Abstract
Neuroticism involves a tendency for enhanced emotional and cognitive processing of negative affective stimuli and a propensity to worry and be anxious. It is known that this trait modulates fear learning and the activation of brain regions involved in it such as the amygdala, hippocampus, and prefrontal cortex and their connectivity. Thirty-nine (21 female) 14-year-old healthy adolescents participated in functional magnetic resonance imaging (fMRI) of aversive pavlovian differential delay conditioning. An unpleasant sound served as unconditioned stimulus (US) and pictures of neutral male faces as conditioned stimuli (CS+ followed by the US in 50% of the cases; CS− never followed by the US). During acquisition (CS+/− differentiation), higher levels of neuroticism were associated with a stronger interaction between the right amygdala and the right hippocampus as well as the right amygdala and prefrontal cortical regions, specifically ventromedial prefrontal cortex, dorsolateral prefrontal cortex, and anterior cingulate cortex. The association of stronger conditionability of fear and connectivity of brain regions related to consolidation of fear associations and neuroticism points to underlying mechanisms of the enhanced propensity for anxiety disorders in highly neurotic participants. This is especially important in adolescence, a vulnerable time for the onset of mental disorders such as anxiety disorders.
Keywords: pavlovian fear conditioning, adolescents, amygdale, hippocampus, prefrontal cortex, neuroticism
INTRODUCTION
Aversive pavlovian conditioning has been shown to be important for the development of several mental disorders, especially anxiety disorders. Previous studies identified a neural network involved in aversive conditioning in both animals (Fendt and Fanselow, 1999; LeDoux, 2000; Maren, 2008) and humans (Büchel and Dolan, 2000; Kim and Jung, 2006; LeDoux, 2000; Sehlmeyer et al, 2009). It is known that especially the amygdala and prefrontal regions such as the anterior cingulate cortex (ACC), ventromedial prefrontal cortex (VMPFC), and dorsolateral PFC (DLPFC) are functionally coupled (Robinson et al, 2012; Kim et al, 2011a; Stein et al, 2007), and that this interaction is essential for emotional processing, for example, the processing of negative facial expressions or unpleasant pictures (Cremers et al, 2010; Heinz et al, 2005), and fear conditioning (see, Marek et al, 2013). In addition, it is known that the amygdala–hippocampal complex has an important role in the acquisition of stimulus-reinforcement contingencies and that greater amygdala–hippocampal activity during fear learning predicts better long-term memory for objects with a learned association (Hooker et al, 2008).
Previous studies in healthy adults have reported an association between amygdala, hippocampus, and prefrontal activation in response to negative stimuli, like unpleasant pictures (Harenski et al, 2009), during observational fear learning (Hooker et al, 2008) and for individual differences in neuroticism (Cremers et al, 2010). Neuroticism is a personality trait, which involves a tendency for enhanced emotional and cognitive processing of negative affective stimuli (Haas et al, 2008), worry, and anxiety (Canli et al, 2004; Wright et al, 2006). It is associated with enhanced activity in the amygdala, anterior cingulate and medial prefrontal cortex, the insula, and the hippocampus and altered connectivity between these regions (Ormel et al, 2013). For example, neuroticism is related to altered amygdala–prefrontal connectivity, with neuroticism being positively correlated with right amygdala-dorsomedial PFC connectivity in response to angry/fearful compared wth neutral faces and negatively correlated with left amygdala–ACC connectivity for angry/fearful/sad compared with neutral faces (Cremers et al, 2010). This may result in an inadequate control of emotional responding such as responses to anxiety-related stimuli. In addition, a negative correlation of amygdala–VMPFC functional connectivity in high-anxious individuals was found (Kim et al, 2011a). These alterations in amygdala–prefrontal coupling might underlie both neuroticism and the associated anxiety and higher emotional responses to aversive stimuli (Ormel et al, 2013). Together, these results indicate a strong association of neuroticism and functional connectivity of learning-related structures such as the amygdala, hippocampus, and prefrontal regions when negative emotional materials are processed (Cremers et al, 2010). This might also be important for fear learning, might affect behavioral adaptations and points to the importance of this trait for the development and maintenance of anxiety disorders (Jorm et al, 2000). However, a direct association of neuroticism and fear learning has so far only been reported for observational learning (Hooker et al, 2008) and only in