Abstract
Ethnopharmacological Relevance
Artemisinin (AN) is produced by Artemisia annua, a medicinal herb long used as a tea infusion in traditional Chinese medicine to treat fever; it is also the key ingredient in current artemisinin-based combination therapies (ACTs) effective in treating malaria. Recently we showed that dried leaves from the whole plant A. annua that produces artemisinin and contains artemisinin-synergistic flavonoids seems to be more effective and less costly than ACT oral malaria therapy; however little is known about how digestion affects release of artemisinin and flavonoids from dried leaves.
Material and Methods
In the current study we used a simulated digestion system to determine how artemisinin and flavonoids are released prior to absorption into the bloodstream. Various delivery methods and staple foods were combined with dried leaves for digestion in order to investigate their impact on the bioavailability of artemisinin and flavonoids. Digestate was recovered at the end of the oral, gastric, and intestinal stages, separated into solid and liquid fractions, and extracted for measurement of artemisinin and total flavonoids.
Results
Compared to unencapsulated digested dried leaves, addition of sucrose, various cooking oils, and rice did not reduce the amount of artemisinin released in the intestinal liquid fraction, but the amount of released flavonoids nearly doubled. When dried leaves were encapsulated into either hydroxymethylcellulose or gelatin capsules, there was >50% decrease in released artemisinin but no change in released flavonoids. In the presence of millet or corn meal, the amount of released artemisinin declined, but there was no change in released flavonoids. Use of a mutant A. annua lacking artemisinin showed that the plant matrix is critical in determining how artemisinin is affected during the digestion process.
Conclusions
This study provides evidence showing how both artemisinin and flavonoids are affected by digestion and dietary components for an orally consumed plant delivered therapeutic and that artemisinin delivered via dried leaves would likely be more bioavailable if provided as a tablet instead of in a capsule.
Keywords: antiprotozoal, Artemisia annua, artemisinin, digestion, flavonoids, gastrointestinal system, malaria
1.0 INTRODUCTION
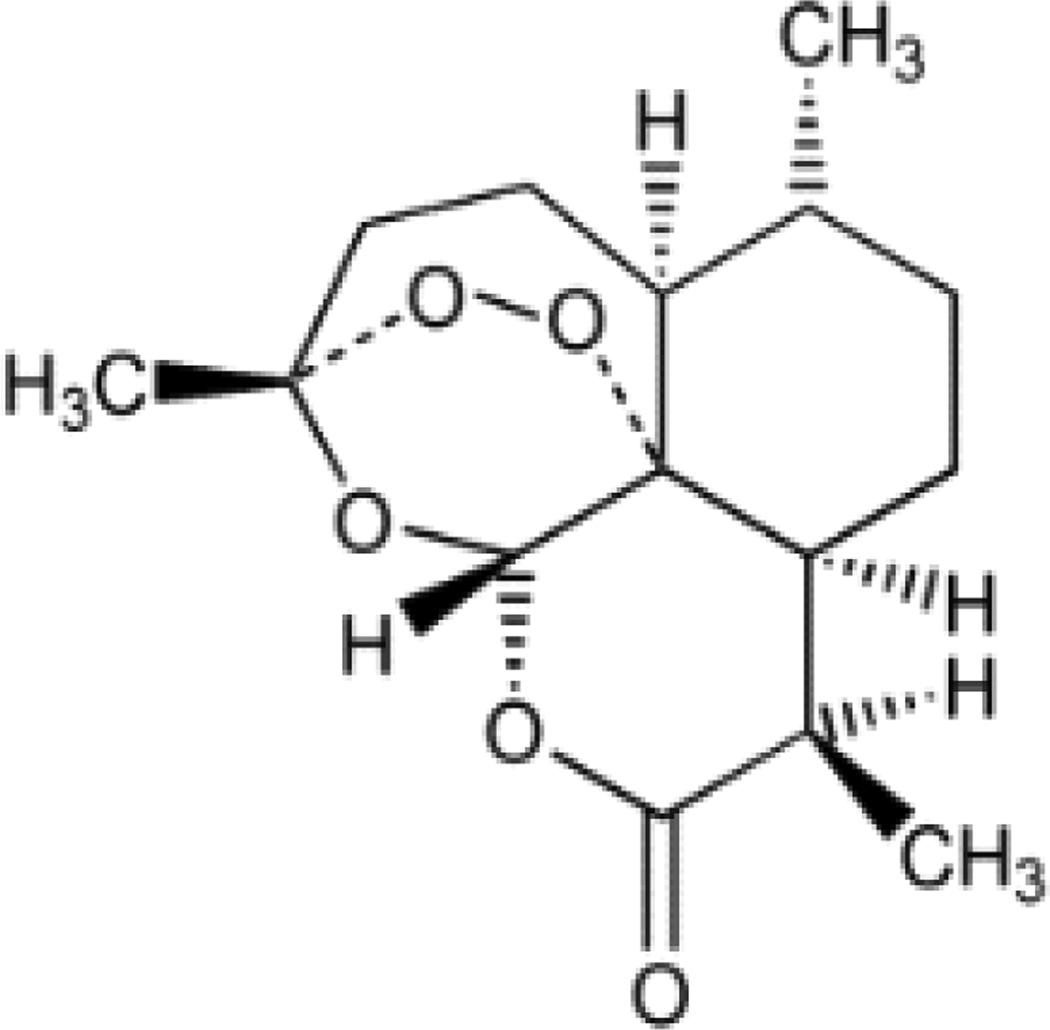
In 2011, there were roughly 215 million cases of malaria, mostly among African children, and an estimated 655,000 deaths in 2010 (WHO, 2010). In 2012, 3.3 billion people, or half of the world population, were at risk of contracting malaria (WHO, 2012). The key drug fraction in malaria treatment is artemisinin (AN; Figure 1), a sesquiterpene lactone that is produced and stored in glandular secretory trichomes in the leaves of Artemisia annua L. (Asteraceae), a generally recognized as safe (GRAS; Duke, 2001) medicinal plant used in traditional Chinese medicine dating to 168 B.CE. AN is also effective against many other diseases (Efferth, 2009)
Figure 1. The chemical structure of artemisinin.
