Abstract
Vaginal pre-exposure prophylaxis has focused heavily on gel formulations. Low adherence linked with frequent dosing and short therapeutic duration has emerged as the major reason for inconsistent efficacy outcomes with gels in clinical trials. Osmotic pumps can achieve versatile drug release profiles however, have not been explored for vaginal delivery. In this report, we describe an osmotic pump tablet (OPT) that can deliver antiretrovirals for several days. We also describe configuring the OPT for pH sensitive delivery where the drug delivery system consistently delivers a antiretroviral at vaginal pH and then gives a burst release triggered by a coitally associated pH increase. We have investigated the vaginal OPT for multiple day delivery of a potent antiretroviral, IQP-0528 in a sheep model. To effectively register spatial drug distribution we also engineered a tool to precisely collect multiple vaginal fluid samples. In a 10-day duration post single application, high micromolar mucosal levels were obtained with peak concentration more than 6 logs higher than IQP-0528 EC50. Overall, our results show successful implementation of the osmotic pump technology for vaginal antiretroviral delivery.
Keywords: Osmotic pump, vaginal, antiretroviral, spatial drug distribution, multiswab device
The pre-exposure prophylaxis (PrEP) drug delivery system (DDS) portfolio is dominated by short-acting coitally associated gel formulations and long-acting intravaginal rings. Currently the field lacks antiretroviral delivery systems that can be used ‘on-demand’ but with durations between that of gels and intravaginal rings. While ‘on demand’ vaginal tablets can be an alternative with higher user acceptance compared to gels (Minkin et al., 2013; Rioux et al., 2000), they have been less explored as HIV prevention technology platforms. Vaginal tablets can be manufactured easily using standard tabletting equipment, are suitable for formulation of water-sensitive drugs and can have long term stability without cold-chain storage requirements (Adams and Kashuba, 2012). However, a common problem among conventional vaginal tablets and gels is the short duration of pharmacokinetics (PK) for most drugs (an exception is Class I drugs (Amidon et al., 1995) with high intracellular half-lives); therefore, they require repeated dosing to ensure consistently protective drug levels. Frequent administration and the potentially associated low adherence ultimately may impact the performance of topical PrEP agents in clinical trials (Marrazzo J et al., 2013). Therefore, we investigated the use of a medium-duration elementary osmotic pump tablet (OPT) that can actively and in a controlled manner deliver topical antiretrovirals for one to multiple days after a single intravaginal application. This, to our knowledge, is the first reported work on the use of an osmotic pump for topical vaginal drug delivery.
The underlying physics behind osmotic pump technology has not changed despite the large amount of work on the design and application in oral and implantable drug delivery (Theeuwes, 1975). Drug release rate from these systems is typically a function of rate of water entry into the device due to an osmotic pressure gradient between the device core and the environment (Theeuwes and Yum, 1976). The osmotic pressure difference can be controlled by the nature and concentration of the osmotic agent, the water vapor transmission rate (P) of the semipermeable membrane (SPM) and geometry of the drug delivery orifices (Fig. 1a). An interplay between these properties, aids in achieving controlled zero order drug release for timed durations. Unless inserted as implants, osmotic pumps are currently utilized for 24-hour controlled oral delivery (Herrlich et al., 2012; Malaterre et al., 2009). We are uncertain why there are no reports on osmotic tablets for vaginal delivery but perhaps researchers assumed there was limited fluid to drive the drug release. Therefore, to achieve multiple day drug delivery, we modified the OPT to ensure retention in the vaginal tract for extended durations by designing an OPT coated with a bioadhesive polymer (Grabovac et al., 2005) that delivers a viscous gel. The gel we hypothesized might aid in retaining the formulation in the vaginal canal and may improve drug distribution in the vaginal tract. Our approach utilized a semi-solid gel forming polymer, hydroxypropyl cellulose (HPC) as the osmoattractant core instead of NaCl or polyethylene glycol. The high molecular weight of this gel-forming polymer is unlikely to cause osmotic stresses to mucosal tissues unlike other systems that have high salt concentrations. Water is driven into the HPC core resulting in polymer swelling and extrusion of a vaginal gel through the orifice and delivery of drug in the vaginal canal (Fig. 1a).