adults. Adolescent fears often subside with age, which may reflect the brain maturation in the service of refined fear learning (Lau et al, 2011). However, the onset frequency of mental disorders increases sharply from childhood to adolescence (Compas et al, 1993) with a lifetime risk for the emergence having its peak at 14 years of age (Kessler et al, 2005). One explanation for the higher vulnerability in adolescents in comparison with adults is the weaker ability to discriminate threat from safety. Lau et al (2011) assessed the ability to categorize threat from safety cues in a discrimination learning paradigm with the aim to investigate differences in fear learning between adolescents and adults. During differential fear learning, the neutral stimulus becomes a threat through its pairing with an aversive stimulus, whereas a second stimulus acquires a safety value by predicting the absence of the aversive stimulus. In this study, adolescents showed deficient discrimination between threat and safety signals in subjective reports, but were more likely than adults to engage early maturing subcortical structures, like the amygdala and hippocampus during threat/safety discrimination learning. Lau et al (2011) suggested that these findings might reflect maturational differences in subcortical and prefrontal regions between adolescent and adult brains that promote age-related deficits in threat/safety discrimination and could thus promote fear. These age-related differences in discrimination may underlie the enhanced vulnerability for the development of anxiety disorders in adolescents (Lau et al, 2011). In line with this, Craske et al (2008) showed that children with anxiety disorders displayed larger skin conductance responses (SCR) to the conditioned stimulus (CS+ danger signal) that was paired with an aversive tone (US) as well as the conditioned stimulus (CS− safety signal) that was never paired with the US relative to healthy controls. Furthermore, Craske et al (2009) reported that higher levels of neuroticism were associated with elevated startle reflex magnitudes, which represent negative amygdala-modulated affect, to safe conditions within the repetition of safe-danger sequences in adolescents. In line with this, Craske et al (2012) found that adolescents, who showed higher startle responses to safe stimuli, were at significantly greater risk of subsequently developing anxiety disorders.
The aim of the present study was to investigate the association of neuroticism and functional coupling between the amygdala and prefrontal regions, like the ACC, VMPFC, DLPFC, and the OFC as well as the amygdala and the hippocampus during aversive learning in a sample of healthy adolescents, where vulnerability factors can be detected. We hypothesized a positive relationship between neuroticism and the coupling between activation in the amygdala and PFC regions such as ACC, VMPF, DLPFC, and OFC as well as the amygdala and hippocampus during fear learning, operationalized as discrimination between CS+ and CS−. In this context it is also of interest to see whether the effect is specific for CS+/CS− discrimination or whether the response patterns are also present for CS+ alone and whether individuals who successfully differentiate between CS+ and CS− differ from those who do not (see, Lau et al, 2011).
MATERIALS AND METHODS
Participants
In the course of the multicenter Imaging Genetics Study (IMAGEN; Schumann et al, 2010), 47 right-handed 14-year-old healthy adolescents (21 females) were recruited from the general public via school visits, flyers, and the city registration office of Mannheim at the Central Institute of Mental Health in Mannheim, Germany. This age was selected because it was assumed that this is a very vulnerable period for the development of mental disorder as confirmed by a twin study that showed that young adolescents (<15 years) with multiple problem behaviors had a lifetime rate of 50–90% for several mental disorders (McGue and Iacono, 2005). The target age group of 14-year-olds was further considered as ideal for the examination of the neurophysiological, genetic, and behavioral correlates of early risk factors for adolescent mental health, because the potential confounding effects of, for example, substance misuse on neurocognitive functioning are minimal (Brown et al, 2000). Exclusion criteria were: any mental disorder as defined by the Development and Well-Being Assessment (DAWBA; Goodman et al, 2000), serious medical conditions, and contraindications for magnetic resonance imaging (MRI) exams such as pregnancy, previous head trauma with unconsciousness, and metal implants. The study was approved by the Ethics Committee of the Medical Faculty Mannheim of Heidelberg University and adhered to the Declaration of Helsinki. All subjects as well as their legal guardians gave written informed consent before participation.
