Abstract
DOCK8 deficiency is a primary immunodeficiency characterized by recurrent sinopulmonary infections, dermatitis with cutaneous infections, elevated serum IgE levels, eosinophilia, and a high incidence of food allergy. Given the seriousness of DOCK8 deficiency, it is important to recognize it early and initiate appropriate therapy. Diagnosis relies on examining DOCK8 protein expression and sequencing of the 48 exons in the DOCK8 gene, but these assays are not always readily available. A major problem facing clinicians is that DOCK8 deficiency shares many clinical and laboratory features with severe atopic dermatitis. Here, we have identified biomarkers routinely measured by flow cytometry on whole blood in clinical immunology laboratories that may be used in distinguishing DOCK8 deficiency from severe atopic dermatitis. The use of these biomarkers may help the clinician identify those patients who are most likely to have DOCK8 mutations and would benefit from further specialized diagnostic testing.
Keywords: DOCK8, Hyper-IgE, atopic dermatitis, T cell, B cell
1. INTRODUCTION
Autosomal recessive Hyper-IgE syndrome is a primary immunodeficiency caused by mutations in the Dedicator of Cytokinesis-8 (DOCK8) gene. It is characterized by recurrent sinopulmonary infections, cutaneous viral infections, dermatitis, elevated serum IgE levels, eosinophilia, a high incidence of food allergies, and early development of squamous cell carcinomas and lymphoid malignancies [1, 2, 3]. There is a growing consensus that hematopoietic stem cell transplantation should be performed early for DOCK8 deficient patients before irreversible complications, including tissue damage and the development of malignancy, occur. Given its seriousness, it is important to recognize DOCK8 deficiency and treat it early, especially since hematopoietic stem cell reconstitution from normal allogeneic donors has proven to be curative [4, 5, 6, 7, 8, 9, 10].
Since most of the patients with DOCK8 deficiency lack DOCK8 protein expression [1, 3], the diagnostic approach is immunoblotting for DOCK8 in cell lysates followed by confirmatory sequencing of the DOCK8 gene. Since immunoblotting is not available at all centers, samples are often sent to interested research laboratories. The majority of DOCK8 deficient patients have been identified in Middle Eastern countries with high degrees of consanguinity [2, 11, 12, 13, 14]. Most of the centers in this region rely on shipping samples to laboratories in Europe or the United States for laboratory confirmation of the clinical diagnosis of DOCK8 deficiency. In our experience, protein degradation in blood cells often occurs during the shipping process. To circumvent this limitation, we derive Epstein Barr Virus transformed cell lines and use them for immunoblotting, which is time consuming and requires tissue culture facilities. Furthermore, sequencing of the DOCK8 gene is onerous due to its large size with 48 exons and is performed only at a few centers.
A major problem facing clinicians is that DOCK8 deficiency shares clinical and laboratory features with severe atopic dermatitis (AD); therefore, patients with DOCK8 deficiency may be misdiagnosed as having severe AD. Conversely, patients with severe AD may be unnecessarily subjected to diagnostic investigations that consume scarce resources. Given that AD affects >10% of children and severe AD affects approximately 0.5% of all children [15], the costs involved in using genetic diagnosis alone to distinguish between severe AD and DOCK8 deficiency are potentially prohibitive. In this study, we examined two cohorts of children with an established genetic diagnosis of DOCK8 deficiency or severe AD to test the hypothesis that aberrations in lymphocyte subsets evaluated by flow cytometry on whole blood would distinguish between these two groups.
2. MATERIAL and METHODS
2.1 Patients
DOCK8 deficient patients were referred to us through the International Consortium for Immunodeficiency, a collaborative network of primary immunodeficiency centers in the Middle East and North Africa where consanguineous marriages are frequent. Blood samples were obtained from patients either during their evaluation at Boston Children’s Hospital or were shipped from collaborators for analysis within 48 hrs. All DOCK8 deficient patients had sequencing of their DOCK8 gene performed and had deleterious mutations or deletions identified (Supplemental Table I). Blood samples from AD patients were obtained during routine visits to the Atopic Dermatitis Center at Boston Children’s Hospital. AD severity was determined using the Rajka-Langeland scoring system [16]. Those with moderate or severe AD (scores ≥ 5) were included (Supplemental Table I). Patients were consented, and samples were collected according institutional IRB guidelines.
2.2 Evaluation of lymphocytes subsets
Flow cytometry was used to measure the percentages of lymphocyte populations in whole blood using the monoclonal antibody conjugates listed in Supplemental Table II. The percentage of each patient's lymphocyte subset was compared with normal controls ranges for age that have either been published [17, 18] or established independently in the Boston Children's flow cytometry laboratory.