Currently, AN-based combination therapy (ACT) is the best available treatment for malaria. Malaria treatments contain AN in combination with an older antimalarial drug to prevent AN drug resistance from emerging. While ACTs are an effective treatment, they are expensive and unattainable to many suffering from malaria in developing countries (Yeung et al., 2008; O’Connell et al., 2011). Resistance to antimalarial medications has also undermined malaria control efforts and continues to be a threat (WHO 2010; Phyo et al., 2012). Emerging research on use of dried leaves of A. annua in what we have called pACT, a plant-based artemisinin combination therapy, suggests that it may be a low cost yet effective solution for the treatment of malaria (Weathers et al., 2011; Elfawal et al., 2012; ICIPE 2005; Onimus et al., 2013) and other diseases (Efferth, 2009).
A. annua contains many other compounds, including flavonoids, which act synergistically with AN (Elford et al., 1987; Lehane and Saliba, 2008; Liu et al., 1992) and increase the potency of AN, lowering the required dosage for treatment. Mouse studies showed that AN delivered orally via gavage of the dried leaves provided at least forty-fold greater bioavailability of AN than that measured from an equally delivered amount of pure AN (Weathers et al., 2011) and was at least five times more effective than pure AN in reducing parasitemia (Elfawal et al., 2012). In a Kenyan human trial, dried A. annua leaf tablets (pACT) fed to 48 malaria patients yielded results similar to trials with pure AN (Weathers et al., 2013), but much less AN was required when the drug was delivered as dried leaves (ICIPE, 2005). Bioavailability can be validated with a better understanding of the progression of AN delivered via dried leaves through the individual stages of the human digestive system.
This study used a simulated digestion system to examine what happens to the plant material as it progresses through the intestinal stage of digestion, but prior to absorption into the blood. It also identified how dietary supplements and delivery methods impacted bioavailability of AN and flavonoids, key components of the proposed pACT malaria treatment.
2.0 METHODS
2.1 Plant Material
Artemisia annua L., SAM cultivar (Weathers and Towler, 2012; voucher MASS 00317314), was field grown in Stow, MA from rooted cuttings planted in May 2012 and harvested mid-September 2012 at full flower bud. All plants were watered regularly and no herbicides or pesticides were used. After harvest, plants were air dried at ambient temperature in light under a greenhouse cover, dried leaves removed and successively processed through 2 and 0.6 mm brass sieves. A single homogeneous batch of dried SAM plant material was used for the entire study. The glandless mutant of A. annua (GLS; vouchers OR State Univ 171772 and 170353) was a gift from Stephen Duke (University of Mississippi, Oxford; Duke et al., 1994). GLS was grown in the lab under continuous light that inhibited flowering. Shoots were harvested, dried, and sieved as described above for the SAM cultivar. Both cultivars were analyzed for their AN and flavonoid levels (Supplemental Table S1).
2.2 Chemicals and Capsules
Unless otherwise specified, all chemicals and enzymes were purchased from Sigma-Aldrich. Methylene chloride was purchased from Thermo Fisher Scientific. Capsules were size “00” vegetable or gelatin capsules and composed completely of hydroxypropylmethylcellulose and water or beef gelatin and water, respectively (Capsule Connection LLC, Prescott, AZ, USA).
