Abstract
Interferon treatment of AKR,C- cells was followed by the establishment of an antiviral state and apparently concomitant morphological, physical, and biochemical alterations of the cell plasma membrane. The density of the plasma membrane was significant altered, and the concentration of some plasma membrane glycoproteins and the number of intramembranous particles observed in freeze-fracture electron micrographs were increased. A parallel increase in the concentration of intramembranous particles and the resistance to viral infection during interferon treatment as well as their parallel decrease upon removal of interferon suggests a relationship between the particle density and the establishment of the antiviral state.
Full text
PDF
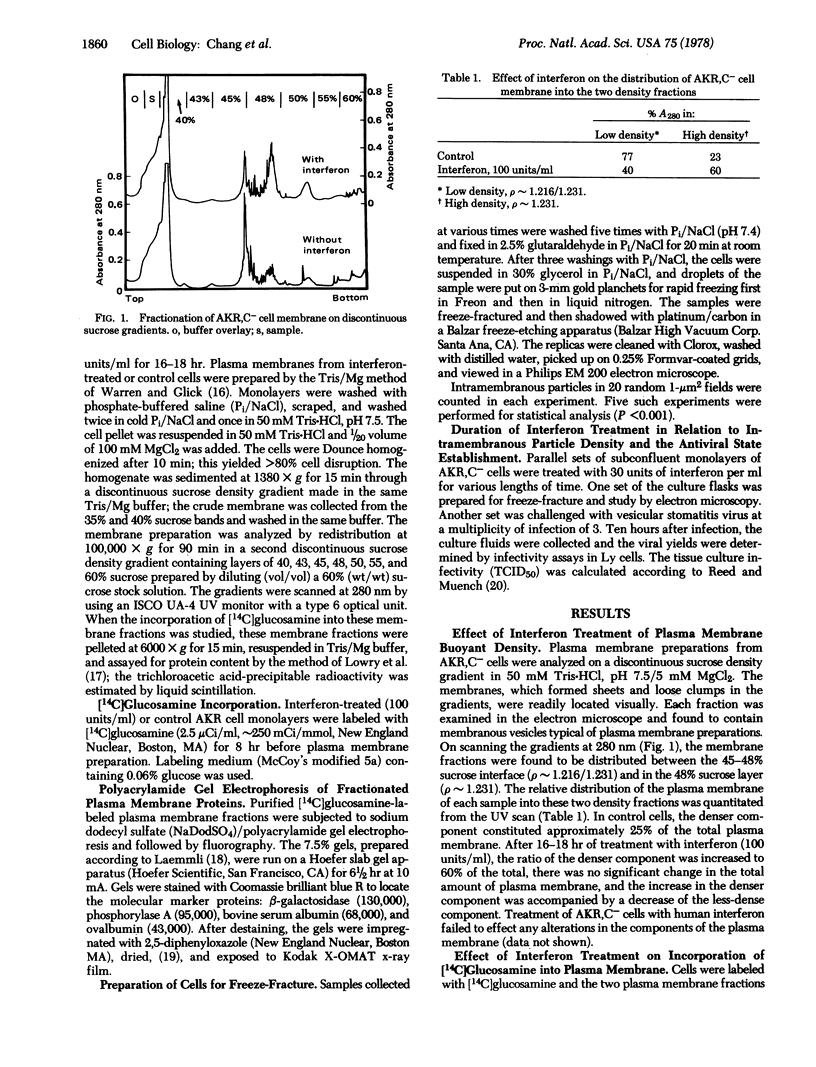

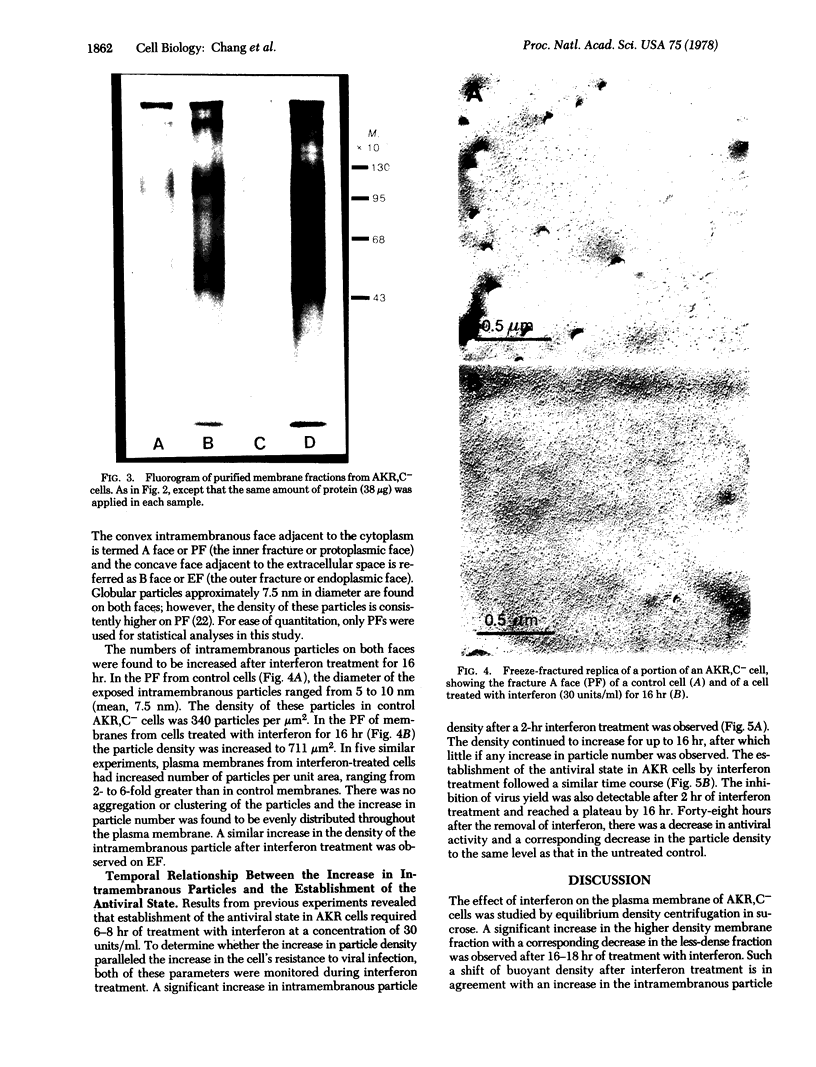
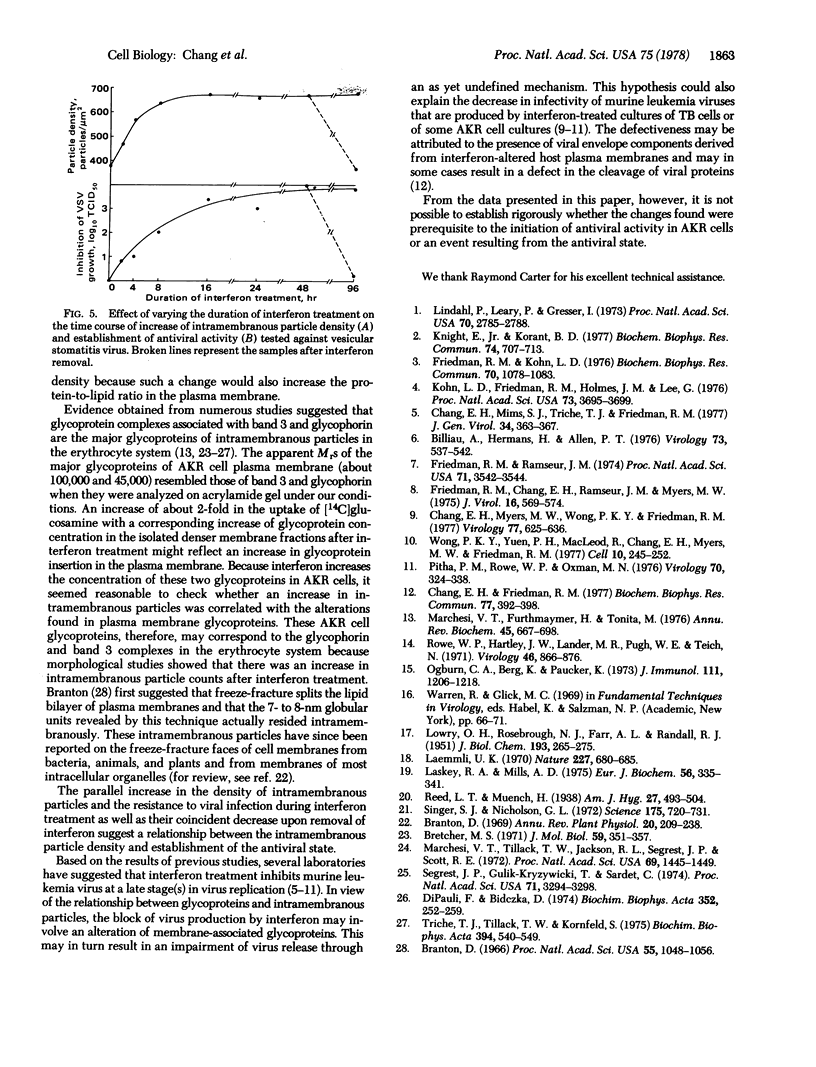
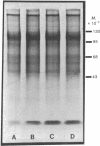
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., Heremans H., Allen P. T., De Maeyer-Guignard J., De Somer P. Trapping of oncornavirus particles at the surface of interferon-treated cells. Virology. 1976 Sep;73(2):537–542. doi: 10.1016/0042-6822(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. A major protein which spans the human erythrocyte membrane. J Mol Biol. 1971 Jul 28;59(2):351–357. doi: 10.1016/0022-2836(71)90055-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Friedman R. M. A large glycoprotein of Moloney leukemia virus derived from interferon-treated cells. Biochem Biophys Res Commun. 