Abstract
The measles virus phosphoprotein (P) gene encodes the P, V, and C proteins, which have multiple functions including type I interferon (IFN) inhibition. With a focus on viral immune modulation, we conducted a study on healthy vaccinees (n = 179) to compare cytokine secretion patterns/cell frequencies and gene expression after in vitro encounter with a highly attenuated strain of measles virus (MVEdmtag), wild-type MV (MVwt) or recombinant MVEdmtag expressing the wild-type P gene (MVwtP). Cytokines were quantified by ELISA and Elispot. Gene expression profiling was performed using real-time PCR. We found differential MV-specific cytokine responses to all detected cytokines characterized by significantly higher cytokine levels (P <0.001) and higher frequencies (P <0.0001) of cytokine-producing cells after stimulation with the highly attenuated MVEdm-tag strain in comparison with MVwt or MVwtP. Furthermore, gene expression profiling revealed significant cytokine suppression at the transcriptional level for viruses encoding the functional wt P gene, compared to attenuated MVEdmtag (P <0.05). Using lentivirus-mediated stable expression of P gene-encoded proteins in human cell lines, we demonstrated that the expression of the functional wt V protein significantly down-modulated the induction of IFNs type I, II, and III in lymphocytes and monocytes. Taken together our results indicate that Th1, Th2, and innate/inflammatory cytokine responses in vaccinees are suppressed both at the protein and transcriptional level by viruses expressing the functional wt P gene products. The functional P gene-encoded viral proteins (particularly V proteins) emerge as crucial immune evasion factors for modulating and shaping the measles virus-specific cytokine responses in humans.
Keywords: measles virus, P gene, MMR vaccine, cellular immunity, cytokines, gene expression
INTRODUCTION
Measles immunity is highly complex, involving different arms of host response, all of which are interrelated and appear to be required for efficient immune response and viral clearance in the course of disease [Wu et al., 1993; Bouche et al., 2002; van Els and Nanan, 2002; Schneider-Schaulies and Dittmer, 2006; Naniche, 2009]. Our group and others have shown that measles-specific cytokine and lymphoproliferative responses are surrogate measures of cell-mediated immunity (CMI) following measles vaccination and disease [Griffin and Ward, 1993; Ward and Griffin, 1993; Ovsyannikova et al., 2003; Dhiman et al., 2005; Ryan et al., 2005; Howe et al., 2005a, b; Naniche, 2009]. As intercellular protein messengers, cytokines are important regulators of innate and adaptive immune responses to both viral infections and vaccination [Chabalgoity et al., 2007; Steinman, 2007; Larosa and Orange, 2008; Hahm, 2009]. Cytokine induction and signaling are targeted by immune evasion/modulation factors evolved by pathogens to achieve efficient replication and spread in the host [Garcia-Sastre and Biron, 2006; Chabalgoity et al., 2007; Randall and Goodbourn, 2008; Hahm, 2009].
Viruses are subject to control by the innate immune system and different strains of measles virus (MV, family Paramyxoviridae, genus Morbillivirus, species Measles virus) are known to differ in their interferon (IFN) antagonistic ability, most likely due to differences in the P/V/C proteins encoded by the viral phosphoprotein (P) gene [Naniche et al., 2000; Gotoh et al., 2002; Ohno et al., 2004; Devaux et al., 2007, 2008; Haralambieva et al., 2007; Fontana et al., 2008; Gerlier and Valentin, 2009; Hahm, 2009]. The global effect of these key immune evasion factors on cellular immune responses and the cytokine milieu has not been studied comprehensively in humans. There are four known amino acid differences between the C proteins of the wild-type IC-B MV strain and the highly attenuated Edmonston tag strain, and 18/16 amino acid differences between the P and V proteins of these two strains, respectively [Nakatsu et al., 2009]. Most of the changes in the Edmonston tag strain are common to Edmonston lineage measles virus strains, however due to tyrosine-to-histidine (Y110H) and cysteine-to-arginine (C272R) substitutions at amino acid positions 110 and 272 (which are not conserved among the Edmonston lineage strains), the V protein of the Edmonston tag strain is considered defective in countering IFN induction and signaling [Ohno et al., 2004; Devaux et al., 2007; Fontana et al., 2008; Gerlier and Valentin, 2009]. Therefore, our study aimed to elucidate the role of the viral phosphoprotein gene in measles-specific innate/inflammatory and adaptive cytokine immune responses in humans. We undertook an extensive study of host cytokine profiling at the gene expression and protein level and compared patterns of cytokine responses and cytokine-specific cell frequencies after in vitro encounter with the highly attenuated Edmonston tag strain of measles virus (MVEdmtag), wild-type MV (MVwt, IC-B derivative), or recombinant Edmtag strain expressing the wild-type P gene (MVwtP). We examined virus-specific Th1, Th2, Th17 and innate/inflammatory cytokine responses in healthy adolescents who had previously received two doses of measles vaccine. Our data demonstrated a global, differential P gene-dependent cytokine response in vaccinees with respect to the different viruses studied and suggest that this might be due to the expression of the strain-specific V protein.
MATERIALS AND METHODS
Study Subjects
We studied a sub-sample (n = 179) of a population-based, age-stratified sample (cohort) of 346 healthy adolescents (aged 12–18 years), from Olmsted County, Minnesota whose demographic and clinical variables have been previously described [Ovsyannikova et al., 2004]. Participants were selected from the original set of 346 subjects if the number of cells available was adequate to assess one or more of the immune outcomes of interest. All participants had a written medical record of having received two doses of measles-mumps-rubella-II (MMR) vaccine, containing the Edmonston strain of MV and had no history of measles infection or known exposure. The Mayo Clinic Institutional Review Board granted approval for the study. Written informed consent and assent (from minors) from subjects and parents/guardians were obtained at the time of enrollment.