Personality Measures
All participants completed the Neuroticism-Extraversion-Openness Five-Factor Inventory (NEO-FFI) to evaluate the personality traits neuroticism, openness, agreeableness, conscientiousness, and extraversion (Costa and McCrae, 1997). Of these personality traits, neuroticism was chosen for further analysis with the emotional learning data. The NEO-FFI was administered in the course of the IMAGEN study several weeks before the MRI test day using psytools software (Delosis, London, UK). The mean neuroticism score of the sample was 1.716 (range: 0.67–2.9, SD=0.497). The results of the Shapiro-Wilk test showed that the distribution of the neuroticism scores was normal. No significant differences were found between males and females.
Aversive Conditioning Paradigm
The aversive differential delay conditioning paradigm consisted of three phases: habituation, acquisition, and extinction. We chose two neutral male faces from the Ekman Series (Ekman, 1982) as conditioned stimuli (CS), which were presented for 6 s and a 80-dB female scream taken from the International Affective Digital Sounds (IADS, Sound No. 276; Bradley and Lang, 1999) as unconditioned stimulus (US), which was presented for 3 s and terminated with the CS. Studies in healthy adults usually employ differential conditioning paradigms with painful electric stimuli or very loud tones as unconditioned stimuli (eg, Büchel and Dolan, 2000). The use of such aversive stimuli in studies with children and adolescents raises ethical issues. These problems can be circumvented by using sounds that are inherently unpleasant but not excessively loud (Neumann et al, 2008).
During the habituation phase, the CS+, CS−, and the US were presented 10 times each in random order. In the acquisition phase, one of the faces was presented before the scream in 50% of the cases (CS+paired), whereas the other was never paired with the scream (CS−). The assignment of the male faces as CS+ and CS− was counterbalanced across subjects. During acquisition 30 CS+ (15 paired, 15 unpaired), 30 CS−, and 15 US were presented. During extinction, the CS+ and CS− were presented 10 times each. The interval between the stimuli varied between 8 and 10 s and a fixation cross was presented. After each phase, the participants rated the valence and arousal for each stimulus using the Self Assessment Manikin (SAM; Bradley and Lang, 1994) by pushing a keypad with the dominant hand, whereas the relevant stimulus was presented. Furthermore, the subjects were asked to indicate via a keypad the probability with which the two male faces were paired with the scream, whereas the relevant stimulus was presented. The participants were told to passively view/hear the stimuli and were uninformed about the contingency between the CSs and US. We assessed online SCRs in all subjects during MRI scanning. The SCRs were measured from two electrodes placed on the thenar and hypothenar eminence of the participants' non-dominant hand using a sampling rate of 16 Hz and a VarioPort recording system (BECKER MEDITEC, Karlsruhe, Germany).
The results of the subjective ratings of valence, arousal, and contingency as well as the results of the SCRs are shown in the Supplementary Material.
MRI Acquisition and Data Analysis
Imaging data were obtained with a 3 Tesla whole body magnetic resonance scanner equipped with a head volume coil (Siemens, Erlangen, Germany). We acquired 40 slices in descending order (2.4 mm, 1 mm gap) using a gradient-echo T2*-weighted sequence (EPI) with the following image parameters: TR=2200 ms; TE=25 ms; and an in-plane resolution of 64 × 64 pixels with a plane of acquisition tilted to the anterior–posterior commissure line (rostral>caudal) to diminish signal dropout in regions with a potentially strong susceptibility gradient (Deichmann et al, 2003). The functional magnetic resonance imaging (fMRI) data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, London, UK, http://www.fil.ion.ucl.ac.uk/spm/). All individual data were slice time corrected in order to minimize temporal differences in slice acquisition, then spatially realigned to correct for head movement, non-linearly warped, and normalized to the standard space of the Montreal Neurological Institute (MNI) brain. To reduce spatial noise, the functional data were smoothed with an 8 × 8 × 10 mm3 Gaussian kernel. The following experimental conditions were modeled: CS+unpaired, CS+paired, CS−, and baseline. Further, the six movement parameters obtained by the realignment procedure were added to the design matrix. The individual contrast images were subsequently included in a second level random effects analysis using the full flexible procedure of SPM8. The problem of non-independent data within subjects as well as error variance heterogeneity was addressed by performing a nonsphericity correction. We chose a family wise-error (FWE)-corrected significance level of p<0.05.
