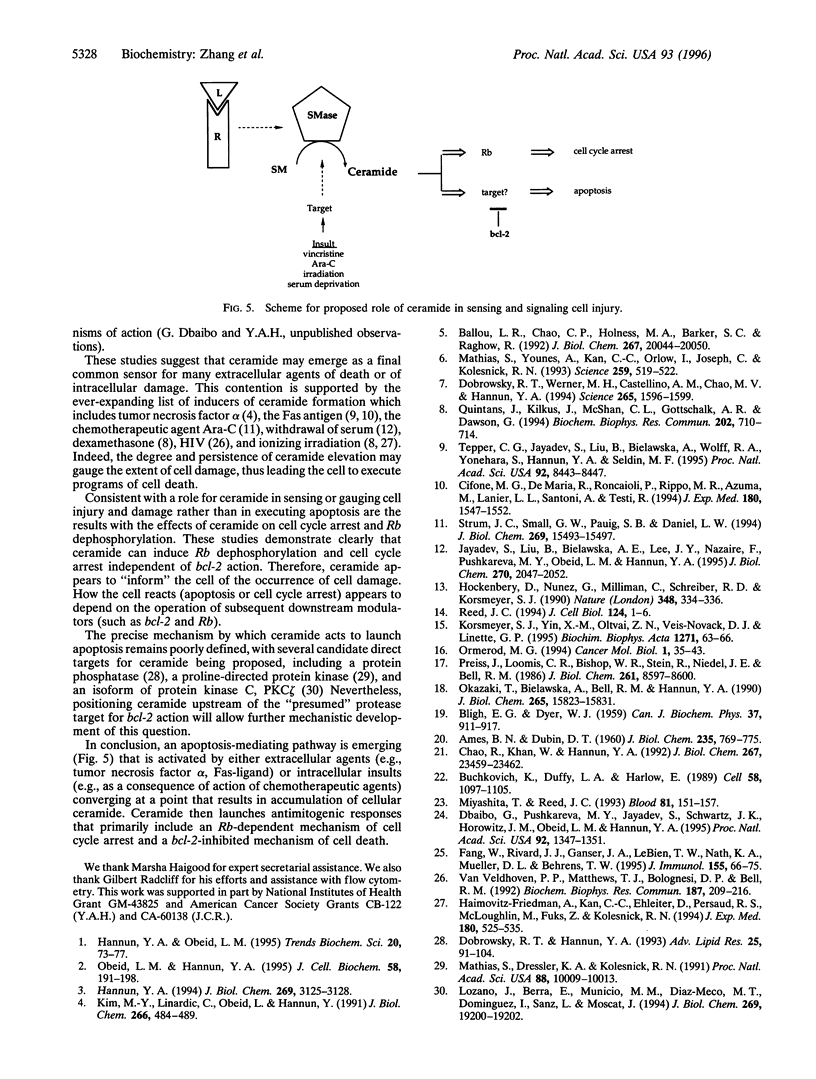
Ceramide, a product of sphingomyelin turn-over, has been proposed as a novel lipid second messenger with specific roles in mediating antiproliferative responses including apoptosis and cell cycle arrest. In this study, we examine the relationship between the ceramide-mediated pathway of growth suppression and the bcl-2 protooncogene. In ALL-697 leukemia cells, the addition of the chemotherapeutic agent vincristine resulted in a time-dependent growth suppression characterized by marked apoptosis. The effects of vincristine on cell death were preceded by a prolonged and sustained accumulation of endogenous ceramide levels reaching -10.4 pmol ceramide/nmol phospholipids at 12 hr following the addition of vincristine--an increase of 220% over vehicle-treated cells. Overexpression of bcl-2 resulted in near total protection of cell death in response to vincristine. However, the ceramide response to vincristine was not modulated by overexpression of bcl-2, indicating that bcl-2 does not interfere with ceramide formation. Overexpression of bcl-2 prevented apoptosis in response to ceramide, suggesting that bcl-2 acts at a point downstream of ceramide. On the other hand, bcl-2 did not interfere with the ability of ceramide to activate the retinoblastoma gene product or to induce cell cycle arrest, suggesting that the effects of ceramide on cell cycle arrest can be dissociated from the effects on apoptosis. These studies suggest that ceramide and bcl-2 partake in a common pathway of cell regulation. The results also cast ceramide as a gauge of cell injury rather than an "executor" of cell death with clearly dissociable biological outcomes of its action depending on downstream factors.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballou L. R., Chao C. P., Holness M. A., Barker S. C., Raghow R. Interleukin-1-mediated PGE2 production and sphingomyelin metabolism. Evidence for the regulation of cyclooxygenase gene expression by sphingosine and ceramide. J Biol Chem. 1992 Oct 5;267(28):20044–20050. [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Chao R., Khan W., Hannun Y. A. Retinoblastoma protein dephosphorylation induced by D-erythro-sphingosine. J Biol Chem. 1992 Nov 25;267(33):23459–23462. [PubMed] [Google Scholar]
- Cifone M. G., De Maria R., Roncaioli P., Rippo M. R., Azuma M., Lanier L. L., Santoni A., Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994 Oct 1;180(4):1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbaibo G. S., Pushkareva M. Y., Jayadev S., Schwarz J. K., Horowitz J. M., Obeid L. M., Hannun Y. A. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1347–1351. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky R. T., Hannun Y. A. Ceramide-activated protein phosphatase: partial purification and relationship to protein phosphatase 2A. Adv Lipid Res. 1993;25:91–104. [PubMed] [Google Scholar]