2.3 Statistical analysis
Fischer’s exact test was used to compare the fraction of patients in the DOCK8 and AD patient groups for whom the individual lymphocytes subsets fell outside the normal range for age in the same direction. In addition, the odds ratio and 95% confidence interval were calculated using GraphPad Prism (San Diego, CA).
3. RESULTS
3.1 T cell subsets
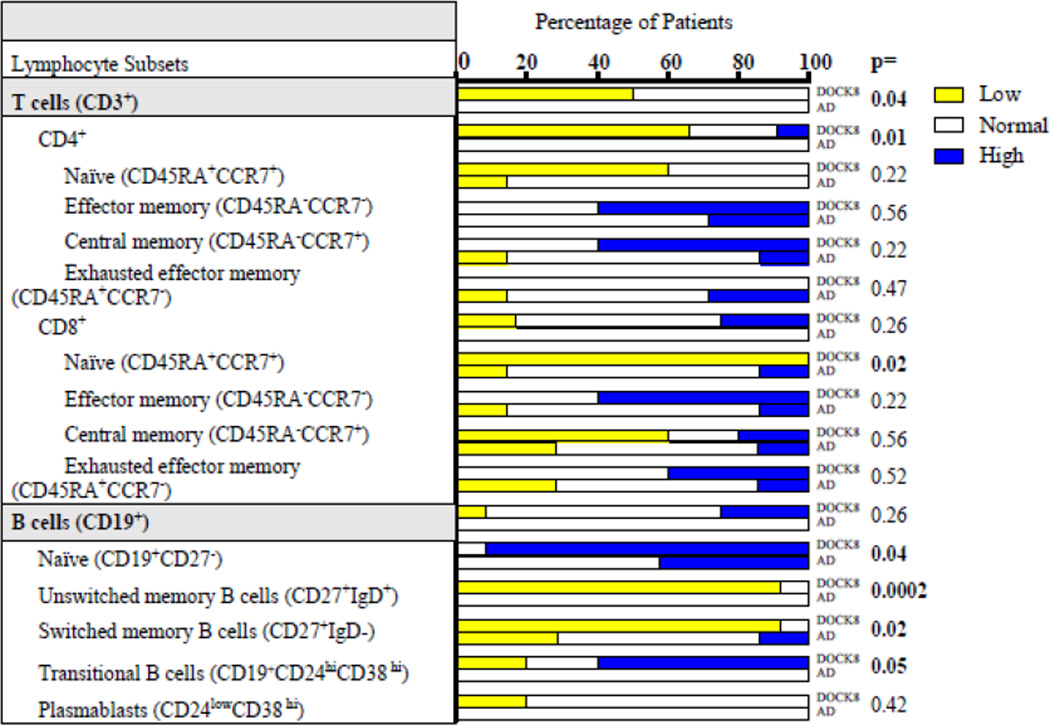
The distribution of individual T lymphocyte subsets in the DOCK8 deficient and AD group of patients relative to the normal range for age is shown in Table I. A significantly higher fraction of DOCK8 deficient patients had percentages of CD3+CD4+and CD8+CD45RA+CCR7+ naïve T cells below the normal range compared to patients with AD. The difference in the fraction of DOCK8 deficient and AD patients with percentages of total CD8+ cells outside the normal range for age was not statistically significant. In addition, when we examined CD4+ subsets (CD45RA+CCR7+ naïve, CD45RA−CCR7− effector memory, CD45RA−CCR7+ central memory, and CD45RA+CCR7− exhausted effector memory), no significant difference was seen. There was a trend towards more DOCK8 deficient patients having elevated percentages of CD8+ memory and exhausted effector memory cells (TEMRA), however this difference was not significant in the number of patients we studied.
Table I.
Evaluated lymphocyte subsets
 |
3.2 B cell subsets
The distribution of individual B lymphocyte subsets in the DOCK8 deficient and the AD groups of patients relative to the normal range for age is shown in Table I. The difference in the fraction of DOCK8 deficient and AD patients with percentages of CD19+ B cells outside the normal range for age was not statistically significant. A significantly higher fraction of patients with DOCK8 deficiency had percentages of CD19+CD27− naive B cells and CD19+CD24hiCD38hi transitional B cells above the normal range compared to patients with AD. Conversely, a significantly higher fraction of DOCK8 deficient compared with AD patients had decreased percentages of CD19+CD27+IgD+ unswitched memory B cells and CD19+CD27+IgD− switched memory B cells. There was not a significant difference in the percentages of plasmablasts between the two groups.