Regions of interest (ROI) analyses were performed using the Wake Forest University (WFU) pickatlas (Maldjian et al, 2003) for the analyses of the regions involved in emotional learning such as the ACC, amygdala, insula, SMA, hippocampus, putamen, pallidum, and the caudate nucleus as well as probabilistic anatomical masks for prefrontal regions, including medial and dorsolateral prefrontal cortex and orbitofrontal cortex, and the nucleus accumbens (Nielsen and Hansen, 2002) that were thresholded with a fractional intensity of ⩾0.5. The VMPFC was defined as a 5 mm sphere around the peak voxel coordinates (x=2, y=41, and z=−6) reported in a study on decision making (Baumgartner et al, 2011). We chose this study as reference as they used a relatively young sample investigating learning-related (goal-directed behavior and decision making) and neuroticism-related (self-interest) aspects. Because of movement (8 participants moved more than 2 mm in any direction) functional data were only available for a subsample of 39 subjects.
PsychoPhysiological Interaction
We used psychophysiological interaction analyses (PPI) to estimate functional connectivity between a source (right amygdala) and further brain regions, especially target ROI of the prefrontal cortex (including ACC, OFC, VMPFC, and DLPFC), during CS+ unpaired vs CS− in the acquisition phase. PPI analysis permits the comparison of the functional coupling of different brain regions dependent on different conditions, capturing the condition-specific modulation of activity in one brain region by activity in another brain region. In the present study, we were specifically interested in amygdala–cortical interactions during successful learning (defined as discrimination between CS+ and CS− in the amygdala indicated by a reliably increased amygdala response to CS+unpaired vs CS−). We used a data-driven individual approach (see, Banks et al, 2007) and used the amygdala as seed ROI. To perform an unbiased analysis on a potential neural vulnerability factor (increased activation of early maturing subcortical structures during threat/safety discrimination), we identified the amygdala activation for each participant examining the contrast of CS+unpaired compared with CS− and included only those participants (N=26) who showed significant activation in the right amygdala at p<0.05 uncorrected. We extracted the BOLD signal time series from a 5 mm radius sphere around the individually defined peak activated voxel in the right amygdala (defined by the WFU pickatlas mask; Maldjian et al, 2003). We chose this approach for several reasons. First, we wanted to identify active voxels associated with an enhanced BOLD response to successful discrimination between CS+ and CS− and thus learning rather than voxels that were deactivated (negative BOLD response). We chose an amygdala-related time series that best reflected the learning-related amygdala response and was thus most reliable in terms of discrimination between CS+ and CS− as the present analysis was intended to identify brain regions selectively related to the strength of discriminative learning and not learning ability per se, and their association with neuroticism. Choosing amygdala voxels that were relatively less active in CS+ than CS− might represent a learning ability (not discrimination) related connectivity pattern. In addition, we also examined connectivity for CS+ and CS− alone and we also computed the PPI for the remaining 13 individuals who did not show a reliable discrimination in the amygdala as well as for all 39 subjects in the analysis. Time series were deconvolved to estimate neuronal time courses using the PPI-deconvolution parameter defaults in SPM8. These time series were multiplied with the modeled hemodynamic response for CS+unpaired and CS− resulting in interaction terms. These interaction terms were reconvolved by the canonical hemodynamic response function and were entered as regressors together with the time series of the right amygdala, the vector coding for the psychological effect (CS+unpaired: 1, CS−: −1), all original regressors of each condition and the realignment parameters in a first level model. In the second level analysis the contrast images for the PPI effects for all participants were entered as regressors. The results reflect regions that displayed stronger functional connectivity with the right amygdala for the CS+/CS− differentiation across all participants. In the next step, the individual scores of neuroticism were entered as a regressor to investigate the modulatory effect of this personality trait on functional connectivity of the right amygdala for CS+/CS− differentiation during the acquisition phase. The conventional fMRI analysis revealed no significant activation in the right amygdala or further ROI in the comparison of CS+ and CS− during the extinction phase. For that reason the PPI analysis were limited to the acquisition phase.
