Abstract
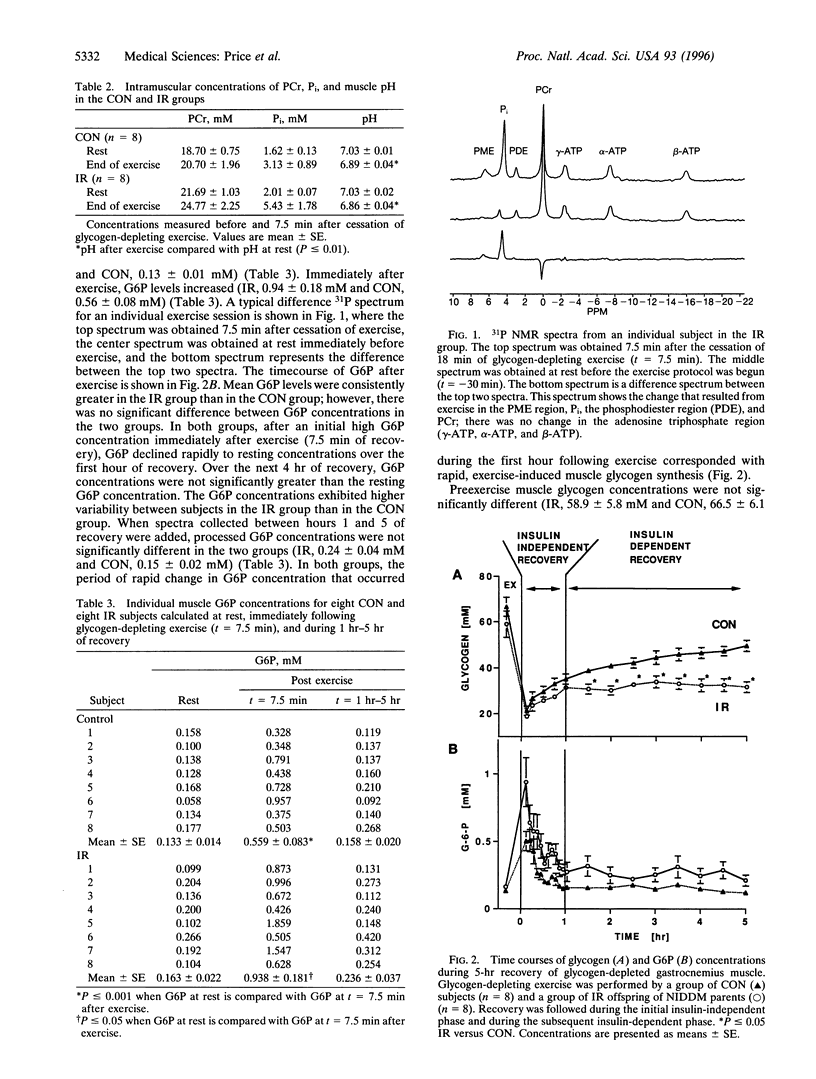
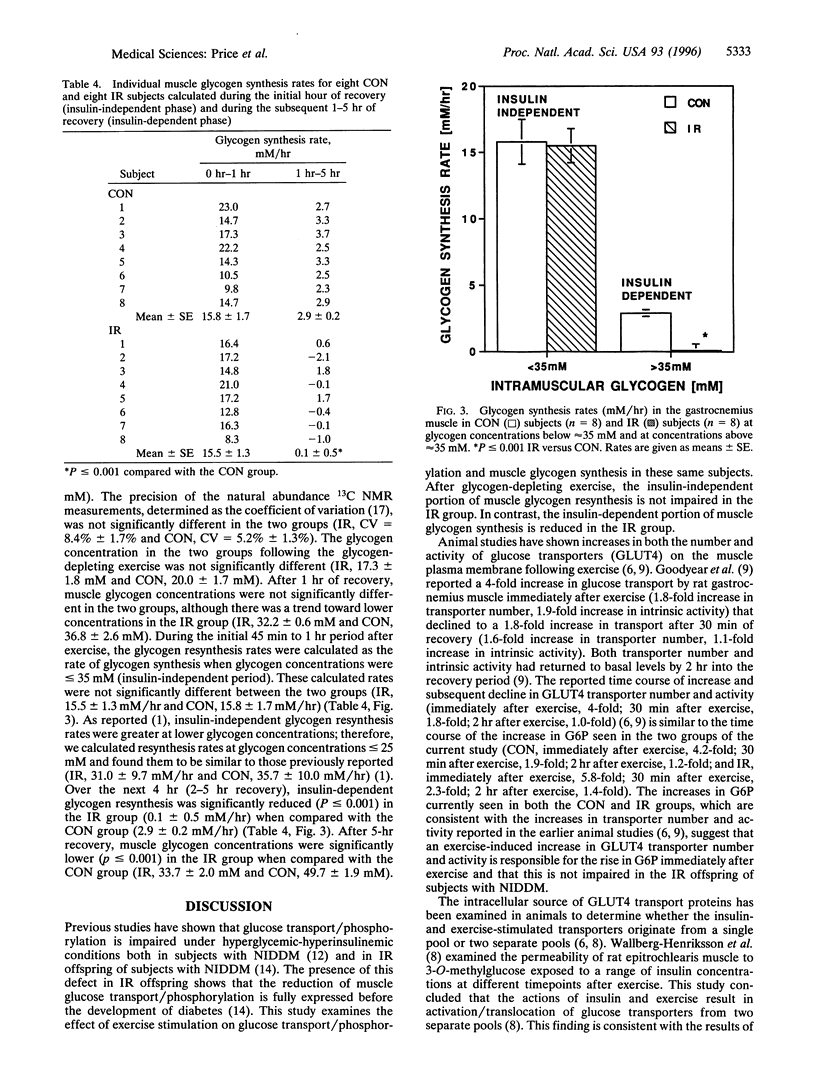
To examine the impact of insulin resistance on the insulin-dependent and insulin-independent portions of muscle glycogen synthesis during recovery from exercise, we studied eight young, lean, normoglycemic insulin-resistant (IR) offspring of individuals with non-insulin-dependent diabetes mellitus and eight age-weight matched control (CON) subjects after plantar flexion exercise that lowered muscle glycogen to approximately 25% of resting concentration. After approximately 20 min of exercise, intramuscular glucose 6-phosphate and glycogen were simultaneously monitored with 31P and 13C NMR spectroscopies. The postexercise rate of glycogen resynthesis was nonlinear. Glycogen synthesis rates during the initial insulin independent portion (0-1 hr of recovery) were similar in the two groups (IR, 15.5 +/- 1.3 mM/hr and CON, 15.8 +/- 1.7 mM/hr); however, over the next 4 hr, insulin-dependent glycogen synthesis was significantly reduced in the IR group [IR, 0.1 +/- 0.5 mM/hr and CON, 2.9 +/- 0.2 mM/hr; (P < or = 0.001)]. After exercise there was an initial rise in glucose 6-phosphate concentrations that returned to baseline after the first hour of recovery in both groups. In summary, we found that following muscle glycogen-depleting exercise, IR offspring of parents with non-insulin-dependent diabetes mellitus had (i) normal rates of muscle glycogen synthesis during the insulin-independent phase of recovery from exercise and (ii) severely diminished rates of muscle glycogen synthesis during the subsequent recovery period (2-5 hr), which has previously been shown to be insulin-dependent in normal CON subjects. These data provide evidence that exercise and insulin stimulate muscle glycogen synthesis in humans by different mechanisms and that in the IR subjects the early response to stimulation by exercise is normal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch G., Chase J. R., Avison M. J., Shulman R. G. In vivo 31P NMR measurement of glucose-6-phosphate in the rat muscle after exercise. Magn Reson Med. 1993 Sep;30(3):347–350. doi: 10.1002/mrm.1910300311. [DOI] [PubMed] [Google Scholar]