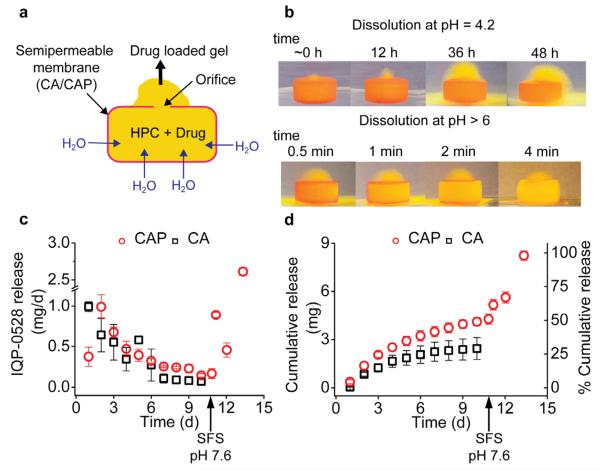
Fig. 1. In vitro IQP-0528 release from OPT.
a) Diagram showing working of an OPT. Water is driven through the semipermeable membrane (CA/CAP) into the core (HPC + Drug) due to osmotic pressure difference, causing polymer swelling and extrusion of a drug loaded gel though the orifice. b) Photograph showing a dye loaded CAP-OPT in release media at pH 4.2 (upper panel) and > 6 (lower panel). Extrusion of a dye-loaded gel can be seen within the first hour. Upon increase in pH > 6, the CAP coating was instantaneously seen to dissolve leaving a gelled core. c) Daily and d) cumulative release of IQP-0528 from CAP- and CA-OPT in 25 mM acetate buffer pH 4.2 (simulated vaginal conditions) followed by mimicking pH increase by changing release media to seminal fluid simulant pH 7.6 (N = 3; Mean ± SD).
In this report we describe the fabrication of a multi-day intravaginal OPT for delivery of IQP-0528, a pyrimidinedione, with potent nanomolar non-nucleoside reverse transcriptase and entry inhibition activity (Buckheit et al., 2008; Johnson et al., 2012), and its evaluation in the sheep vaginal model. Vaginal fluid samples were collected using a new multiswab device to determine spatial drug distribution by placing several circular sponges at precise positions along a penis shaped rod. This allowed us to map the drug distribution in the vaginal tract as a function of time and distance. In addition, the ability of IQP-0528 present in vaginal swabs to inhibit HIV-1 infection was evaluated in vitro.
Vaginal tablets with 10 wt% IQP-0528 in HPC matrix were fabricated using a standard pellet press, spray-coated with SPM forming polymers, cellulose acetate phthalate (CAP) (Daugherity and Nause, 2009b) or cellulose acetate (CA) (Daugherity and Nause, 2009a) and a drug delivery orifice was manually drilled (S.Fig. 1). CAP, an enteric coating polymer is insoluble at the approximate vaginal pH 4, but is soluble at seminal pH of 6-8 (Daugherity and Nause, 2009b), a feature we utilized for engineering a semen-triggered burst drug release system. Samples were tested for in vitro drug elution under simulated vaginal conditions. Next, two uncoated tablets (N = 3) or CA-OPT (N = 5) were administered in adult female sheep using a custom-designed applicator (Supplementary methods). To study vaginal drug distribution, we engineered a multiswab device (S.Fig. 2) to allow us to collect spatially registered vaginal fluid samples at varying time-points up to 10 days. Vaginal swabs were tested for drug levels and antiviral activity (Supplementary methods).
To study the advantage of an OPT over conventional tablet formulations, we maintained an identical core composition for IQP-0528 pellets (S.Fig. 1; 8.4 ± 0.6 wt%, N = 10). As expected, uncoated tablets exhibited faster swelling with almost complete drug dissolution (91.8 ± 4.1% (N = 5) in 48 h; S.Fig. 3a). In contrast, OPTs showed multi-day IQP-0528 release in vitro. Slow drug release was noted early on with 0.9 ± 0.06 mg (10.7 ± 0.7%) and 0.4 ± 0.09 mg (4.6 ± 1%) drug released on day 1 from CA- and CAP-OPTs respectively. A total of 29.5 ± 8.4% (N = 4) and 47.1 ± 3.3% (N = 6) [P = 0.0015] of the drug load was delivered by day 10 from CA- and CAP-OPTs respectively. (Fig. 1c and d) The variation in the amount of drug released was significantly different and can be attributed to the difference in the water vapor transmission rate (P) from the two membranes. Since membrane thickness and area and orifice area were constant for both the OPTs, the membrane permeability will control water uptake by the core and resulting drug release (PCAP=0.52 g.m−2/24 h or 0.002 mg.cm−2h−1 and PCA=5 mg.cm−2h−1 (Crawford and Esmerian, 1971; Sprockel et al., 1990). Although the data indicated that release could have continued for more than 10 days we believe that short to extended duration delivery systems that last on the order of 2 - 5 days will be of the most utility in the HIV prevention field.