3.3 Odd ratios
We next calculated the odds ratio for the seven lymphocyte subsets for which the DOCK8 deficient and AD groups significantly differed (Table II). This analysis showed that when all seven subsets are out of the normal range, the odds ratio is 26.3 (9.4 – 73.4) in favor of a DOCK8 diagnosis. When only the four B cell subsets that differed between the patient groups were analyzed, the odds ratio was 33.1 (8.6 – 127.2) in favor of a DOCK8 diagnosis.
Table II.
Odds ratios for lymphocyte subset and group analysis
| Odds Ratio | 95% confidence interval |
|
|---|---|---|
| CD3+ T cells | 15 | 0.7 – 320.9 |
| CD4+ T cells | 28.3 | 1.3 – 618.4 |
| Naïve CD8+ T cells (CD8+CD45RA+CCR7+) | 47.7 | 1.6 – 1424 |
| Naïve B cells (CD19+CD27−) | 14.7 | 1.2 – 185.4 |
| Unswitched memory B cells (CD27+IgD+) | 115 | 4.1 – 3216 |
| Switched memory B cells (CD27+IgD−) | 27.5 | 2.0 – 379.1 |
| Transitional B cells (CD19+CD24hiCD38hi) | 21 | 0.78 – 564.6 |
| All 7 subsets above | 26.3 | 9.4 – 73.4 |
| B cell subsets | 33.1 | 8.6 – 127.2 |
4. DISCUSSION
We have identified a set of biomarkers measured by flow cytometry on whole blood that distinguishes DOCK8 deficiency from severe AD. The use of these biomarkers will help the clinician, with access to a standard clinical immunology flow cytometry laboratory, distinguish between DOCK8 deficiency and severe AD.
The percentage of CD3+ and CD4+ T cells and CD8+CD45RA+ CCR7+ naïve T cells, three of eight T cell subsets that are routinely measured in a clinical immunology laboratory on whole blood, was found to be significantly more likely to be outside the normal range in DOCK8 deficient patients than in AD patients. All three discriminating T cell subsets were more likely to be below the normal range in DOCK8 deficient patients than in AD patients. The decrease in CD3+ total T cells and CD4+ T cells is consistent with previous findings that the percentages of CD4+ T cells, the major T cell subset, is significantly lower in patients with DOCK8 deficiency as well in DOCK8 deficient mice [2, 10, 11, 12, 19, 20, 21]. The decrease in peripheral CD4+ T cells in DOCK8 deficient mice was shown to be due to poor survival of mature DOCK8 deficient CD4+ T cells compared with wild-type T cells [21]. The decrease in the percentage CD8+CD45RA+ CCR7+ naïve T cells in the presence of normal percentages of CD8+ T cells is consistent with previous findings [20, 21]. It likely reflects the increase in the percentage of CD8+CD45RA+ CCR7− TEMRA cells previously noted in DOCK8 deficient patients [20], which was also observed in our study. However, an abnormal percentage of TEMRA cells did not discriminate between DOCK8 deficient and AD patients in our study, possibly because the percentage of TEMRA cells was also outside the normal range in some AD patients.
When the B cell percentage was measured on whole blood, there was no significant difference between the fraction of DOCK8 deficient patients and AD patients with percentages outside of the normal range. However, the percentage of all four B cell subsets that are routinely measured in a clinical immunology laboratory was found to be significantly more likely to be outside the normal range in DOCK8 deficient patients than in AD patients. Both subsets of memory B cells, the CD27+IgD+ unswitched memory B cells and the CD27+IgD− switched memory B cells, were significantly more likely to be below the normal range in DOCK8 deficient patients than in AD patients. A low percentage for both subsets has been reported in DOCK8 deficient patients and is consistent with the impaired antibody response in these patients [14, 22]. The majority of the CD27+IgD+ unswitched memory B cells are thought to be marginal zone (MZ)-like B cells [23]. The presence of low numbers of MZ B cells is well documented in DOCK8 deficient mice [19]. MZ B cells are the major producers of antibodies to polysaccharide antigens [24]. The decrease in MZ-like B cells in DOCK8 deficient patients is consistent with the impaired response of these patients to bacterial polysaccharide antigens and their susceptibility to infection with encapsulated bacteria [3, 14]. The decrease in CD27+IgD− switched memory B cells in DOCK8 deficient patients is consistent with their well document impaired memory responses to commonly given vaccines [12, 13, 14].