Viruses, Cell Culture, and Infection Assays
All recombinant MV strains: a highly attenuated strain of MV Edmonston tag (MVEdmtag), wild-type MV (MVwt, IC-B derivative) and recombinant MVEdm-tag strain, expressing the wild-type P gene (MVwtP), were a kind gift from Dr. Kah-Whye Peng (Mayo Clinic, Rochester, MN) and were propagated and titered on Vero or Vero-hSLAM cells as previously described [Duprex et al., 1999; Takeda et al., 2000; Ono et al., 2001; Haralambieva et al., 2007] (Fig. 1). For all recombinant viruses the transcription unit for green fluorescent protein (GFP) was inserted in front of the N gene to ensure the same level of attenuation (Fig. 1). The generation of MVwtP, a chimeric MVEdmtag encoding the wild-type P gene, has been described in detail previously [Haralambieva et al., 2007]. This recombinant virus is essentially the same as the highly attenuated MVEdmtag strain except that it expresses the P, V, and C proteins of wild-type MV. All viral stocks were free of lipopolysaccharide (LPS) contamination as verified by the Limulus Amebocyte Lysate PYROGEN test kit (Cambrex, Walkersville, MD). The human monocytic cell line THP-1 was obtained from the American Type Culture Collection (ATCC, Manassas, VA), and the EBV-negative human Burkitt’s lymphoma cell line (BJAB) was a gift from Dr. M. Peter (University of Chicago, IL). VSV-G pseudotyped pHR-SIN-CSGWdlNotI-based lentiviral vectors (from Dr. Y. Ikeda, Mayo Clinic, Rochester, MN) were created to individually encode the P, V, or C proteins of the highly attenuated Edmonston tag strain of measles virus (MVEdmtag) or wild-type strain of measles virus MVwt (IC-B derivative), all obtained from Dr. Kah-Whye Peng, Mayo Clinic, Rochester, MN [Haralambieva et al., 2007]. There are 18 known amino acid differences between the P proteins of the Edmonston tag strain (MVEdmtag) and the IC-B strain (MVwt). The V proteins of those two strains are known to differ by 16 amino acids, and the C proteins by 4 amino acids [Nakatsu et al., 2009] (Fig. 1). The cDNAs encoding the P, V, and C proteins of the MVEdmtag and MVwt were obtained by RT-PCR (SuperScript™ One-step RT-PCR, Invitrogen, Carlsbad, CA) from virus-infected cells using specific primers. The cDNAs were cloned into the BamHI–NotI site of pHR-SIN-CSGWdlNotI to replace GFP of the lentiviral vector and their sequences were verified by DNA sequencing. The V cDNAs were identified by the presence of a non-templated nucleotide insertion at the RNA editing site. Vector stocks were used to generate BJAB and THP-1 cell lines, stably expressing individually each one of the proteins of interest, as confirmed by Western blot (data not shown) [Haralambieva et al., 2007]. We used these stable cell lines (along with normal BJAB/THP-1 cells) to determine the effects of P, V, or C proteins from the highly attenuated MVEdmtag strain or from the wild-type MV strain, on the induction of IFNs. Measles-specific secreted IFN type I (IFN-α), IFN type II (IFN-γ), and IFN type III (IFN-λ1 and IFN-λ2) were quantified by ELISA in supernatants from the generated stable cell lines expressing the corresponding proteins, after MVEdmtag stimulation at a multiplicity of infection (MOI) of 0.5 for 48 hr. The levels of stimulated IFN induction in each cell line (after subtraction of the background levels of uninfected cells) were compared to the levels of a normal BJAB/THP-1 control.
Fig. 1.
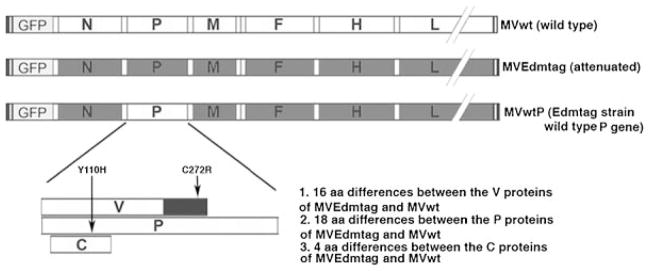
Schematic representation of the viruses used in the study and P/V/C protein differences. Strain-specific amino acid (aa) substitutions (Y110H in P/V proteins and C272R in V protein), known to affect IFN signaling are shown with arrows.
Measles-Specific Cytokines
Measles-specific secreted cytokines (13), and IL-10 and IFN-γ Elispot assays were performed on a sub-sample of the study subjects depending on cell availability. The number of GFP-positive infected PBMCs was quantified (by flow cytometry) for each assay, for each one of the viruses used, and no statistically significant differences between the different viruses were found (data not shown). Thirteen measles-specific secreted cytokines (Th1: IL-2, IFN-γ, IL-12p40; Th2: IL-4, IL-5, IL-10; Th17: IL-17; and innate/inflammatory: IL-1β, IL-6, TNF-α, IFN-α, IFN-λ1, IFN-λ2) were quantified in cultured PBMCs in triplicate after live virus stimulation with one of the three viruses using a MOI of 0.5 for all cytokines except for IL-4 and IL-5, which were assayed at a MOI of 0.1 as previously described [Ovsyannikova et al., 2005]. Cell-free supernatants were harvested 48 hr later and assayed by ELISA using kit-specific manufacturer’s recommendations (R&D Systems, Minneapolis, MN, for IL-17, IFN-λ1 and IFN-λ2; PBL Biomedical Laboratories, Piscataway, NJ for IFN-α, and BD Biosciences Pharmingen, San Diego, CA for the rest of the cytokines). Stimulation with inactivated viruses (using the same MOI and time of incubation) were included as controls. PHA (5 μg/ml) was used as a positive control.
Human IL-10 Elispot assays (BD Biosciences, San Diego, CA) and IFN-γ Elispot assays (R&D Systems) were performed as previously described [Ryan et al., 2005] and following the manufacturer’s protocol after live virus stimulation with one of the three viruses at a MOI of 0.5. PHA (5 μg/ml) was used as a positive control. The plates were processed after initial incubation for 24 hr (for IL-10) or 42 hr (for IFN-γ) at 37°C, in 5% CO2. All plates were scanned and analyzed on an Immuno-Spot® S4 Pro Analyzer (Cellular Technology Ltd, Cleveland, OH, USA) using ImmunoSpot® version 4.0 software (Cellular Technology Ltd).
Gene Expression Profiling
Quantitative mRNA expression analyses of 88 human cytokine and cytokine-related genes were performed using the 96-plex human common cytokines RT2 Profiler™ PCR array system (SABiosciences, Frederick, MD), customized to add four additional genes: IL29 (IFN-λ1), MX1, OAS1, and EIF2AK2 (PKR). Total RNA was isolated from PBMCs (from three high cytokine responders) after mock stimulation (medium Opti-MEM) or after stimulation with live highly attenuated MVEdmtag virus, wild-type measles virus MVwt, or attenuated virus expressing the wild-type P gene MVwtP, at a MOI of 0.5. After 24 hr cells were stabilized using RNAprotect cell reagent (Qiagen, Valencia, CA), total RNA was extracted using the RNAeasy Plus Mini kit (Qiagen) and the RNA concentration and quality were assessed by Nanodrop spectrophotometry (Thermo Fisher Scientific, Wilmington, DE) and Nano LabChip analysis on an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA), respectively. Equal amounts of RNA (0.5 μg) from each sample were then converted to cDNA using the RT2 First Strand Kit (SABiosciences) following the manufacturer’s instructions. Real-time PCR was performed using the RT2 SYBR Green/ROX qPCR Master Mix (SABiosciences), as recommended by the manufacturer, on an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Carlsbad, CA) with the following cycling conditions: an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. mRNA expression of each gene was normalized using the average Ct value of all five housekeeping genes (ACTB, GAPDH, B2M, RPL13A, HPRT1), as recommended.