2.3 Simulated Three Stage Digestion
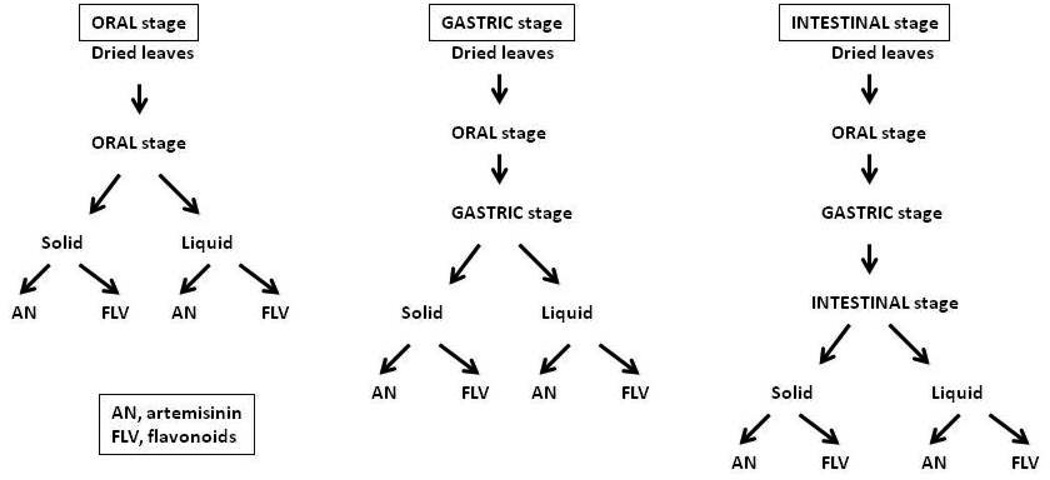
The simulated digestion protocol used in this study was based on the method of Kean et al., (2011) modified from the original reported by Garrett et al., (1999). The method is schematically illustrated in Figure 2 and simulates food progressing through the three stages of the digestive system: oral, gastric, and intestinal. Since this experiment involved the ingestion of plant material, as opposed to porridge used in the Kean et al., (2011) protocol, the volumes were scaled down based on the mass of plant material used.
Figure 2. Schematic illustrating the process of simulated digestion.
Dried leaves of A. annua were subjected to successive stages of digestion and then separated into the solid and liquid fractions, which were each extracted with methylene chloride for analysis of artemisinin (AN) and total flavonoids (FLV).
For the oral stage, an oral base solution was prepared containing 0.179 g potassium chloride, 0.177 g anhydrous dibasic sodium phosphate, 0.114 g anhydrous sodium sulfate, 0.059 g sodium chloride, and 0.338 g sodium bicarbonate in 100 mL of water. A 10 mL aliquot of the oral base solution was mixed in a beaker with 4 mg urea, 0.3 mg uric acid, and 0.5 mg mucin. A 1.5 mL aliquot of this solution was added to a 50 mL centrifuge tube containing 0.36 g of dry A. annua sieved leaves (see above) and 1.64 mL of distilled water. In the capsule delivery experiments, two capsules were used per reaction tube with each containing 0.18 g of A. annua dried leaves. In the dietary supplement experiments, an additional 0.36 g of cereal grains ground in a coffee grinder to yield grain meals, various oils, or common table sugar (sucrose) was added to the reaction tube directly following the addition of 0.36 g of A. annua leaves. Oils tested included canola, red palm, peanut, and sunflower. All cereal meals and oils were purchased from local food stores. In the experiments containing the cereal meals (millet, corn, or white rice), the addition of water was doubled. To each reaction tube, 46.8 mg of α-amylase was added and the tube was vortexed for 2 minutes. The tubes were blanketed with N2 gas, capped, and then placed in a 37°C water bath and shaken at 90 rpm for 10 minutes. During the 10 minute incubation, a solution of 10 mg mL−1 pepsin in 0.1M HCl was prepared for the gastric stage. The reaction tubes were removed from the bath and immediately placed on ice. A 4 mL aliquot of saline (0.9% w/v sodium chloride) was added to bring the volume to 7.5 mL. Using 1M HCl, the pH of each reaction tube was adjusted to 4.0 ± 0.1, and 0.5 mL of the pepsin solution was added. The pH was readjusted to 2.5 ± 0.1 using 1M HCl, and the volume of each reaction tube was brought to 10 mL with saline. The tubes were blanketed with N2 gas, capped, placed in the 37°C water bath, and incubated at 90 rpm for 1 hour. During this time, a solution containing 30 mg mL−1 bile extract in 100 mM sodium bicarbonate solution was prepared and sonicated for 30 minutes. Then 45 minutes into the incubation period, 1.5 mL of a pancreatin-lipase solution was prepared containing 20 mg mL−1 pancreatin and 10 mg mL−1 lipase in 100 mM NaHCO3. After the hour-long gastric phase incubation, the reaction tubes were removed and immediately placed on ice for preparation of the intestinal stage. The pH was adjusted to 4.0 ± 0.1 using 1M NaHCO3, and 0.5 mL of the pancreatin-lipase solution was added, followed by 0.75 mL of the bile extract solution. The pH was then adjusted to 6.5 ± 0.1 using 1M NaHCO3, and each volume was brought to 12.5 mL with saline. The samples were blanketed with N2 gas, capped, and incubated in the 37°C water bath at 90 rpm for 2 hours for completion of the simulated digestion process.