Very recently, a single diagnostic laboratory started offering a flow cytometry assay for DOCK8 expression using a commercially available mAb to DOCK8 (http://www.seattlechildrens.org/research/immunity-and-immunotherapies/immunology-diagnostic-laboratory/ December 15, 2013). We have also developed a flow cytometry assay for DOCK8 expression in lymphocytes using a commercially available mAb, and demonstrated that this assay discriminates readily between normal individuals and DOCK8 deficient patients who are known to lack DOCK8 expression as determined by immunoblotting (S. Pai et al.: manuscript submitted). Although this assay is highly specific, it has several limitations. First, its availability will be restricted to laboratories that are proficient in performing intracellular staining, an expertise not readily available in countries where DOCK8 deficiency is more common. Second, the flow cytometry assay for DOCK8 will not be useful in the few DOCK8 deficient patients with a missense mutation in DOCK8 and near normal expression of the mutant DOCK8 protein as determined by immunoblotting of lysates from peripheral blood mononuclear cells. Third, and most importantly, the assay may not be useful for testing shipped blood samples in which extensive degradation of normal DOCK8 protein can occur as evidenced by immunoblotting.
In summary, a lymphocyte profile on whole blood of CD3+ and CD4+ T cell lymphopenia and decreased naive CD8+ T cells, along with a preserved total B cell percentage in conjunction with a decrease in memory B cells is strongly suggestive of DOCK8 deficiency rather than AD in a patient with severe eczema. The use of these biomarkers, which are readily measured using whole blood in a standard clinical immunology laboratory, may help the clinician identify those patients who are most likely to have DOCK8 mutations and who would benefit from further diagnostic testing by immunoblotting and/or DNA sequencing, thus saving scarce and costly resources.
Supplementary Material
HIGHLIGHTS.
The clinical phenotype of DOCK8 deficiency and severe atopic dermatitis can be similar
Flow cytometry of T and B subsets distinguishes between DOCK8 deficiency and atopic dermatitis
DOCK8 deficient patients have CD3+ and CD4+ T cell lymphopenia with decreased naïve CD8+ T cells
DOCK8 deficient patients have decreased memory and increased naïve and transitional B cells
Acknowledgments
Sources of Funding
This work is supported by the Harvard-Dubai Foundation for Medical Research, USPHS grant AI100315-01A1, and the Jeffrey Modell Foundation.
Abbreviations
- DOCK8
Dedicator of Cytokinesis-8
- AD
atopic dermatitis
- TEMRA
exhausted effector memory cells
- MZ
marginal zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflict of interest.
REFERENCES
- 1.Zhang Q, Davis JC, Dove CG, Su HC. Genetic, clinical, and laboratory markers for DOCK8 immunodeficiency syndrome. Dis Markers. 2010;29:131–139. doi: 10.3233/DMA-2010-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanal O, Jing H, Ozgur T, Ayvaz D, Strauss-Albee DM, Ersoy-Evans S, Tezcan I, Turkkani G, Matthews HF, Haliloglu G, Yuce A, Yalcin B, Gokoz O, Oguz KK, Su HC. Additional diverse findings expand the clinical presentation of DOCK8 deficiency. J Clin Immunol. 2012;32:698–708. doi: 10.1007/s10875-012-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su HC. Dedicator of cytokinesis 8 (DOCK8) deficiency. Curr Opin Allergy Clin Immunol. 2010;10:515–520. doi: 10.1097/ACI.0b013e32833fd718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald DR, Massaad MJ, Johnston A, Keles S, Chatila T, Geha RS, Pai SY. Successful engraftment of donor marrow after allogeneic hematopoietic cell transplantation in autosomal-recessive hyper-IgE syndrome caused by dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2010;126:1304–1305. e3. doi: 10.1016/j.jaci.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh S, Schuster FR, Fuchs I, Laws HJ, Borkhardt A, Meisel R. Treosulfan-based conditioning in DOCK8 deficiency: complete lympho-hematopoietic reconstitution with minimal toxicity. Clin Immunol. 2012;145:259–261. doi: 10.1016/j.clim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Boztug H, Karitnig-Weiss C, Ausserer B, Renner ED, Albert MH, Sawalle-Belohradsky J, Belohradsky BH, Mann G, Horcher E, Rummele-Waibel A, Geyeregger R, Lakatos K, Peters C, Lawitschka A, Matthes-Martin S. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol. 2012;29:585–594. doi: 10.3109/08880018.2012.714844. [DOI] [PubMed] [Google Scholar]