Statistical Analysis
Measurement of cytokine secretion (by ELISA) and frequencies of cytokine positive cells (by Elispot) resulted in 12 recorded values per individual for each outcome: 3 without in vitro viral stimulation (control), 3 post in vitro stimulation with MVEdmtag, 3 post in vitro stimulation with MVwt, and 3 post in vitro stimulation with MVwtP. A single summary measurement per individual per virus was obtained for each outcome by subtracting the median of the three unstimulated values from the median of the three stimulated values (negative values indicate that stimulated values were on average smaller than unstimulated values). Data were descriptively displayed across individuals using medians and inter-quartile ranges.
All statistical tests were two-sided. Within-subject differences in cytokine measures across virus type were evaluated using paired t-tests. Three comparisons were carried out for each cytokine secretion and Elispot outcome: one comparing MVEdmtag to MVwt, one comparing MVEdmtag to MVwtP, and one comparing MVwt to MVwtP. P-values <0.05 were considered significant.
All gene expression data analyses (including QA/QC) were performed using the PCR Array Data Analysis Web Portal (SABiosciences), and the ΔΔCt method with a fourfold change as a threshold for up/down-regulation and a P <0.05 as a threshold for statistical significance.
RESULTS
Measles Viruses Expressing Functional/Wild-Type P Gene Products Induce Lower Cytokine Secretion in Vaccinees Than the Highly Attenuated MVEdmtag
We determined the innate/inflammatory, Th1, Th2, and Th17 cytokine secretion response in study participants after in vitro exposure to each of three measles viruses (MVEdmtag, MVwt, and MVwtP). We were able to detect 8 of the 13 measured cytokines induced at least by one of the viruses (no secretion of IL-17, IL-4, IL-5, IL-12p40, or IL-28A/IFN-λ2 was detected) (Table I). Comparison within individuals revealed significantly higher cytokine levels (P <0.001) after stimulation with the attenuated MVEdmtag strain in comparison with either MVwt or MVwtP for all eight measured cytokines (IL-2, IFN-γ, IL-10, IL-1β, IL-6, TNF-α, IFN-α, IFN-λ1/IL29). Stimulation with inactivated viruses revealed low levels of detected cytokines (for all three viruses), comparable with unstimulated values (data not shown).
TABLE I.
Cytokine Secretion and Gene Expression in Vaccinees Upon Different MV Encounter
| Virus | Cytokine/protein | Secretion, median (IQR) pg/ml | Gene symbol | Gene expression |
|---|---|---|---|---|
| MVEdmtag | 87 (70, 207) | ↑↑↑ | ||
| MVwt | IFN-α | 5 (1, 9) | IFNA | NC |
| MVwtP | 5 (2, 8) | (1, 2, 4, 5, 8) | ↑ | |
|
| ||||
| MVEdmtag | 184 (110, 244) | ↑ | ||
| MVwt | IFN-γ | −10 (−25, 1) | IFNG | NC |
| MVwtP | −10 (−27, 0) | NC | ||
|
| ||||
| MVEdmtag | 36 (27, 59) | ↑ | ||
| MVwt | IFN-λ1/IL-29 | 1 (−11, 6) | IL29/IFNL1 | NC |
| MVwtP | 4 (−1, 12) | NC | ||
|
| ||||
| MVEdmtag | 58 (22, 126) | ↑↑ | ||
| MVwt | IL-1β | 45 (8, 60) | IL1B | ↑ |
| MVwtP | 3 (1, 8) | ↑ | ||
|
| ||||
| MVEdmtag | 36 (24, 62) | ↑ | ||
| MVwt | IL-2 | 6 (1, 12) | IL2 | NC |
| MVwtP | 6 (3, 17) | NC | ||
|
| ||||
| MVEdmtag | 634 (534, 721) | ↑↑ | ||
| MVwt | IL-6 | 131 (29, 168) | IL6 | NC |
| MVwtP | 24 (7, 78) | NC | ||
|
| ||||
| MVEdmtag | 46 (32, 51) | NC | ||
| MVwt | IL-10 | 8 (3, 13) | IL10 | NC |
| MVwtP | 2 (−6, 6) | NC | ||
|
| ||||
| MVEdmtag | 31 (17, 63) | ↑ | ||
| MVwt | TNF-α | 2 (0, 4) | TNF | NC |
| MVwtP | 2 (1, 6) | NC | ||
|
| ||||
| MVEdmtag | ND | IL12B | ↑ | |
| MVwt | IL-12p40 | ND | NC | |
| MVwtP | ND | NC | ||
|
| ||||
| MV (all strains) | IL-4 | ND | IL4 | NC |
| MV (all strains) | IL-5 | ND | IL5 | NC |
| MV (all strains) | IL-17 | ND | IL17A, IL17B, IL17C | NC |
| MV (all strains) | IFN-λ2/IL-28A | ND | IL28A/IFNL2 | N |
IQR, interquartile range; NC, not changed (for gene expression); ND, not detectable (for cytokine secretion); N, not done.
↑, Upregulated gene expression 4- to 10-fold; ↑↑, upregulated 10- to 100-fold; ↑↑↑, upregulated more than 100-fold. All negative cytokine secretion values indicate that unstimulated levels were, on average, higher than stimulated secretion levels.
Measles Viruses Expressing the Functional/Wild-Type P Gene Products Elicit Lower Frequencies of IFN-γ/IL-10-Producing Cells in Vaccinees Than MVEdmtag
We also compared the frequencies of MV-specific IFN-γ and IL-10 producing cells in subjects’ PBMCs after in vitro stimulation. Consistent with the cytokine secretion data we detected lower frequencies of IL-10-producing cells in PBMCs, stimulated with MVwt (median 1 [IQR −3, 6] spots per 2 × 105 cells) or MVwtP (median 4 [IQR 2, 13] spots per 2 × 105 cells) in comparison with MVEdmtag virus (median 103 [IQR 60, 191] spots per 2 × 105 cells) (P <0.0001, Fig. 2A). Similarly, our data demonstrated significantly higher frequencies of IFN-γ-producing cells when subjects’ PBMCs were stimulated with MVEdmtag virus (median 16 [IQR 7, 24] spots per 2 × 105 cells) compared to MVwt or MVwtP stimulation (P <0.0001, Fig. 2B). In summary, our results quantified the presence and frequencies of measles-specific IL-10 (0.1%) and IFN-γ (0.02%) producing cells, but only upon stimulation with the highly attenuated MVEdmtag strain, and not with viruses expressing the functional wild-type P gene.
Fig. 2.