- Bonadonna R. C., Del Prato S., Saccomani M. P., Bonora E., Gulli G., Ferrannini E., Bier D., Cobelli C., DeFronzo R. A. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest. 1993 Jul;92(1):486–494. doi: 10.1172/JCI116592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. A., Hardy C. J., Roemer P. B., Mueller O. M. Proton-decoupled, Overhauser-enhanced, spatially localized carbon-13 spectroscopy in humans. Magn Reson Med. 1989 Dec;12(3):348–363. doi: 10.1002/mrm.1910120307. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Rastogi S., Bilan P. J., Cartee G. D., Vranic M., Holloszy J. O., Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990 Aug 15;265(23):13427–13430. [PubMed] [Google Scholar]
- Fleckenstein J. L., Canby R. C., Parkey R. W., Peshock R. M. Acute effects of exercise on MR imaging of skeletal muscle in normal volunteers. AJR Am J Roentgenol. 1988 Aug;151(2):231–237. doi: 10.2214/ajr.151.2.231. [DOI] [PubMed] [Google Scholar]
- Garetto L. P., Richter E. A., Goodman M. N., Ruderman N. B. Enhanced muscle glucose metabolism after exercise in the rat: the two phases. Am J Physiol. 1984 Jun;246(6 Pt 1):E471–E475. doi: 10.1152/ajpendo.1984.246.6.E471. [DOI] [PubMed] [Google Scholar]
- Goodyear L. J., Hirshman M. F., King P. A., Horton E. D., Thompson C. M., Horton E. S. Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J Appl Physiol (1985) 1990 Jan;68(1):193–198. doi: 10.1152/jappl.1990.68.1.193. [DOI] [PubMed] [Google Scholar]
- Gruetter R., Prolla T. A., Shulman R. G. 13C NMR visibility of rabbit muscle glycogen in vivo. Magn Reson Med. 1991 Aug;20(2):327–332. doi: 10.1002/mrm.1910200216. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Hermansen L. Resynthesis of muscle glycogen stores during recovery from prolonged exercise in non-diabetic and diabetic subjects. Acta Paediatr Scand Suppl. 1980;283:33–38. doi: 10.1111/j.1651-2227.1980.tb15307.x. [DOI] [PubMed] [Google Scholar]
- Kadish A. H., Sternberg J. C. Determination of urine glucose by measurement of rate of oxygen consumption. Diabetes. 1969 Jul;18(7):467–470. doi: 10.2337/diab.18.7.467. [DOI] [PubMed] [Google Scholar]
- Lund S., Holman G. D., Schmitz O., Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5817–5821. doi: 10.1073/pnas.92.13.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlum S., Høstmark A. T., Hermansen L. Synthesis of muscle glycogen during recovery after prolonged severe exercise in diabetic and non-diabetic subjects. Scand J Clin Lab Invest. 1977 Jun;37(4):309–316. doi: 10.3109/00365517709092634. [DOI] [PubMed] [Google Scholar]