RESULTS
Ratings, SCRs, and Functional Imaging Data
The results indicate successful aversive pavlovian conditioning on a subjective, peripheral, and neural level. In addition to significant CS+/− differentiation in emotional valence, arousal, contingency, and SCRs, we found significant activation in the right amygdala, SMA, and prefrontal regions including bilateral ACC, bilateral medial prefrontal cortex as well as the right caudate nucleus, bilateral pallidum, bilateral putamen, and nucleus accumbens during aversive learning (see Supplementary Data). No significant association of neuroticism and either ratings of valence, arousal, contingency, SCRs, or brain responses to CS+/− were observed, neither during acquisition nor extinction.
Functional Connectivity: PPI Analysis
The PPI analysis revealed no significant interaction between the right amygdala and hippocampus or between the right amygdala and frontal regions including the VMPFC, DLPFC, OFC, or ACC for the differentiation of CS+/CS− during the acquisition phase. Furthermore, the results revealed no significant interaction between the right amygdala and hippocampus or between right amygdala and frontal regions including the VMPFC, DLPFC, OFC, or ACC for the differentiation of CS+/baseline and CS−/baseline during learning. However, we found a positive correlation between neuroticism and the interaction between the right amygdala and the VMPFC, ACC, and DLPFC and the right amygdala and the right hippocampus for the differentiation of CS+/CS− during learning. Table 1 and Figure 1 show the association of neuroticism and the connectivity of the right amygdala (for scatter plots see Supplementary Information Supplementary Figures S1 and S2). We found no significant association between neuroticism and these neural connectivity patterns to either CS+ or CS− alone (for scatter plots see Supplementary Information Supplementary Figures S3–S6). In addition, we observed no significant correlation between neuroticism and the interaction between the right amygdala and the OFC (t=3.27; pcorrected=0.421) and also not for the interaction between the left amygdala and the DLPFC, VMPFC, ACC, or hippocampus.
Table 1. Associations between Neuroticism and Functional Connectivity with the Right Amygdala for the CS+/CS− Differentiation (p<0.05, Family Wise-Error (FWE) Corrected) in the Acquisition Phase.
| Regions of interest | Laterality | Coordinates (x y z) | Tmax | pcorr | Brodmann area (BA) |
|---|---|---|---|---|---|
| Anterior cingulate cortex | Left | −7, 4, 28 | 3.51 | 0.05 | BA 32, 33 |
| Dorsolateral prefrontal cortex | Bilateral | 41,31,15 | 3.88 | 0.04 | BA 9/46 |
| Ventromedial prefrontal cortex | Right | 14, 21, 42 | 4.67 | 0.009 | BA 32 |
| Hippocampus | Right | 38, −27, −13 | 4.48 | 0.006 |
Figure 1.
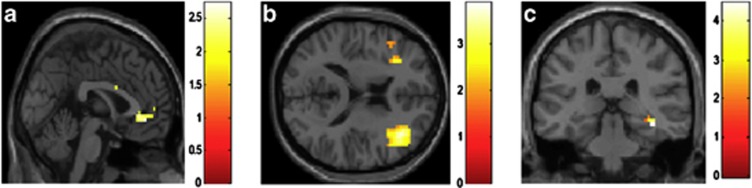
Brain regions displaying a positive association between neuroticism and functional connectivity with the right amygdala for CS+unpaired in comparison with CS− in the acquisition phase (p<0.05, family wise-error (FWE) corrected, N=26). Results are shown for the left anterior cingulate cortex (a), the dorsolateral prefrontal cortex (b), and the right hippocampus (c).
When those individuals who did not match the amygdala response criterion (p<0.05, uncorrected) for successful CS+/CS− discrimination were included in the analysis, this association was no longer significant. The additional analysis of this group without significant amygdala CS+/− differentiation yielded no significant functional coupling of the amygdala with the prefrontal or orbitofrontal regions or with the hippocampus.