1977 Jul 11;77(1):392–398. doi: 10.1016/s0006-291x(77)80210-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Mims S. J., Triche T. J., Friedman R. M. Interferon inhibits mouse leukaemia virus release: an electron microscope study. J Gen Virol. 1977 Feb;34(2):363–367. doi: 10.1099/0022-1317-34-2-363. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Myers M. W., Wong P. K., Friedman R. M. The inhibitory effect of interferon on a temperature-sensitive mutant of Moloney murine leukemia virus. Virology. 1977 Apr;77(2):625–636. doi: 10.1016/0042-6822(77)90487-1. [DOI] [PubMed] [Google Scholar]
- Di Pauli G., Brdiczka D. Localization of glycoproteins within erythrocyte membranes of sheep. A freeze-etching and biochemical study. Biochim Biophys Acta. 1974 Jun 13;352(2):252–259. doi: 10.1016/0005-2736(74)90216-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Chang E. H., Ramseur J. M., Myers M. W. Interferon-directed inhibition of chronic murine leukemia virus production in cell cultures: lack of effect on intracellular viral markers. J Virol. 1975 Sep;16(3):569–574. doi: 10.1128/jvi.16.3.569-574.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Kohn L. D. Cholera toxin inhibits interferon action. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1078–1084. doi: 10.1016/0006-291x(76)91012-3. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. A cell surface alteration in mouse L cells induced by interferon. Biochem Biophys Res Commun. 