- Price T. B., McCauley T. R., Duleba A. J., Wilkens K. L., Gore J. C. Changes in magnetic resonance transverse relaxation times of two muscles following standardized exercise. Med Sci Sports Exerc. 1995 Oct;27(10):1421–1429. [PubMed] [Google Scholar]
- Price T. B., Rothman D. L., Avison M. J., Buonamico P., Shulman R. G. 13C-NMR measurements of muscle glycogen during low-intensity exercise. J Appl Physiol (1985) 1991 Apr;70(4):1836–1844. doi: 10.1152/jappl.1991.70.4.1836. [DOI] [PubMed] [Google Scholar]
- Price T. B., Rothman D. L., Taylor R., Avison M. J., Shulman G. I., Shulman R. G. Human muscle glycogen resynthesis after exercise: insulin-dependent and -independent phases. J Appl Physiol (1985) 1994 Jan;76(1):104–111. doi: 10.1152/jappl.1994.76.1.104. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Enhanced muscle glucose metabolism after exercise: modulation by local factors. Am J Physiol. 1984 Jun;246(6 Pt 1):E476–E482. doi: 10.1152/ajpendo.1984.246.6.E476. [DOI] [PubMed] [Google Scholar]
- Roch-Norlund A. E., Bergström J., Hultman E. Muscle glycogen and glycogen synthetase in normal subjects and in patients with diabetes mellitus. Effect of intravenous glucose and insulin administration. Scand J Clin Lab Invest. 1972 Sep;30(1):77–84. doi: 10.3109/00365517209081094. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Magnusson I., Cline G., Gerard D., Kahn C. R., Shulman R. G., Shulman G. I. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L., Shulman R. G., Shulman G. I. 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate. Evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J Clin Invest. 1992 Apr;89(4):1069–1075. doi: 10.1172/JCI115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Sternlicht E., Barnard R. J., Grimditch G. K. Exercise and insulin stimulate skeletal muscle glucose transport through different mechanisms. Am J Physiol. 1989 Feb;256(2 Pt 1):E227–E230. doi: 10.1152/ajpendo.1989.256.2.E227. [DOI] [PubMed] [Google Scholar]
- Taylor R., Price T. B., Rothman D. L., Shulman R. G., Shulman G. I. Validation of 13C NMR measurement of human skeletal muscle glycogen by direct biochemical assay of needle biopsy samples. Magn Reson Med. 1992 Sep;27(1):13–20. doi: 10.1002/mrm.1910270103. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Constable S. H., Young D. A., Holloszy J. O. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol (1985) 1988 Aug;65(2):909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]