2.4 Filtration and Extraction of Digesta
Using replicate digestions, tubes were extracted after the oral stage, the gastric stage, or the intestinal stage was completed (see Figure 2). The resulting digesta from each of the oral, gastric, and intestinal stages were vortexed and filtered through Whatman #1 filter paper to separate liquid and solid fractions. The solid and liquid fractions from each stage were extracted with methylene chloride in a sonicating water bath for 30 minutes. After sonication, the solid fraction was vacuum filtered. All methylene chloride extracts were dried under a stream of N2 gas.
2.5 Assays
AN was quantified in extracts by GCMS according to the method detailed in Weathers and Towler (2012). Total flavonoids were assayed using the AlCl3 method of Arvouet-Grand et al., (1994) with quercetin as the standard. Briefly, an aliquot of standard or sample was dried and then resuspended in 3 mL of a 1:1 solution of 2% AlCl3 (w/v in MeOH) to MeOH and incubated for 25 minutes. Absorbance at 415 nm was then measured and flavonoid content of each digestate extract was calculated by using the quercetin standard curve. Flavonoids were expressed as quercetin equivalents.
2.6 Statistical Analysis
Digestions were performed at least in triplicate, and averages and standard deviations were calculated for each digestate fraction. Using the statistical software SPSS, post-hoc Tukey tests and Student’s t-tests were used to determine statistical differences between samples.
3.0 THEORY
Often, victims of malaria are unable to consume food, which limits their treatment options. The role of diet and its effect on the role of dried leaf consumption of A. annua have yet to be studied. Variables such as staple foods should be compared in order to determine whether they inhibit or enhance bioavailability of AN and flavonoids. Studies conducted on A. annua in the past have provided information on the efficacy of dried leaf treatment based on the concentration of AN found in the serum (Weathers et al., 2011). More information on how this plant is digested can provide insight into how endogenous therapeutic chemicals in the plant are released and passed through the digestive tract and into the bloodstream, where parasites reside after infection.
4.0 RESULTS and DISCUSSION
4.1 Some Artemisinin Is Lost after Dried Leaf Digestion
When the whole plant material of A. annua (dried leaves) was extracted prior to digestion, it contained 7.65 mg g−1 and 2.97 mg g−1 DW of AN and flavonoids, respectively (Table 1). After digestion, the amounts of AN recovered in the liquid fraction of each digestion stage decreased, with about half of the initially available AN remaining in the intestinal stage (Table 1, S+L data column). However, most of the AN resided in the solid phase, with only 22% of the originally available AN in the liquid phase where it will be most bioavailable (Table 1). Although about 50% of the initial total AN (S+L) was lost, the amount of AN released into the intestinal liquid phase nearly tripled from that in the liquid phase of the oral stage of digestion (Table 1). AN water solubility is about 50 mg L−1 (van der Kooy and Verpoorte, 2011). The amount of recovered AN in the liquid phase after the intestinal stage of digestion is about 50 mg L−1, suggesting overall AN solubility did not increase.
Table 1.
Artemisinin and flavonoids in solid and liquid phases after each stage of digestion of dried leaves.
| Plant material | Digested samples of A. annua dried leaves | |||||
|---|---|---|---|---|---|---|
| Artemisinin (mg g−1 DW) |
Flavonoids (mg g−1 DW) |
|||||
| Undigested dried leaves | 7.65 ± 0.92 | 2.967 ± 0.082 | ||||
| Digested leaves: | S | L | S+L | S | L | S+L |
| Oral Phase | 5.46 ± 2.50 | 0.61 ± 0.21 | 6.07 | 1.158 ± 0.089 | 0.071 ± 0.035 | 1.229 |
| Gastric Phase | 5.55 ± 0.73 | 0.81 ± 0.05 | 6.36 | 0.899 ± 0.121 | 0.064 ± 0.016 | 0.963 |
| Intestinal Phase | 2.24 ± 0.31* | 1.72 ± 0.54* | 3.96* | 0.983 ± 0.046 | 0.087 ± 0.028 | 1.070 |
Statistically different at p ≤ 0.05 for n = 3 when compared to oral stage; data are shown ± SD. S, solid phase of digestion stage; L, liquid phase of digestion stage.
Flavonoids in foodstuffs are usually present as glycosides; enzymatic hydrolysis is often needed for passage into the blood plasma, and serum levels are usually low, e.g. for 68–307 mg ingested quercetin glycosides, maximum serum levels (Cmax) ranged from 0.7–7.6 µmol L−1 (Manach and Donovan, 2004). Absorption of quercetin-3-glycoside yields about tenfold more flavonoid in the serum than the aglycone (Hollman, 2004). Although quercetin is not detectable in freshly harvested A. annua leaves, it is present at ~0.02 mg g−1 DW in the SAM cultivar after the leaves are dried. Recovery of flavonoids in the liquid phase of the intestinal stage of digestion was <4% of the original flavonoids in the starting plant material (Table 1). The amount of total flavonoids recovered from both the liquid and solid phases (S+L) through each stage of digestion did not vary significantly (Table 1), and ultimately about one third of the start value was present at the end of digestion.