- Dobrowsky R. T., Werner M. H., Castellino A. M., Chao M. V., Hannun Y. A. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994 Sep 9;265(5178):1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Fang W., Rivard J. J., Ganser J. A., LeBien T. W., Nath K. A., Mueller D. L., Behrens T. W. Bcl-xL rescues WEHI 231 B lymphocytes from oxidant-mediated death following diverse apoptotic stimuli. J Immunol. 1995 Jul 1;155(1):66–75. [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Obeid L. M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995 Feb;20(2):73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994 Feb 4;269(5):3125–3128. [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jayadev S., Liu B., Bielawska A. E., Lee J. Y., Nazaire F., Pushkareva MYu, Obeid L. M., Hannun Y. A. Role for ceramide in cell cycle arrest. J Biol Chem. 1995 Feb 3;270(5):2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- Kim M. Y., Linardic C., Obeid L., Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor alpha and gamma-interferon. Specific role in cell differentiation. J Biol Chem. 1991 Jan 5;266(1):484–489. [PubMed] [Google Scholar]
- Korsmeyer S. J., Yin X. M., Oltvai Z. N., Veis-Novack D. J., Linette G. P. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta. 1995 May 24;1271(1):63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- Lozano J., Berra E., Municio M. M., Diaz-Meco M. T., Dominguez I., Sanz L., Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994 Jul 29;269(30):19200–19202. [PubMed] [Google Scholar]
- Mathias S., Dressler K. A., Kolesnick R. N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S., Younes A., Kan C. C., Orlow I., Joseph C., Kolesnick R. N. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1 beta. Science. 1993 Jan 22;259(5094):519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- Obeid L. M., Hannun Y. A. Ceramide: a stress signal and mediator of growth suppression and apoptosis. J Cell Biochem. 1995 Jun;58(2):191–198. doi: 10.1002/jcb.240580208. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Bielawska A., Bell R. M., Hannun Y. A. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990 Sep 15;265(26):15823–15831. [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Quintans J., Kilkus J., McShan C. L., Gottschalk A. R., Dawson G. Ceramide mediates the apoptotic response of WEHI 231 cells to anti-immunoglobulin, corticosteroids and irradiation. Biochem Biophys Res Commun. 1994 Jul 29;202(2):710–714. doi: 10.1006/bbrc.1994.1988. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum J. C., Small G. W., Pauig S. B., Daniel L. W. 1-beta-D-Arabinofuranosylcytosine stimulates ceramide and diglyceride formation in HL-60 cells. J Biol Chem. 1994 Jun 3;269(22):15493–15497. [PubMed] [Google Scholar]
- Tepper C. G., Jayadev S., Liu B., Bielawska A., Wolff R., Yonehara S., Hannun Y. A., Seldin M. F. Role for ceramide as an endogenous mediator of Fas-induced cytotoxicity. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8443–8447. doi: 10.1073/pnas.92.18.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Matthews T. J., Bolognesi D. P., Bell R. M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992 Aug 31;187(1):209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]