DISCUSSION
The aim of the present study was to investigate the association of neuroticism and fear learning-related brain mechanisms, specifically amygdala–prefrontal and amygdala–hippocampal connectivity during aversive differential fear conditioning in a sample of healthy 14-year-old adolescents. We found that higher levels of neuroticism were associated with higher functional connectivity between the right amygdala and prefrontal regions and between the right amygdala and right hippocampus in response to CS+ vs CS− in the acquisition phase of aversive fear learning. These results extend the results of previous studies conducted in adults showing an association of neuroticism and activation of learning-related regions such as the amygdala, hippocampus, and PFC and their connectivity in response to negative stimuli and during observational fear learning (Cremers et al, 2010; Harenski et al, 2009; Hooker et al, 2008; Wright et al, 2006) to pavlovian fear conditioning. Understanding the relationship between personality and activation in and connectivity between brain regions involved in fear learning may provide insights into why some individuals are more vulnerable to certain disorders than others. This investigation may help to elucidate neurobiological vulnerability markers for the development of anxiety disorders in healthy individuals. In the present study, we observed no significant interaction between the activation in the right amygdala and further ROI such as the hippocampus and frontal regions like DLPFC, VMPFC, and ACC for the differentiation of CS+/baseline and CS−/baseline during learning, but only to the CS+ vs CS− contrast. This is in line with the study of Lau et al (2011) that found increased activation in early maturing subcortical structure during discrimination learning in adolescents compared with adults, although not on a verbal level. The specificity of the effects for the CS+/− differentiation suggest that it is differential conditioning not the general emotional response to fearful stimuli as indicted by the CS+ that involves neuroticism. Moreover, the reported neural response pattern could not be observed in individuals who did not show significant discrimination of CS+ vs CS− in the amygdala. However, those individuals who successfully differentiated on a neural level (increased amygdala activation to CS+ vs CS−) did not significantly differ in their ratings related to CS+ vs CS− from those subjects without successful amygdala differentiation. Both groups differentiated significantly in their valence, arousal, and contingency rating of CS+ vs CS−. Based on the study of Lau et al (2011) one would rather suggest a difference in the ratings between amygdala-based high and low discriminating individuals. The present data, however, indicates that the vulnerability might be best seen in brain activation rather than behavior, in accordance with the suggestion that brain-based phenotypes might be more sensitive to detect vulnerability (Meyer-Lindenberg and Weinberger, 2006).
The present study conducted in adolescents revealed a positive correlation between neuroticism and the coupling of the right amygdala with several prefrontal regions. The role of the VMPFC has often been discussed in view of its regulatory role of the VMPFC in emotion processing and in diminishing fear in response to aversive stimuli (Kim et al, 2011a; Etkin et al, 2011) as well as in the context of inhibiting amygdala activation, particularly during fear extinction (Quirk et al, 2000). Nieuwenhuis and Takashima (2011) described that the VMPFC has an important role in the integration of information, which is represented in separate parts of the limbic system (hippocampus and amygdala). Furthermore, Kim et al (2011b) suggested that the functional coupling of the amygdala and the VMPFC is related to trait anxiety in both task-related and resting-state studies. However, the role of the VMPFC in the acquisition of conditioned fear and the underlying consolidation and storage of fear memory (CS−US association) remained so far quite unclear. Our findings point to a potential important role of this amygdala–VMPFC interaction in highly neurotic persons for their enhanced vulnerability for the development of anxiety disorders.
An important role of the ACC in the processing of fear and anxiety has previously been demonstrated (eg, Etkin et al, 2011). We found a positive correlation between individual neuroticism scores and the right amygdala–ACC interaction during fear acquisition. Both structures were often shown to be involved in fear learning and are also functionally coupled (Cremers et al, 2010; Kienast et al, 2008; Pezawas et al, 2005). Previous studies investigated the coupling between the amygdala and the ACC and its association with neuroticism during the processing of negative materials (Cremers et al, 2010; Kienast et al, 2008). They found that neuroticism and also trait anxiety correlate negatively with functional connectivity between the amygdala and the ACC. However, there are no studies that investigated the association of neuroticism and the interaction between these regions during the acquisition of fear in either adults or adolescents. The CS–US association during the acquisition phase involves learning processes like the encoding, consolidation and storage of a fear memory (Etkin et al, 2011). From studies conducted in animals, it is known that the ACC has an important role in the acquisition and formation of fear memories (Malin and McGaugh, 2006; Tang et al, 2005). Whereas dorsal-caudal ACC regions may be involved in the expression and appraisal of negative emotion rather than fear learning (Maier et al, 2012), ventral-dorsal parts in generating emotional responses (Etkin et al, 2011). Therefore, it is likely that participants higher in neuroticism show stronger fear memory encoding rather than storage or consolidation, reflected by the stronger interaction between the right amygdala and the more dorsal part of the ACC (BA32).