4.2 Capsules Inhibit Dried Leaf Digestion
When dried leaves were encased in vegetable or gelatin capsules, AN recovery from the intestinal liquid phase significantly decreased (Table 2). AN declined from 1.72 mg g−1 DW for unencapsulated digested dried leaves to 0.23 and 0.74 mg g−1 DW for vegetable and gelatin capsules, respectively (Table 2). While most of this loss in the vegetable capsule occurred during the intestinal stage, there was no significant change among digestion stages for gelatin-encapsulated dried leaves (Supplemental Table S2).
Table 2.
Digestion of dried leaves and effect of encapsulation on release of artemisinin and flavonoids through the intestinal stage.
| Dried leaves | Artemisinin | Flavonoids | ||
|---|---|---|---|---|
| mg g−1 DW | as % of digested dried leaves |
mg g−1 DW | as % of digested dried leaves |
|
| undigested | 7.65 ± 0.92 | not applicable | 2.967 ± 0.082 | not applicable |
| digested | 1.72 ± 0.54 | 100a (22)b | 0.087 ± 0.028 | 100a (3)b |
| in vegetable capsule | 0.23 ± 0.19* | 13a | 0.147 ± 0.011 | 169a |
| in gelatin capsule | 0.74 ± 0.12* | 43a | 0.150 ± 0.030 | 172a |
| + empty vegetable capsule, undigested | 4.92 ± 0.86* | 64b | not applicable | not applicable |
| + empty gelatin capsule, undigested | 8.05 ± 1.02 | 105b | not applicable | not applicable |
As % of digested dried leaves, which is normalized to 100%.
As % of original undigested dried leaves.
Statistically significantly different at p ≤ 0.05; n = 3 when compared to digested dried leaves.
To examine if the reduction in AN when dried leaves were encased in a capsule was due to the presence of the hydroxymethylcellulose or the gelatin, the main material used to produce vegetable and gelatin capsules, respectively, each type of capsule was added to dried leaves and then immediately extracted with no digestion. When dried leaves were added to an empty gelatin capsule and extracted, the AN recovered was equivalent to that recovered from undigested dried leaf extracts (Table 2). This suggested that it was not the gelatin alone with A. annua that made AN unrecoverable but the combination of the digestion process and gelatin (Table 2). In contrast, when dried leaves were added to an undigested vegetable capsule and immediately extracted (Table 2), about 30% less AN was recovered compared to undigested dried leaves (Table 2) suggesting that the hydroxymethylcellulose comprising the vegetable capsule added to A. annua partially degrades, binds to, or masks AN. Although it can bind to terpenoid lactones (Xu et al., 2001), gelatin did not seem to affect AN when it was added without digestion. We are not aware of any similar reports regarding terpenoid binding to cellulose to explain the AN losses in the presence of undigested vegetable (cellulosic) capsules.
When the total flavonoid content of the liquid fraction of the intestinal stage was measured, there was no significant difference between capsule types and digested dried leaves in the amount of flavonoids recovered (Table 2). Although there was a slight increase in flavonoid recovery in the liquid intestinal phase as digestion progressed from oral through intestinal stage, the difference was not statistically significant among the three digestion stages for either capsule type (Supplemental Table S2). Together these results suggested that encapsulation of dried leaves is not recommended, but rather compressed leaf tablets should instead be used.
4.3 Dried Leaves and Dietary Supplementation
Although a pharmacokinetic study by Dien et al. (1997) showed that food intake did not affect AN absorption after oral consumption of pure AN, assessment of the impact of food is critical to understanding how dried leaves are digested. To determine the effect of food, common dietary components such as sugar (sucrose), four oils, and three cereal meals were included in digestion tests. When sucrose was added to dried leaves to decrease its bitter taste, there was no change in AN recovered from the intestinal stage (Table 3). Peters et al. (2010) also noticed that sucrose had no negative effect on catechin bioavailability from green tea.
Table 3.