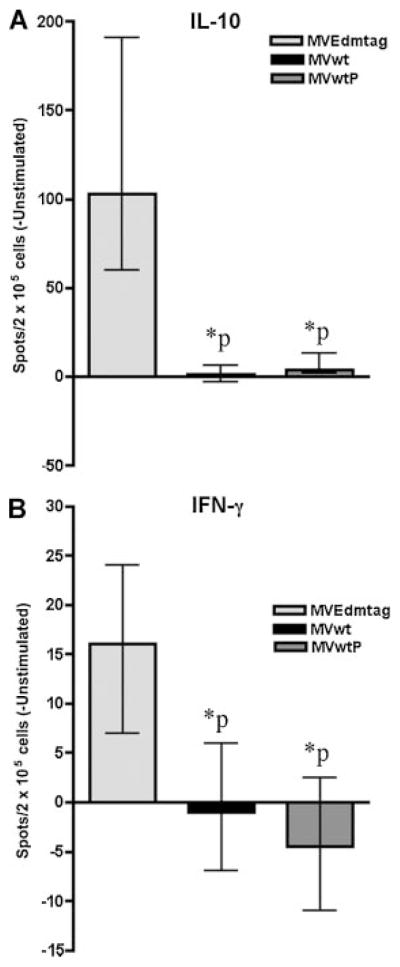
Total IFN-γ and IL-10 Elispot cell frequencies in vaccinees after encounter with different MV strains. Frequencies of IL-10-producing cells (A), and IFN-γ-producing cells (B) in vaccinees upon stimulation with MVEdmtag, MVwt, or MVwtP viruses. Results are presented as median cytokine-positive spots per 2 × 105 cells ± interquartile range. *P <0.0001 for wtP encoding viruses in comparison to MVEdmtag (paired t-test).
Measles Viruses Expressing the Functional/Wild-Type P Gene Products Elicit Differential and Reduced Cytokine Gene Expression Compared to the Highly Attenuated MVEdmtag Strain in Vaccinees
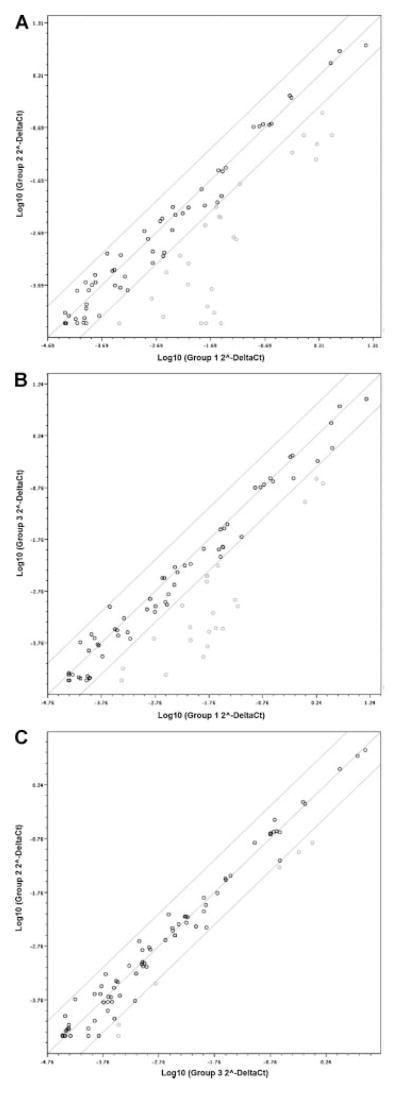
To obtain a more comprehensive view of the overall MV-specific gene expression in target PBMCs, we used the PCR-based RT2 Profile PCR array system, comprising 88 cytokine and cytokine-related genes. The expression profile of MVEdmtag-stimulated, MVwt-stimulated, and MVwtP-stimulated PMBCs was compared to the mock-stimulated PBMC control after normalization. In our analyses we used a threshold of fourfold for up/down regulation and a level of significance of 0.05. The data revealed 30 upregulated (more than fourfold) cytokine and cytokine related genes upon in vitro MVEdmtag infection, and 29 of those had expression significantly different from the control (P <0.05) (Table II). These included a wide range of growth factors, interleukins, TGF-β family and TNF superfamily members, interferons type I, II, and III, as well as three represented IFN-induced antiviral protein genes (EIF2AK2/PKR, MX1, OAS1). However, stimulation with the viruses expressing the wild-type P gene, lead to the upregulation of only 4 (of which only 3 genes were with P <0.05) and 11 of these genes with MVwt and MVwtP stimulation, respectively, and all demonstrated a lower level of expression (Table II). All IFN genes (IFNB1, IFNG, IFNL1/IL29 and five IFNA genes) were significantly upregulated upon stimulation with the attenuated MVEdmtag strain, but not with the MVwt strain, and only three of these eight genes were significantly upregulated (although 30-fold less than MVEdmtag) upon MVwtP stimulation (Table II). When expression of cytokine and cytokine-related genes were compared in the MVwt-stimulated group versus the MVEdmtag-stimulated group we found 27 genes to be down-regulated (more than fourfold) with the wild-type strain in comparison with the attenuated strain (P <0.05) (Fig. 3A). Similarly, stimulation with MVwtP (same as MVEdmtag, but with a wild-type P gene) demonstrated 22 down-regulated genes compared to MVEdmtag (P <0.05) (Fig. 3B), while the difference between the MVwt versus MVwtP-stimulated group (6 down-regulated genes, P <0.05) appeared to be smaller (Fig. 3C). Gene expression was in accordance with the observed protein secretion of the corresponding cytokines and for most of the genes with detectable cytokine secretion we found gene upregulation with the attenuated MVEdmtag strain, and no change or upregulation to a lower extent with the viruses expressing the wild-type P gene (Table I). A significant number of cytokine and cytokine-related genes not studied at the secretion/protein level also displayed similar differential gene expression patterns including IFN-induced antiviral proteins (CSF1, CSF2, FAM3B, IL1A, IL1F5, IL1F9, IL7, IL8, IL15, IL19, TGFA, INHBA, TNFSF10, TNFSF13B, EIF2AK2, MX1, OAS1) (Table II).
TABLE II.