1977 Jan 24;74(2):707–713. doi: 10.1016/0006-291x(77)90360-6. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Friedman R. M., Holmes J. M., Lee G. Use of thyrotropin and cholera toxin to probe the mechanism by which interferon initiates its antiviral activity. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3695–3699. doi: 10.1073/pnas.73.10.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogburn C. A., Berg K., Paucker K. Purification of mouse interferon by affinity chromatography on anti-interferon globulin-sepharose. J Immunol. 1973 Oct;111(4):1206–1218. [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Gulik-Krzywicki T., Sardet C. Association of the membrane-penetrating polypeptide segment of the human erythrocyte MN-glycoprotein with phospholipid bilayers. I. Formation of freeze-etch intramembranous particles. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3294–3298. doi: 10.1073/pnas.71.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Triche T. J., Tillack T. W., Kornfeld S. Localization of the binding sites for the Ricinus communis, Agaricus bisporus and wheat germ lectins on human erythrocyte membranes. Biochim Biophys Acta. 1975 Jul 18;394(4):540–549. doi: 10.1016/0005-2736(75)90139-x. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Yuen P. H., MacLeod R., Chang E. H., Myers M. W., Friedman R. M. The effect of interferon on de novo infection of Moloney murine leukemia virus. Cell. 1977 Feb;10(2):245–252. doi: 10.1016/0092-8674(77)90218-5. [DOI] [PubMed] [Google Scholar]