Effect of dietary constituents of artemisinin and flavonoids released after digestion of dried leaves through the intestinal stage.
| Dried leaves | Artemisinin | Flavonoids | ||
|---|---|---|---|---|
| mg g−1 DW | as % of digested dried leavesa |
mg g−1 DW | as % of digested dried leavesa |
|
| digested alone | 1.72 ± 0.54 | 100 | 0.087 ± 0.028 | 100 |
| + sucrose | 1.59 ± 0.20 | 92 | 0.207 ± 0.019* | 205 |
| + canola oil | 1.40 ± 0.26 | 81 | 0.203 ± 0.052* | 201 |
| + red palm oil | 1.12 ± 0.37 | 65 | 0.201 ± 0.026* | 199 |
| + sunflower oil | 1.02 ± 0.23b | 59 | 0.234 ± 0.072b | 232 |
| + peanut oil | 1.61 ± 0.47 | 94 | 0.161 ± 0.009b | 159 |
| + white rice meal | 1.36 ± 1.00 | 79 | 0.156 ± 0.003 | 154 |
| + corn meal | 0.73 ± 0.21b | 42 | 0.205 ± 0.070b | 203 |
| + millet meal | 0.99 ± 0.20b | 58 | 0.116 ± 0.023 | 115 |
Digested dried leaves are normalized to 100%.
Statistically significantly different at p ≤ 0.05; n = 3 when compared to dried leaves alone digested.
p = 0.11, 0.06, 0.11 for AN in dried leaves + sunflower oil, corn meal, and millet meal, respectively;
p = 0.09, 0.11, 0.11 for flavonoid in dried leaves + sunflower oil, peanut oil, and corn meal, respectively.
Although AN is not particularly soluble in either oil or aqueous solutions, we also tested addition of oils to dried leaves. When compared to dried leaves alone, none of the four tested oils altered the amount of AN recovered from the intestinal stage (Table 3). Using canola oil, each digestion stage was individually measured and there was a significant increase in AN content in the liquid phase from the oral to the gastric stage, but no increase from the gastric to the intestinal stage (Supplemental Table S3). In contrast, combining dried leaves with sucrose showed little change in AN content in the liquid fraction from the oral to the gastric stage, but an increase from the gastric to the intestinal stage (Supplemental Table S3).
A. annua is a bitter tasting herb, so either sweetening it with sugar and/or adding it to porridge (Bonati et al., 2011) could facilitate oral consumption, especially for pediatric patients. Addition of different cereal meals to dried leaves yielded varying AN levels in the intestinal liquid fractions. The dried leaves + white rice combination showed no significant change in the release of AN. However, recovery of AN decreased in the intestinal stage of both dried leaves + millet and dried leaves + corn meal combinations (Table 3). In a small Kenyan study that mixed pure AN or A. annua dried leaves with Uji, a popular millet porridge made with hot water, AN was fully recovered from all of the test combinations, demonstrating that AN was stable in this common food (Bonati et al., 2011). The dried leaves + corn meal combination showed the greatest reduction with an average 0.73 mg AN g−1 DW in the intestinal liquid fraction, a 50% decrease compared to the dried leaves alone.
When sucrose or oil was added to dried leaves, overall flavonoid recovery in the intestinal stage was double that of dried leaves alone (Table 3; Supplemental Tables S4 and S5). Although the higher yield of flavonoids when oil was present suggested that more flavonoids were released when oils were present, it is possible there were flavonoids in these oils; however we are unaware of any such reports. Total flavonoid analysis of each of the four undigested oils proved technically challenging and thus was not measured. On the other hand, some of the oils used in this study enhanced the extraction of the flavonoid, quercetin, from St. John’s wort, and this could account for the increased recovery of flavonoids in the dried leaves + oil samples (Arsić et al., 2010). Flavonoid content of the three undigested grain meals was 0.0, 0.02, and 0.04 mg g−1 DW for rice, millet, and corn, respectively, which is less than 1.5% of that in dried leaves of the SAM cultivar. The cereal meal experiments showed no statistically significant difference in flavonoid content present in the liquid fraction in any of the three digestion stages when compared to dried leaves (Supplemental Table S5).
4.4 Effect of Digestion on Pure AN and the Role of the Plant Matrix
Because about a third of the total available AN in digested dried leaves disappeared after the intestinal stage (Table 1, S+L), this suggested that AN may have been degraded or otherwise altered during the digestion process. To determine the effect of the digestion process on pure AN, we added an amount of AN equal to that in the starting dried leaf material (7.65 mg g−1 DW) and observed that compared to digested dried leaves, significantly more AN was recovered in the liquid phase after the intestinal stage of digestion: 2.36 mg g−1 DW for pure AN, vs. 1.72 mg g−1 DW for AN from dried leaves (Supplemental Table S6). However, when the amount of AN in the solid phase was also included, then total recovery of pure digested AN declined by about 40%, indicating that the process of digestion partially destroyed AN. Although AN is susceptible to degradation by extremes of pH, in simulated gastric conditions (aqueous 0.01 M HCl, 36°C) Baker et al. (1993) reported that arteether decomposed slowly (441 min half-life) to dihydroartemisinin, an active antimalarial, suggesting that gastric conditions of pH and temperature are not likely responsible for AN losses. AN does bind to plasma proteins (Li et al., 1982; Ashton et al., 1998), so if AN bound to any of the proteins that make up the three-stage digestion complex, e.g. amylase, lipase etc., this could account for the lack of full recovery of pure AN after the intestinal digestion stage.