Cytokine-Related Gene Upregulation in Vaccinees Upon Different MV Encounter
| Family | Gene symbol | MVEdmtag
|
MVwt
|
MVwtP
|
|||
|---|---|---|---|---|---|---|---|
| a Fold regulation | P-value | a Fold regulation | P-value | a Fold regulation | P-value | ||
| Growth factors/other | CSF1 | 10.6 | 0.002 | 1.8 | 0.163 | 2.2 | 0.074 |
| CSF2 | 30.6 | <0.001 | 2.1 | 0.234 | 3.9 | 0.004 | |
| FAM3B | 12.3 | 0.002 | 1.4 | 0.522 | 6.0 | 0.006 | |
|
| |||||||
| Interferons type I, II, and III | IFNA1 | 317.9 | 0.002 | 0.7 | 0.274 | 2.8 | 0.034 |
| IFNA2 | 608.0 | 0.002 | 0.7 | 0.274 | 6.3 | 0.023 | |
| IFNA4 | 369.7 | 0.003 | 0.9 | 0.774 | 5.2 | 0.005 | |
| IFNA5 | 198.5 | 0.005 | 0.9 | 0.647 | 3.2 | 0.021 | |
| IFNA8 | 263.8 | 0.003 | 0.7 | 0.274 | 1.8 | 0.127 | |
| IFNB1 | 197.2 | 0.003 | 1.6 | 0.092 | 6.2 | <0.001 | |
| IFNG | 7.8 | 0.014 | 1.1 | 0.950 | 1.3 | 0.718 | |
| IFNL1/IL29 | 47.0 | 0.014 | 1.3 | 0.651 | 2.1 | 0.035 | |
|
| |||||||
| Interleukins | IL1A | 13.5 | 0.003 | 5.7 | 0.014 | 2.2 | 0.151 |
| IL1B | 51.6 | <0.001 | 7.0 | 0.018 | 4.4 | 0.017 | |
| IL1F5 | 8.1 | 0.018 | 0.7 | 0.274 | 0.6 | 0.133 | |
| IL1F9 | 53.0 | <0.001 | 1.0 | 0.922 | 0.8 | 0.603 | |
| IL2 | 6.3 | 0.070 | 1.7 | 0.119 | 2.2 | 0.041 | |
| IL6 | 75.2 | <0.001 | 2.3 | 0.222 | 1.6 | 0.220 | |
| IL7 | 6.5 | 0.002 | 1.1 | 0.627 | 1.4 | 0.165 | |
| IL8 | 55.0 | <0.001 | 9.4 | 0.001 | 5.0 | 0.017 | |
| IL12B | 8.4 | 0.008 | 3.5 | 0.014 | 1.1 | 0.916 | |
| IL15 | 7.2 | 0.001 | 1.0 | 0.861 | 2.0 | 0.004 | |
| IL19 | 47.7 | 0.002 | 3.1 | 0.237 | 1.2 | 0.715 | |
|
| |||||||
| TGF-β family | TGFA | 5.5 | 0.005 | 1.3 | 0.327 | 1.1 | 0.682 |
| INHBA | 281.3 | <0.001 | 7.0 | 0.062 | 4.2 | 0.039 | |
|
| |||||||
| TNF superfamily | TNF | 6.6 | 0.001 | 1.6 | 0.052 | 1.8 | 0.031 |
| TNFSF10 | 42.8 | <0.001 | 1.2 | 0.265 | 6.2 | <0.001 | |
| TNFSF13B | 7.7 | <0.001 | 1.0 | 0.866 | 3.1 | 0.007 | |
|
| |||||||
| Antiviral proteins | EIF2AK2 | 10.5 | <0.001 | 1.1 | 0.693 | 4.3 | 0.002 |
| MX1 | 31.0 | <0.001 | 1.3 | 0.495 | 8.9 | 0.002 | |
| OAS1 | 21.9 | <0.001 | 1.2 | 0.664 | 6.7 | 0.001 | |
All genes upregulated fourfold or more by at least one of the viruses are presented. Values above 4 are highlighted in bold. P values show the level of significance for difference from the control group (mock stimulated PBMCs), P <0.05 is considered as statistically significant.
Fig. 3.
Cytokine gene down-modulation in vaccinees upon stimulation with MVs, encoding the functional wild-type P gene. Normalized expression levels (2−ΔCt ) for cytokine genes in the two groups (stimulated with different viruses) are represented on each scatter plot. Values are plotted on the log scale. The line of identity and the lines representing fourfold change as a threshold for difference are shown. Dots represent individual genes. Dots within the diagonal lines represent genes not changed between groups. Dots, which fall to the right of the lower line indicate gene down-regulation (more than fourfold) of: (A) Group 2 (MVwt-stimulated group, y-axis) versus Group 1 (MVEdmtag-stimulated group, x-axis); (B) Group 3 (MVwtP-stimulated group, y-axis) versus Group 1 (MVEdmtag-stimulated group, x-axis); (C) Group 2 (MVwt-stimulated group, y-axis) versus Group 3 (MVwtP-stimulated group, x-axis). All down-regulated genes are significantly different between groups (P <0.05).
The Functional Wild-Type V Protein Significantly Attenuates the Induction of IFNs Type I, II, and III in Human Lymphocytes and Monocytes
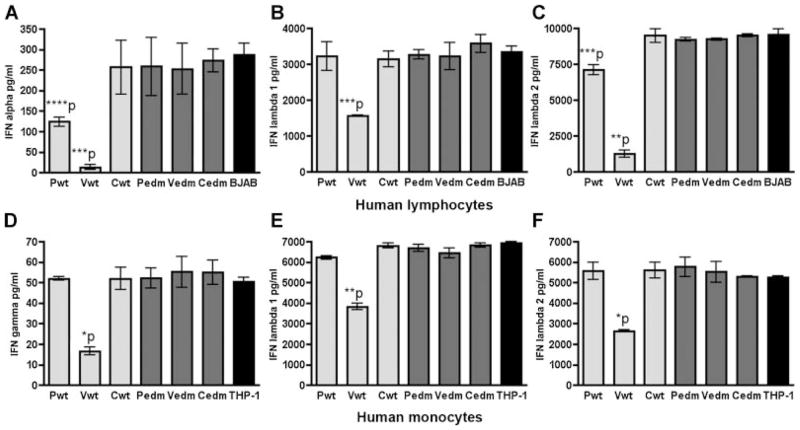
We further explored which of the proteins (P, V, or C) is involved in the inhibition of all three types of IFNs in human cells, by using stable MV-protein expressing BJAB and THP-1 cell lines in comparative experiments of IFN induction. The results demonstrated that only the expression of the functional wild-type V protein (and to a lesser extent the wild-type P protein, which shares the N terminal with the V protein) is able to significantly down-modulate (P <0.01) the stimulated induction of IFN-α, IFN-λ1, and IFN-λ2 in human BJAB cells (Fig. 4A–C, respectively). Similarly, we found that expression of the functional wild-type V protein (but not the P or C wt proteins or P, V, or C MVEdmtag proteins) significantly inhibits stimulated IFN-γ, IFN-λ1, and IFN-λ2 in human monocytic THP-1 cells (P <0.001) (Fig. 4D–F, respectively). Thus, the functional wild-type V protein appears to be the major factor which counters the induction of IFN type I (IFN-α,), type II (IFN-γ), and type III (IFN-λ1 and IFN-λ2), in human lymphocytes and monocytes.
Fig. 4.
The functional wild-type V protein significantly attenuates the induction of IFNs in human lymphocytes and monocytes. A–C: The P, V, and C proteins of wild-type (wt, lighter gray bars) MV and attenuated Edmtag MV (edm, darker gray bars) were stably expressed in BJAB cell line (normal BJAB is represented by black bars) and used in comparative IFN induction experiments (see the Materials and Methods Section). **P <0.0001; ***P <0.01; ****P <0.05 (paired t-test). D–F: The P, V, and C proteins of wild-type (wt, lighter gray bars) MV and attenuated Edmtag MV (edm, darker gray bars) were similarly expressed in the human monocytic cell line THP-1 (normal THP-1 is represented by black bars) and used in comparative IFN induction experiments (see the Materials and Methods Section). *P <0.001; **P <0.0001 (paired t-test). Background levels of cytokine secretion in cultures (for all stable cell lines) not stimulated with measles virus were subtracted from the levels of MVEdmtag-induced responses to produce the “corrected” secretion values shown. Values represent mean (from triplicate) ± SD. The data shown are representative of three experiments (each cell type) with similar results.