Semen can cause a large pH change in the vaginal canal that can be used to design semen triggered release of drugs (Clark et al., 2011; Gupta et al., 2007; Zhang et al., 2013). To test the suitability of the CAP-OPT as a semen-triggered DDS we changed the release media from acetate buffer pH 4.2 (simulated vaginal conditions) to seminal fluid simulant pH 7.6 on day 10. Dissolution of the CAP coating occurred within minutes of the pH change, exposing a gel core followed by 49% of the drug load being delivered in the next 2.5 days amounting to 97% total drug release (Fig. 1d). To visualize the working of the CAP-OPT, we formulated a yellow dye-loaded HPC core with a rhodamine B-CAP coating (Fig. 1b). A bright yellow gel labeled with the dye was seen to extrude out of the drug delivery orifice under osmotic forces when the system was placed in media (pH 4.2; Fig. 1b). Change in pH to > 6 resulted in rapid dissolution of the CAP SPM, exposing the hydrated gel core (Fig. 1b).
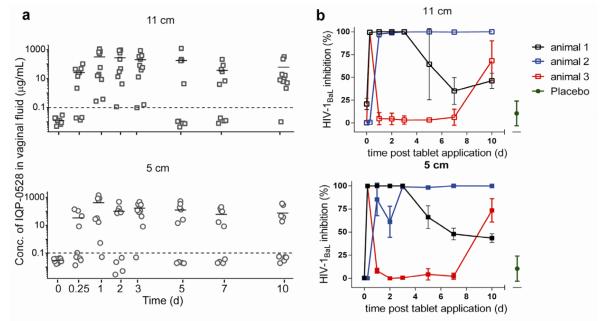
We further evaluated IQP-0528 vaginal distribution from uncoated tablets (N = 3) and CA-OPT (N = 5) in the sheep model. Due to lack of a convenient animal model where vaginal pH is in the acidic range, we were unable to study pH triggered release from the CAP-OPT in vivo. Spatially registered swabs were collected at 11 and 5 cm from the introitus using the multiswab device. (S.Fig. 2) Intravaginal administration of 2 uncoated tablets per animal resulted in Cmax of 3.5 μg/mL (mean; range 12-0.04 μg/mL) and 0.6 μg/mL (mean; range 2.9-0.02 μg/mL) distal and proximal to the introitus respectively, at 6 h (N = 3) with no drug detected by 48 h (S.Fig. 3b). In the first CA-OPT study (N = 3), only 1/6 OPTs inserted were retained in the first 6 hours. We attribute this finding to the small amounts of gel released early on being insufficient to retain these systems in the canal. Thus to increase retention, we asymmetrically dip-coated the face of the OPT opposite to the orifice with Carbopol 974P (3wt% in methanol) followed by 24-hour vacuum drying and wetted the OPTs before insertion. This CA-OPT formulation resulted in an average IQP-0528 concentration of 145 μg/mL in a 10-day application. The drug was well distributed in the vaginal tract with 152 μg/mL (mean, range 1176.3-0.001 μg/mL) at 11 cm and 137.2 μg/mL (mean, range 1404-0.003 μg/mL) at 5 cm from the introitus. Peak drug levels were noted on day 1, 309.3 μg/mL (mean, range 1087.3-0.3 μg/mL) distal and 420 μg/mL (mean, range 1404-0.05 μg/mL) proximal to the introitus. (Fig. 2a) Similar to drug levels, the antiviral activity of the material eluted from the swabs showed high variability, but except for 1 swab collected at 11 cm on day 2, all samples were above the EC50 (range 105 - 2× EC50) with the Cmax greater than 106×EC50 (Fig. 2b). Vaginal secretions from 2/3 animals showed greater than 50% inhibition for the first 3 days of OPT administration with samples from animal 2 resulting in HIV-1BaL inhibition for the complete 10-day duration. Overall, samples with high drug concentrations showed potent antiviral activity.