- 7.Metin A, Tavil B, Azik F, Azkur D, Ok-Bozkaya I, Kocabas C, Tunc B, Uckan D. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. Pediatr Transplant. 2012;16:398–399. doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 8.Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan JL, Michel G, Fischer A, Picard C. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011;128:420–422. e2. doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, Wintergerst U, Hauser M, Klein B, Schwarz K, Schmid I, Albert MH. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;222:351–355. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- 10.Gatz SA, Benninghoff U, Schutz C, Schulz A, Honig M, Pannicke U, Holzmann KH, Schwarz K, Friedrich W. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46:552–556. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, Chen A, Kim HS, Lloret MG, Schulze I, Ehl S, Thiel J, Pfeifer D, Veelken H, Niehues T, Siepermann K, Weinspach S, Reisli I, Keles S, Genel F, Kutukculer N, Camcioglu Y, Somer A, Karakoc-Aydiner E, Barlan I, Gennery A, Metin A, Degerliyurt A, Pietrogrande MC, Yeganeh M, Baz Z, Al-Tamemi S, Klein C, Puck JM, Holland SM, McCabe ER, Grimbacher B, Chatila TA. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–1302. e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, Holland SM, Su HC. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsum Z, Hawwari A, Alsmadi O, Al-Hissi S, Borrero E, Abu-Staiteh A, Khalak HG, Wakil S, Eldali AM, Arnaout R, Al-Ghonaium A, Al-Muhsen S, Al-Dhekri H, Al-Saud B, Al-Mousa H. Clinical, immunological and molecular characterization of DOCK8 and DOCK8-like deficient patients: single center experience of twenty-five patients. J Clin Immunol. 2013;33:55–67. doi: 10.1007/s10875-012-9769-x. [DOI] [PubMed] [Google Scholar]
- 14.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, Rauter I, Benson H, Schneider L, Baxi S, Recher M, Notarangelo LD, Wakim R, Dbaibo G, Dasouki M, Al-Herz W, Barlan I, Baris S, Kutukculer N, Ochs HD, Plebani A, Kanariou M, Lefranc G, Reisli I, Fitzgerald KA, Golenbock D, Manis J, Keles S, Ceja R, Chatila TA, Geha RS. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24:476–486. doi: 10.1111/pai.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 17.Piatosa B, Wolska-Kusnierz B, Pac M, Siewiera K, Galkowska E, Bernatowska E. B cell subsets in healthy children: reference values for evaluation of B cell maturation process in peripheral blood. Cytometry B Clin Cytom. 2010;78:372–381. doi: 10.1002/cyto.b.20536. [DOI] [PubMed] [Google Scholar]
- 18.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, Wara DW, Douglas SD, Luzuriaga K, McFarland EJ, Yogev R, Rathore MH, Levy W, Graham BL, Spector SA. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Randall KL, Lambe T, Johnson AL, Treanor B, Kucharska E, Domaschenz H, Whittle B, Tze LE, Enders A, Crockford TL, Bouriez-Jones T, Alston D, Cyster JG, Lenardo MJ, Mackay F, Deenick EK, Tangye SG, Chan TD, Camidge T, Brink R, Vinuesa CG, Batista FD, Cornall RJ, Goodnow CC. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, Tan A, Arkwright PD, Al Suwairi W, Lugo Reyes SO, Yamazaki-Nakashimada MA, Garcia-Cruz Mde L, Smart JM, Picard C, Okada S, Jouanguy E, Casanova JL, Lambe T, Cornall RJ, Russell S, Oliaro J, Tangye SG, Bertram EM, Goodnow CC. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambe T, Crawford G, Johnson AL, Crockford TL, Bouriez-Jones T, Smyth AM, Pham TH, Zhang Q, Freeman AF, Cyster JG, Su HC, Cornall RJ. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol. 2011;41:3423–3435. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.aan de Kerk DJ, van Leeuwen EM, Jansen MH, van den Berg JM, Alders M, Vermont CL, van Lier RA, Pals ST, Kuijpers TW. Aberrant humoral immune reactivity in DOCK8 deficiency with follicular hyperplasia and nodal plasmacytosis. Clin Immunol. 2013;149:25–31. doi: 10.1016/j.clim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 24.Zouali M, Richard Y. Marginal zone B-cells, a gatekeeper of innate immunity. Front Immunol. 2011;2:63. doi: 10.3389/fimmu.2011.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