DISCUSSION
Generation of an immune response is primarily dependent on the dynamic interactions between pathogenic viral factors and host determinants. The measles virus P gene is the only gene in the MV genome which encodes three proteins: the P protein, an important viral polymerase cofactor, and the V and C non-structural proteins, encoded by a process of RNA editing and by an alternative translational initiation in a different reading frame, respectively (Fig. 1). The functions of these proteins are still not completely understood, but they are known as IFN antagonists [Gotoh et al., 2002; Yanagi et al., 2006; Hahm, 2009]. It has been demonstrated that V proteins are more potent inhibitors of IFN-inducible reporter gene expression than C proteins, and this effect was unrelated to whether the protein originated from an attenuated or wild-type strain [Fontana et al., 2008]. The importance of tyrosine at position 110 and cysteine at position 272 in the inhibition of IFNα/β and IFNγ signaling has also been revealed [Ohno et al., 2004; Devaux et al., 2007; Fontana et al., 2008; Gerlier and Valentin, 2009]. The changes at these two positions in the Edmonston tag strain are not unique and are also found in other lineages of MV vaccine strains, as well as cultured, cell-adapted MV strains [Nakatsu et al., 2009]. Therefore, we studied the P gene of the Edmonston tag strain as a representative P gene of highly attenuated MV strains [Nakatsu et al., 2009] and compared it to the functional (wild-type) P gene.
Our study comprehensively investigates and compares measles-specific cytokine secretion profiles and cytokine gene expression and T cell responses in vaccinees, induced by different MV strains (highly attenuated, wild-type, or chimeric) with the ultimate goal of elucidating the viral-encoded factors involved in cytokine immune response modulation. Our results conclusively demonstrate global P-gene-dependent differential cytokine responses, across all measured cytokines, after stimulation with the highly attenuated MVEdmtag strain compared to strains expressing the functional/wild-type P gene products (MVwt and MVwtP). The observed effects were likely dependent on viral replication and/or the expression of virus-encoded gene products. Cytokines and cellular immune responses play a critical role in the outcome of antigen-specific T cell immunity and those were consistently different in immunized subjects depending on the genetic makeup (P gene) of the encountered virus. Hence, it is likely that viral factors play a role in the nature and magnitude of cytokine responses. For the highly attenuated MVEdmtag virus we observed a mixed activation of both measles-specific Th1 (IFN-γ, IL-2) and Th2 (IL-10) immune responses, innate/inflammatory cytokine responses (including secretion of measles-specific type III IFN-λ1) and no Th17-related response. However, the viruses expressing the functional/wild-type P gene elicited predominantly inflammatory cytokine responses, characterized by moderate to low levels of IL-6 and IL-1β secretion and no or minimal secretion above background levels for other detected cytokines (including IFNs), which is consistent with the lack of global protein shutdown in the infected cells. Our data are in agreement with our previous, and other studies, which examine the quality, quantity and dynamics of Th1 and Th2 cytokine responses in the course of disease or after measles immunization, and demonstrate the involvement of Th1 (IFN-γ, IL-2), Th-2 (IL-4, IL-10, IL-13), or mixed Th1/Th2 responses in measles-specific immunity [Griffin and Ward, 1993; Ward and Griffin, 1993; Griffin et al., 1994; Nanan et al., 2000; Moss et al., 2002; Ovsyannikova et al., 2003; Dhiman et al., 2005; Ovsyannikova et al., 2005; Howe et al., 2005a,b; Yu et al., 2008; Naniche, 2009]. Suppression of IL-12 secretion has recently been reported in a cotton rat model, where infection of bone marrow-derived macrophages with a wild-type MV, but not with a vaccine virus, reduced IL-12 secretion, thus confirming the notion that MV might disturb cytokine production [Carsillo et al., 2009]. The interaction of MV hemagglutinin with the human receptor Signaling Lymphocytic Activation Molecule (SLAM) was demonstrated to suppress TLR4-mediated IL-12 induction in dendritic cells [Hahm et al., 2006]. Our gene expression data (since we were not able to detect IL-12p40 secretion) suggest that the functional/wild-type P gene might also contribute to the transcriptional suppression of IL12 as well as affect the expression of numerous other cytokine genes. Interestingly, the ability to activate TLR2 signaling and proinflammatory cytokine response (like IL-6) in human monocytes was found to be confined to lymphotropic MV wild-type strains [Bieback et al., 2002]. However, our data are more in concert with an overall suppression of the cytokine response by wild-type viruses, conferred at least partly by the functional wild-type P gene-encoded viral factors (mostly the V protein). Interestingly, our data also demonstrate higher inflammatory responses (IL-1β and IL-6 secretion) upon stimulation with the wild-type MV compared to MVwtP, which suggests that other factors such as the wild-type hemagglutinin protein might be involved in proinflammatory cytokine activation through TLR2 signaling [Bieback et al., 2002].
The cytokine-related immunological changes were studied at the transcriptional level (using real-time PCR arrays) and the gene expression patterns for the three viruses used in the study closely paralleled the observed protein expression profiles (Table I). Microarray gene expression patterns comparable to the MVEdmtag-induced gene expression were observed in immature monocyte-derived dendritic cells infected with the Chicago-1 strain of MV (grown on Vero cells and hence likely attenuated), including the induction of IFNs, interleukins, chemokines, TNF superfamily members and IFN-responsive antiviral genes in addition to other genes associated with maturation of antigen-presenting function and migration to lymph nodes [Zilliox et al., 2006]. In contrast, wild-type MV elicited only a few biologically relevant changes in PBMC gene expression consistent with an inflammatory response (mostly IL1B, IL1A, and IL8 gene upregulation), in agreement with gene expression data in the literature related to wild-type MV in children with measles [Zilliox et al., 2007]. Similar to the wild-type virus, the chimeric construct, expressing the functional (wild-type) P gene on the backbone of the highly attenuated Edmtag strain (MVwtP) resulted in the down-modulation of 22 cytokine-related genes compared to the parental Edmtag strain (Fig. 3B), and substantially attenuated the expression level and number of upregulated genes (Table II), indicating viral interference with immune system activation at the transcriptional level. Our findings are further supported by studies in rhesus monkeys showing that inflammatory cytokine response (IL-6 and TNF-α) and IFN response are better controlled by wild-type MV, compared to V- or C-defective measles viruses [Devaux et al., 2008]. The closely related Morbillivirus infection (Canine Distemper Virus/CDV) in a ferret model revealed a general lack of cytokine mRNA induction in animals (infected with lethal CDV strains) that later succumbed to the disease, compared to survivors (infected with non-lethal strains), providing evidence that Morbillivirus pathogenesis and disease outcome are likely dependent on immune activation and cytokine regulation [Svitek and von Messling, 2007].