It is also possible, however, that the plant matrix itself was interfering with the release of or destroying AN, so to asses this we used the A. annua glandless mutant (GLS). GLS has no glandular trichomes and produces no AN (Duke et al., 1994); it also contains less than a quarter of the total flavonoids measured in the SAM cultivar that is used for dried leaves (Supplemental Table S1). When pure AN was added to GLS and digested, AN recovered from the liquid phase of the intestinal stage declined from 1.72 mg g−1 in digested dried leaves to 0.57 mg g−1 in the presence of GLS, about a 65% loss. Interestingly, 2.98 mg AN g−1 DW, about a third of the AN added to the GLS at the beginning of digestion remained with the intestinal solid phase, suggesting AN was unavailable for digestion. Together these data showed that both the process of digestion as well as the presence of the plant matrix affected AN losses and possibly also bioavailability.
5.0 CONCLUSIONS
Using a simulated digestion of dried leaves of A. annua as used in pACT, about 22% of AN and 4% of flavonoids were recovered from the liquid phase of the intestinal stage of digestion. Although capsules are readily soluble and apparently inert, encapsulation of dried leaves resulted in as much as 87% loss of AN compared to unencapsulated dried leaves, but with no significant change in recovery of flavonoids. Neither sugar nor four types of common culinary oils affected the amount of AN in the liquid phase of the intestinal stage of digestion, but the amount of flavonoids doubled. Three grain meals, on the other hand, appeared to have either no effect or reduced the amount of AN released in the intestinal stage, but had little effect on flavonoid release. It appeared that both the plant matrix and the process of digestion per se affected the amount of AN that appeared in the liquid intestinal phase. Together these results demonstrate how some common dietary constituents and encapsulation may affect the therapeutic efficacy of oral consumption of the dried leaf herbal medicine, pACT, which could prove useful for the treatment of malaria and other diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Prof. Mario Feruzzi and Tristan Lipkie of Purdue University for advice on simulated digestion and to Laura Sandford for technical assistance. We are also grateful to Worcester Polytechnic Institute and University of Massachusetts Center for Clinical and Translational Science (CCTS) for funding this project. The project also was partially supported by Award Number NIH-2R15GM069562-03 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Glossary
- AN
artemisinin
- pACT
dried A. annua leaves (SAM cultivar)
- GLS
glandless A. annua null AN mutant
- DW
dry weight
- FLV
flavonoids
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arsić IA, Žugic AR, Runjaić-Antić D, Zdunić G, Dekanski D, Marković GM, Tadić VM. Gastroprotective activity of Hypericum perforatum extracts prepared with different vegetable oils. Planta Medica. 2010 76-LS8. [Google Scholar]
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardization of a propolis extract and identification of the main constituents. Journal de pharmacie de Belgique. 1994;49:462–468. [PubMed] [Google Scholar]
- Ashton M, Nguyen DS, Nguyen VH, Trinh NH, Dinh XH, Nguyen TN, Le DC. Artemisinin kinetics and dynamics during oral and rectal treatment of uncomplicated malaria. Clinical Pharmacological Therapy. 1998;63:482–493. doi: 10.1016/S0009-9236(98)90044-3. [DOI] [PubMed] [Google Scholar]
- Baker JK, McChesney JD, Chi HT. Decomposition of artemether in simulated stomach acid yielding compounds retaining antimalarial activity. Pharmaceutical Research. 1993;10:662–666. doi: 10.1023/a:1018943329109. [DOI] [PubMed] [Google Scholar]
- Bonati M, Severino F, Bagnati R, Carrà A, Fanelli R. Millet-porridge with Artemisia annua as first aid for African children with malaria? Journal of Alternative and Complementary Medicine. 2011;17:1–3. doi: 10.1089/acm.2010.0252. [DOI] [PubMed] [Google Scholar]
- Dien TK, de Vries PJ, Khanh NX, Koopmans R, Binh LEN, Duc DD, Kager PA, van Boxtel CJ. Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrobial Agents and Chemotherapeutics. 1997;41:1069–1072. doi: 10.1128/aac.41.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke JA. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL: CRC Press LLC; 2001. p. 70. [Google Scholar]
- Duke MG, Paul RN, Elsohly HN, Sturtz G, Duke SO. Localization of artemisinin and artemisitene in foliar tissues of glanded and glandless biotypes of Artemisia annua L. International Journal of Plant Science. 1994;155:185–209. [Google Scholar]
- Efferth T. Chapter 11 Artemisinin: A Versatile Weapon from Traditional Chinese Medicine. In: Ramawat KG, editor. Herbal Drugs: Ethnomedicine to Modern Medicine. Springer Berlin Heidelberg: DGR; 2009. pp. 173–189. [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLos ONE. 2012;7:e52746. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford BC, Roberts MF, Phillipson D, Wilson RJM. Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Transactions of the Royal Society of Tropical Medical Hygiene. 1987;81:434–436. doi: 10.1016/0035-9203(87)90161-1. [DOI] [PubMed] [Google Scholar]