Fig. 2. IQP-0528 release from CA-OPT in vivo.
a) IQP-0528 concentrations in sheep vaginal fluid collected using multiswab device (S.Fig. 2) at two locations, 11 and 5 cm from the introitus (N = 5). The bars represent the mean and the dotted line represents the LLOQ (0.1 μg/mL). Drug concentrations below the LLOQ was plotted as 1/10th the calculated LLOQ for that sample. b) The ability of vaginal swabs from 3 animals treated with CA-OPTs to inhibit HIV-1BaL infection was assessed in TZM-bl cells. Results are presented as percentage inhibition of infection relative to control wells and each data point represents the average of 2 experiments conducted in triplicate (N=3 animals and 2 swabs/animal).
Overall, we present a new medium duration vaginal DDS that can be tailored for topical PrEP. This is exemplified by the CA-OPT that provide continuous protective mucosal drug concentrations. Since infected semen is a major source of HIV-1 sexual transmission (Royce et al., 1997), we also present the concept of semen-triggered drug delivery by utilizing a pH sensitive coating; the CAP-OPT generating sustained vaginal drug levels followed by high IQP-0528 concentrations upon a simulated coitally-associated pH change. We have shown extended drug PK from the CA-OPT over uncoated tablet formulations. Furthermore, this gel delivering OPT assists in drug distribution in the vaginal canal resulting in high antiretroviral concentrations all throughout the reproductive tract.
Topical PrEP needs practical ARV eluting delivery systems that increase adherence and generate protective drug PK. This is the first proof-of-concept study exploring the potential of osmotic pump technology for drug delivery to the reproductive tract for extended durations. We have shown the tunability of this technology for designing ‘on-demand’ vaginal antiretroviral delivery. Nonetheless this platform can also replace gels for other conditions, example, cervical ripening (Swamy, 2012) where high drug concentrations have to be achieved locally for short to moderate durations. Our further OPT designs will be directed at modeling and modifying these systems to deliver ARV for 2-5 days by changing parameters such as SPM thickness, orifice area, tablet geometry and ARV identity and loading. Further, we will explore approaches to improve vaginal retention, for example, tablet convexity to increase contact area and alternate mucoadhesive SPM coatings. Finally, a priority is to conduct more detailed studies to determine PK and safety in non-human primates that include both fluid and tissue sampling.
Supplementary Material
S.Fig.1. Photograph showing a 10 wt% IQP-0528 loaded OPT and an uncoated tablet.
S.Fig.2. Photograph showing a multiswab device fitted with swabs (insert) at 11 and 5 cm used for collection of vaginal fluid from ewes.
S.Fig.3. IQP-0528 delivery from uncoated tablets. a) In vitro drug release under simulated vaginal conditions (N = 5; mean ± SD) and b) drug levels in vaginal swabs after administration of 2 uncoated tablets (N = 3; 2 swabs/animal) collected at 11 and 5 cm from the introitus. The bars represent the mean for that data-set and the dotted line represents the LLOQ (0.1 μg/mL). Drug concentration below the LLOQ was plotted as 1/10th the calculated LLOQ for that sample.
Highlights.
First pH-sensitive vaginal osmotic pump for ‘on-demand’ antiretroviral delivery.
First vaginal osmotic pump for intermittent antiretroviral delivery to mucosa.
New tool to collect vaginal fluid samples to determine spatial drug distribution.