A number of studies have shown that paramyxovirus P, V, and C proteins inhibit IFN α/β induction, and/or IFN (IFN α/β or IFNγ) signaling by different mechanisms including MDA5 interaction to block dsRNA binding, STAT degradation, interference with STAT phosphorylation, nuclear translocation and distribution, and suppression of JAK1 phosphorylation. In addition, the attenuated measles virus phenotype was demonstrated to be P/V protein-dependent [Gotoh et al., 2002; Palosaari et al., 2003; Shaffer et al., 2003; Takeuchi et al., 2003; Andrejeva et al., 2004; Ohno et al., 2004; Berghall et al., 2006; Nakatsu et al., 2006; Caignard et al., 2007; Childs et al., 2007; Devaux et al., 2007, 2008; Fontana et al., 2008; Ramachandran et al., 2008; Childs et al., 2009; Gerlier and Valentin, 2009; Nakatsu et al., 2009]. Using lentivirus-mediated stable expression of P gene-encoded viral proteins in human cell lines we were able to identify the functional (wild-type) V protein as the major immune evasion factor, countering the induction of all three types of IFNs (including IFN-λ1 and IFN-λ2) in lymphocytes and monocytes. Reports from the literature suggest that the P or C proteins might also act as IFN antagonists, although the V proteins are considered more potent IFN inhibitors [Shaffer et al., 2003; Takeuchi et al., 2005; Nakatsu et al., 2006, 2009; Devaux et al., 2007; Fontana et al., 2008]. Although the mechanism by which wild-type MV or other strains with functional P/V/C proteins induce transcriptional down-modulation in numerous cytokine genes, and suppress cytokine secretion in human cells remains unknown, our studies add new insights into the immunological changes that occur during MV infection and viral interference for immune evasion. The P gene-encoded viral proteins (particularly the functional V protein) emerge as crucial immune avoidance resources for modulating and shaping the virus-specific cytokine response in humans, thereby affecting the quality and quantity of T cell activation. Further functional studies to delineate the specific underlying mechanisms are warranted.
Acknowledgments
Grant sponsor: NIH; Grant numbers: AI 48793, AI 33144, 1 UL1 RR024150-01; Grant sponsor: National Center for Research Resources (NCRR); Grant sponsor: National Institutes of Health; Grant sponsor: NIH Roadmap for Medical Research.
We thank the parents and children who participated in the study and the Mayo Clinic Vaccine Research Group nurses for subject recruitment. We thank the following for their generous gifts of reagents: Yasuhiro Ikeda and Kah-Whye Peng (Mayo Clinic, Rochester, MN), and Marcus Peter (University of Chicago, IL). We thank Christopher P. Kolbert and Vernadette A. Simon (Mayo Clinic, Advanced Genomic Technology Center Microarray Shared Resource) for their help in performing the gene profiling for the study. We also thank Tiffany Phan for technical assistance in preparing the manuscript. Financial disclosure: Dr. Poland is the chair of a DMSB for novel vaccines undergoing clinical study by Merck Research Laboratories. Dr. Jacobson serves on a Safety Review Committee for a post-licensure study of Gardasil for Kaiser-Permanente.
References
- Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghall H, Siren J, Sarkar D, Julkunen I, Fisher PB, Vainionpaa R, Matikainen S. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 2006;8:2138–2144. doi: 10.1016/j.micinf.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche FB, Ertl OT, Muller CP. Neutralizing B cell response in measles. Viral Immunol. 2002;15:451–471. doi: 10.1089/088282402760312331. [DOI] [PubMed] [Google Scholar]
- Caignard G, Guerbois M, Labernardiere JL, Jacob Y, Jones LM, Wild F, Tangy F, Vidalain PO Infectious Mapping Project I-MAP. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology. 2007;368:351–362. doi: 10.1016/j.virol.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Carsillo M, Klapproth K, Niewiesk S. Cytokine imbalance after measles virus infection has no correlation with immune suppression. J Virol. 2009;83:7244–7251. doi: 10.1128/JVI.00148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabalgoity JA, Baz A, Rial A, Grille S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 2007;18:195–207. doi: 10.1016/j.cytogfr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Childs K, Stock N, Ross C, Andrejeva J, Hilton L, Skinner M, Randall R, Goodbourn S. MDA-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Childs KS, Andrejeva J, Randall RE, Goodbourn S. Mechanism of MDA-5 inhibition by paramyxovirus V proteins. J Virol. 2009;83:1465–1473. doi: 10.1128/JVI.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, Hodge G, McChesney MB, Cattaneo R. Attenuation of V- or C-defective measles viruses: Infection control by the inflammatory and interferon responses of rhesus monkeys. J Virol. 2008;82:5359–5367. doi: 10.1128/JVI.00169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P, von Messling V, Songsungthong W, Springfeld C, Cattaneo R. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology. 2007;360:72–83. doi: 10.1016/j.virol.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Dhiman N, Ovsyannikova IG, Ryan JE, Jacobson RM, Vierkant RA, Pankratz SV, Jacobsen SJ, Poland GA. Correlations among measles virus-specific antibody, lymphoproliferation and Th1/Th2 cytokine responses following MMR-II vaccination. Clin Exp Immunol. 2005;142:498–504. doi: 10.1111/j.1365-2249.2005.02931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana JM, Bankamp B, Bellini WJ, Rota PA. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology. 2008;374:71–81. doi: 10.1016/j.virol.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: A lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gerlier D, Valentin H. Measles virus interaction with host cells and impact on innate immunity. In: Griffin DE, Oldstone MB, editors. Measles—History and basic biology. Berlin, Heidelberg: Springer-Verlag; 2009. pp. 163–191. [DOI] [PubMed] [Google Scholar]
- Gotoh B, Komatsu T, Takeuchi K, Yokoo J. Paramyxovirus strategies for evading the interferon response. Rev Med Virol. 2002;12:337–357. doi: 10.1002/rmv.357. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Ward BJ. Differential CD4 T cell activation in measles. J Infect Dis. 1993;168:275–281. doi: 10.1093/infdis/168.2.275. [DOI] [PubMed] [Google Scholar]
- Griffin DE, Ward BJ, Esolen LM. Pathogenesis of measles virus infection: An hypothesis for altered immune responses. J Infect Dis. 1994;170:S24–S31. doi: 10.1093/infdis/170.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- Hahm B. Hostile communication of measles virus with host innate immunity and dendritic cells. Curr Top Microbiol Immunol. 2009;330:271–287. doi: 10.1007/978-3-540-70617-5_13. [DOI] [PubMed] [Google Scholar]