- Ferreira JFS, Luthria DL, Sasaki D, Heyerick A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 2010;15:3135–3170. doi: 10.3390/molecules15053135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DA, Failla ML, Sarama RJ. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. Journal of Agriculture and Food Chemistry. 1999;47:4301–4309. doi: 10.1021/jf9903298. [DOI] [PubMed] [Google Scholar]
- Hollman PCH. Absorption, bioavailability, and metabolism of flavonoids. Pharmaceutical Biology. 2004;42:74–83. [Google Scholar]
- ICIPE. Whole-leaf Artemisia annua-based antimalarial drug: report on proof-of-concepts studies, unpublished report. [Retrieved on July 20, 2013];2005 from http://www.google.com/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=2&ved=0CDgQFjAB&url=http%3A%2F%2Fwww.iwerliewen.org%2Findex.php%2Fcomponent%2Fedocman%2F%3Ftask%3Ddocument.download%26id%3D96%26Itemid%3D181&ei=J2miUbnFNo-80QGoi4GACw&usg=AFQjCNHoLJmPt4n0AkKyBlXPSyl5W7rc6w&sig2=ppM08X1tZglQLLiaojZx1w&bvm=bv.47008514,d.dmQ. [Google Scholar]
- Kean EG, Bordenave N, Edjeta G, Hamaker B, Ferruzzi M. Carotenoid bioaccessibility from whole grain and decorticated yellow endosperm sorghum porridge. Journal of Cereal Science. 2011;54:450–459. [Google Scholar]
- Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Research Notes. 2008;1:26. doi: 10.1186/1756-0500-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WH, Shu HL, Xu GY, Zeng YL. The binding of qinghaosu (artemisinine) and its derivatives to plasma protein [Chinese] Acta Pharmacetica Sinica. 1982;17:783–786. [PubMed] [Google Scholar]
- Liu KC-S, Yang SL, Roberts ME, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Reports. 1992;11:637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radical Research. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Gatakaa H, Poyer S, Njogu J, Evance I, Munroe E, Solomon T, Goodman C, Hanson K, Zinsou C, Akulayi L, Raharinjatovo J, Arogundade E, Buyungo P, Mpasela F, Adjibabi CB, Agbango JA, Ramarosandratana BF, Coker B, Rubahika D, Hamainza B, Chapman S, Shewchuk T, Chavasse D. Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malaria Journal. 2011;10:326. doi: 10.1186/1475-2875-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimus M, Carteron S, Lutgen P. The surprising efficiency of Artemisia annua powder capsules. Medicinal and Aromatic Plants. 2013;2:3. [Google Scholar]
- Peters CM, Green RJ, Janle EM, Feruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability. Food Research International. 2010;43:95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NPJ, White NJ, Anderson TJC, Nosten F. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung S, van Damme W, Socheat D, White NJ, Mills A. Cost of increasing access to artemisinin combination therapy: the Cambodian experience. Malaria Journal. 2008;7:84. doi: 10.1186/1475-2875-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooy F, Verpoorte R. The content of artemisinin in the Artemisia annua tea infusion. Planta Medica. 2011;77:1754–1756. doi: 10.1055/s-0030-1271065. [DOI] [PubMed] [Google Scholar]
- Verret WJ, Arinaitwe E, Humphrey W, Victor B, Kakuru A, Kamya M, Tappero JW, Sandison T, Dorsey G. Effect of nutritional status on response to treatment with artemisinin-based combination therapy in young children with malaria. Antimicrobial Agents and Chemotherapeutics. 2011;55:2629–2635. doi: 10.1128/AAC.01727-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW. Artemisinin Production in Artemisia annua : Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochemistry Reviews. 2010;10:173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers PJ, Towler MJ. The flavonoids casticin and artemetin are poorly extracted and are unstable in an Artemisia annua tea infusion. Planta Medica. 2012;78:1024–1026. doi: 10.1055/s-0032-1314949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers P, Reed K, Hassanali A, Lutgen P, Engeu PO. Chapter 3. Whole plant approaches to therapeutic use of Artemisia annua, L. (Asteraceae) In: Aftab T, Feirrera JFS, editors. Artemisia annua. Springer Berlin: GDR; 2013. in press. [Google Scholar]
- WHO. 10 Facts on Malaria. 2012 http://www.who.int/features/factfiles/malaria/en/index.html.
- WHO. Global Malaria Programme: Good procurement practices for artemisinin-based antimalarial medicines. 2010 http://whqlibdoc.who.int/publications/2010/9789241598927_eng.pdf.
- Xu M, Shi Z, Feng L, Liu J, Shi R, Xu M, Lu Y, He B. Synthesis of gelatin-PVA adsorbent and its applications in the separation of gingko flavonol glycosides and terpene lactones. Reactive Functional Polymers. 2001;46:273–282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.