ACKNOWLEDGEMENTS
The work was supported by grant funded by National Institutes of Health Grant U19 AI076980. We want to thank Drs. D.R. Friend and M.R. Clark for organizing the 2013 CONRAD MPT workshop where this work was first presented.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JL, Kashuba AD. Formulation, pharmacokinetics and pharmacodynamics of topical microbicides. Best Pract Res Clin Obstet Gynaecol. 2012;26(4):451–462. doi: 10.1016/j.bpobgyn.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- Buckheit RW, Jr., Hartman TL, Watson KM, Chung SG, Cho EH. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob Agents Chemother. 2008;52(1):225–236. doi: 10.1128/AAC.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Aliyar HA, Lee CW, Jay JI, Gupta KM, Watson KM, Stewart RJ, Buckheit RW, Kiser PF. Enzymatic triggered release of an HIV-1 entry inhibitor from prostate specific antigen degradable microparticles. Int J Pharm. 2011;413(1-2):10–18. doi: 10.1016/j.ijpharm.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RR, Esmerian OK. Effect of plasticizers on some physical properties of cellulose acetate phthalate films. J Pharm Sci. 1971;60(2):312–314. doi: 10.1002/jps.2600600238. [DOI] [PubMed] [Google Scholar]
- Daugherity P, Nause R. Cellulose acetate. In: Rowe RC, Sheskey PJ, Quinn ME, editors. Handbook of Pharmaceutical Excipients. 6th ed Pharmaceutical Press; London, UK: 2009a. pp. 141–143. [Google Scholar]
- Daugherity P, Nause R. Cellulose acetate phthalate. In: Rowe R, Sheskey P, Quinn M, editors. Handbook of Pharmaceutical Excipients. 6th ed Pharmaceutical Press; London, UK: 2009b. pp. 143–146. [Google Scholar]
- Grabovac V, Guggi D, Bernkop-Schnurch A. Comparison of the mucoadhesive properties of various polymers. Adv Drug Deliv Rev. 2005;57(11):1713–1723. doi: 10.1016/j.addr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gupta KM, Barnes SR, Tangaro RA, Roberts MC, Owen DH, Katz DF, Kiser PF. Temperature and pH sensitive hydrogels: an approach towards smart semen-triggered vaginal microbicidal vehicles. J Pharm Sci. 2007;96(3):670–681. doi: 10.1002/jps.20752. [DOI] [PubMed] [Google Scholar]
- Herrlich S, Spieth S, Messner S, Zengerle R. Osmotic micropumps for drug delivery. Adv Drug Deliv Rev. 2012;64(14):1617–1627. doi: 10.1016/j.addr.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Srinivasan P, Albright TH, Watson-Buckheit K, Rabe L, Martin A, Pau CP, Hendry RM, Otten R, McNicholl J, Buckheit R, Jr., Smith J, Kiser PF. Safe and Sustained Vaginal Delivery of Pyrimidinedione HIV-1 Inhibitors from Polyurethane Intravaginal Rings. Antimicrob Agents Chemother. 2012;56(3):1291–1299. doi: 10.1128/AAC.05721-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaterre V, Ogorka J, Loggia N, Gurny R. Oral osmotically driven systems: 30 years of development and clinical use. Eur J Pharm Biopharm. 2009;73(3):311–323. doi: 10.1016/j.ejpb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito Taljaard M, Piper J, Gomez Feliciano K, M., C. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine or vaginal tenofovir gel in the VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. p. 26LB. [Google Scholar]
- Minkin MJ, Maamari R, Reiter S. Improved compliance and patient satisfaction with estradiol vaginal tablets in postmenopausal women previously treated with another local estrogen therapy. Int J Womens Health. 2013;5:133–139. doi: 10.2147/IJWH.S41897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JE, Devlin C, Gelfand MM, Steinberg WM, Hepburn DS. 17beta-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream. Menopause. 2000;7(3):156–161. doi: 10.1097/00042192-200007030-00005. [DOI] [PubMed] [Google Scholar]
- Royce RA, Sena A, Cates W, Jr., Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- Sprockel OL, Prapaitrakul W, Shivanand P. Permeability of cellulose polymers: water vapour transmission rates. J Pharm Pharmacol. 1990;42(3):152–157. doi: 10.1111/j.2042-7158.1990.tb05375.x. [DOI] [PubMed] [Google Scholar]
- Swamy GK. Current methods of labor induction. Semin Perinatol. 2012;36(5):348–352. doi: 10.1053/j.semperi.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Theeuwes F. Elementary osmotic pump. J Pharm Sci. 1975;64(12):1987–1991. doi: 10.1002/jps.2600641218. [DOI] [PubMed] [Google Scholar]
- Theeuwes F, Yum SI. Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Ann Biomed Eng. 1976;4(4):343–353. doi: 10.1007/BF02584524. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang C, Agrahari V, Murowchick JB, Oyler NA, Youan BB. Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antiviral Res. 2013;97(3):334–346. doi: 10.1016/j.antiviral.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S.Fig.1. Photograph showing a 10 wt% IQP-0528 loaded OPT and an uncoated tablet.
S.Fig.2. Photograph showing a multiswab device fitted with swabs (insert) at 11 and 5 cm used for collection of vaginal fluid from ewes.
S.Fig.3. IQP-0528 delivery from uncoated tablets. a) In vitro drug release under simulated vaginal conditions (N = 5; mean ± SD) and b) drug levels in vaginal swabs after administration of 2 uncoated tablets (N = 3; 2 swabs/animal) collected at 11 and 5 cm from the introitus. The bars represent the mean for that data-set and the dotted line represents the LLOQ (0.1 μg/mL). Drug concentration below the LLOQ was plotted as 1/10th the calculated LLOQ for that sample.