- Hahm B, Cho JH, Oldstone MB. Measles virus-dendritic cell interaction via SLAM inhibits innate immunity: Selective signaling through TLR4 but not other TLRs mediates suppression of IL-12 synthesis. Virology. 2006;358:251–257. doi: 10.1016/j.virol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe RC, Dhiman N, Ovsyannikova IG, Poland GA. Induction of CD4 T cell proliferation and Th1-like cytokine responses in vitro to measles virus. Clin Exp Immunol. 2005a;140:333–342. doi: 10.1111/j.1365-2249.2005.02766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe RC, Ovsyannikova IG, Pinsky NA, Poland GA. Identification of Th0 cells responding to measles virus. Hum Immunol. 2005b;66:104–115. doi: 10.1016/j.humimm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Larosa DF, Orange JS. 1. Lymphocytes. J Allergy Clin Immunol. 2008;121:S364–S369. doi: 10.1016/j.jaci.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Moss WJ, Ryon JJ, Monze M, Griffin DE. Differential regulation of interleukin (IL)-4, IL-5, and IL-10 during measles in Zambian children. J Infect Dis. 2002;186:879–887. doi: 10.1086/344230. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y, Takeda M, Iwasaki M, Yanagi Y. A highly attenuated measles virus vaccine strain encodes a fully functional C protein. J Virol. 2009;83:11996–12001. doi: 10.1128/JVI.00791-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu Y, Takeda M, Ohno S, Koga R, Yanagi Y. Translational inhibition and increased interferon induction in cells infected with C protein-deficient measles virus. J Virol. 2006;80:11861–11867. doi: 10.1128/JVI.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanan R, Rauch A, Kämpgen E, Niewiesk S, Kreth HW. A novel sensitive approach for frequency analysis of measles virus-specific memory T-lymphocytes in healthy adults with a childhood history of natural measles. J Gen Virol. 2000;81:1313–1319. doi: 10.1099/0022-1317-81-5-1313. [DOI] [PubMed] [Google Scholar]
- Naniche D. Human immunology of measles virus infection. Curr Top Microbiol Immunol. 2009;330:151–171. doi: 10.1007/978-3-540-70617-5_8. [DOI] [PubMed] [Google Scholar]
- Naniche D, Yeh A, Eto D, Manchester M, Friedman RM, Oldstone BA. Evasion of host defenses by measles virus: Wild-type measles virus infection interferes with induction of Alpha/Beta interferon production. J Virol. 2000;74:7478–7484. doi: 10.1128/jvi.74.16.7478-7484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Ono N, Takeda M, Takeuchi K, Yanagi Y. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J Gen Virol. 2004;85:2991–2999. doi: 10.1099/vir.0.80308-0. [DOI] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Hidaka Y, Tomonobu A, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001;75:4399–4401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Vierkant RA, Jacobsen SJ, Pankratz VS, Poland GA. The contribution of HLA class I antigens in immune status following two doses of rubella vaccination. Hum Immunol. 2004;65:1506–1515. doi: 10.1016/j.humimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Reid KC, Jacobson RM, Oberg AL, Klee GG, Poland GA. Cytokine production patterns and antibody response to measles vaccine. Vaccine. 2003;21:3946–3953. doi: 10.1016/s0264-410x(03)00272-x. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Ryan JE, Vierkant RA, Pankratz SV, Jacobson RM, Poland GA. Immunologic significance of HLA class I genes in measles virus-specific IFN-gamma and IL-4 cytokine immune responses. Immunogenetics. 2005;57:828–836. doi: 10.1007/s00251-005-0061-6. [DOI] [PubMed] [Google Scholar]
- Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J Virol. 2003;77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Parisien JP, Horvath CM. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J Virol. 2008;82:8330–8338. doi: 10.1128/JVI.00831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Ryan JE, Ovsyannikova IG, Dhiman N, Pinsky NA, Vierkant RA, Jacobson RM, Poland GA. Inter-operator variation in ELISPOT analysis of measles virus-specific IFN-gamma secreting T cells. Scand J Clin Lab Invest. 2005;65:681–690. doi: 10.1080/00365510500348252. [DOI] [PubMed] [Google Scholar]
- Schneider-Schaulies S, Dittmer U. Silencing T cells or T-cell silencing: Concepts in virus-induced immunosuppression. J Gen Virol. 2006;87:1423–1438. doi: 10.1099/vir.0.81713-0. [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Bellini WJ, Rota PA. The C protein of measles virus inhibits the type I interferon response. Virology. 2003;315:389–397. doi: 10.1016/s0042-6822(03)00537-3. [DOI] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Svitek N, von Messling V. Early cytokine mRNA expression profiles predict Morbillivirus disease outcome in ferrets. Virology. 2007;362:404–410. doi: 10.1016/j.virol.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Takeuchi K, Miyajima N, Kobune F, Ami Y, Nagata N, Suzaki Y, Nagai Y, Tashiro M. Recovery of pathogenic measles virus from cloned cDNA. J Virol. 2000;74:6643–6647. doi: 10.1128/jvi.74.14.6643-6647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Kadota SI, Takeda M, Miyajima N, Nagata K. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 2003;545:177–182. doi: 10.1016/s0014-5793(03)00528-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Takeda M, Miyajima N, Ami Y, Nagata N, Suzaki Y, Shahnewaz J, Kadota S, Nagata K. Stringent requirement for the C protein of wild-type measles virus for growth both in vitro and in macaques. J Virol. 2005;79:7838–7844. doi: 10.1128/JVI.79.12.7838-7844.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Els CA, Nanan R. T cell responses in acute measles. Viral Immunol. 2002;15:435–450. doi: 10.1089/088282402760312322. [DOI] [PubMed] [Google Scholar]
- Ward BJ, Griffin DE. Changes in cytokine production after measles virus vaccination: Predominant production of IL-4 suggests induction of a Th2 response. Clin Immunol Immunopathol. 1993;67:171–177. doi: 10.1006/clin.1993.1061. [DOI] [PubMed] [Google Scholar]
- Wu VH, McFarland H, Mayo K, Hanger L, Griffin DE, Dhib-Jalbut S. Measles virus-specific cellular immunity in patients with vaccine failure. J Clin Microbiol. 1993;31:118–122. doi: 10.1128/jcm.31.1.118-122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Takeda M, Ohno S. Measles virus: Cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- Yu XL, Cheng YM, Shi BS, Qian FX, Wang FB, Liu XN, Yang HY, Xu QN, Qi TK, Zha LJ, Yuan ZH, Ghildyal R. Measles virus infection in adults induces production of IL-10 and is associated with increased CD4+ CD25+ regulatory T cells. J Immunol. 2008;181:7356–7366. doi: 10.4049/jimmunol.181.10.7356. [DOI] [PubMed] [Google Scholar]
- Zilliox MJ, Moss WJ, Griffin DE. Gene expression changes in peripheral blood mononuclear cells during measles virus infection. Clin Vaccine Immunol. 2007;14:918–923. doi: 10.1128/CVI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilliox MJ, Parmigiani G, Griffin DE. Gene expression patterns in dendritic cells infected with measles virus compared with other pathogens. Proc Natl Acad Sci USA. 2006;103:3363–3368. doi: 10.1073/pnas.0511345103. [DOI] [PMC free article] [PubMed] [Google Scholar]