In addition to the positive correlation between neuroticism and the interaction between the right amygdala and the VMPFC and ACC, we also found a positive correlation between the interaction of the right amygdala and the DLPFC during learning and individual neuroticism scores. It is known that activity in the amygdala is modulated by inputs from structures of the PFC including the DLPFC (Cremers et al, 2010; Liu et al, 2011), and our present finding is in line with the findings by Cremers et al (2010) on a positive correlation of neuroticism and amygdala–DPFC coupling in response to negative faces and puts the important role of these structures and neuroticism into a learning context. Siegle et al (2007) suggested that there are no direct connections between the DLPFC and the amygdala and the influence from the DLPFC may be mediated by connections from the VMPFC, the ACC, and the OFC to the amygdala. As mentioned above, these regions may have an important role in the encoding, storage, and consolidation of fear memories during the acquisition of a fear response (US–CS association). Thus, the higher interaction between the right amygdala and the DLPFC might also indicate a better encoding and/or storage of the US–CS association dependent on the individual neuroticism score.
We also found a positive correlation between neuroticism and the interaction between the right amygdala and the right hippocampus. It is known that the amygdala–hippocampal complex has an important role for the acquisition of stimulus-reinforcement contingencies (LaBar et al, 1995). Phelps (2004) suggested that amygdala activity during aversive learning enhances the encoding and consolidation of learned associations in the hippocampus by modulating encoding and storage of memories associated with hippocampal function. Furthermore, Hooker et al (2008) showed that neuroticism was positively correlated with amygdala and hippocampal activity during observational fear learning. To indicate whether this activity during fear learning was related to better memory performance, they correlated in a further step the neural activity during fear learning with the accuracy and speed of the participants in identifying fear-related and neutral objects in a post-test. Greater activation in the amygdala and the hippocampus was related to better long-term memory of learned associations. Thus, adolescents higher in neuroticism may show stronger consolidation of learned fear associations reflected by stronger amygdala–hippocampus connectivity.
We could not find a significant coupling of hippocampus or PFC with the left amygdala, which is in line with previous studies that found bilateral, left, or right of the amygdala and hippocampus or PFC (Cremers et al, 2010; Hooker et al, 2008).
When the neuroticism scores and the coupling of the amygdala with ACC, DLPFC, VMPFC, and hippocampus were examined, scatter plots (see Supplementary Figures S1–S6) showed a very similar pattern for each interaction—the variance did not significantly differ and the graphs indicate that the effects were not driven by only a few individuals.
Previous studies reported that females show higher neuroticism scores than males (Weisberg et al, 2011). In addition, gender differences have also been shown for pavlovian conditioning (Dala and Shors, 2009). However, in the present study we did not find gender-specific effects, neither for neuroticism nor for any of the learning measures (ratings, SCR, neural activation, and coupling), nor for the association of neuroticism and brain responses during learning.
Adolescence is a period marked by typical physical and behavioral changes (Ernst and Fudge, 2009), including a heightened experience of persistent negative labile mood states and enhanced responsiveness to emotional cues (Somerville et al, 2010) and incentives (Ernst et al, 2005; Knutson et al, 2001). Because of this it may be especially important to investigate the association of neuroticism and pavlovian learning and the underlying brain correlates in this target age group and so far studies investigating the association of neuroticism and brain connectivity are still missing. This is important because adolescent development appears to involve significant functional reorganization of frontal and limbic systems (Ernst et al, 2005; Cullen et al, 2009; Hulvershorn et al, 2011; Yurgelun-Todd and Killgore, 2006). Yurgelun-Todd and Killgore (2006) showed that during the processing of fear-related stimuli (facial expressions of fear) in adolescents, age was significantly correlated with greater functional activity in the prefrontal cortex, whereas no significant relationship was observed between age and activity in the amygdala. The authors suggested that prefrontal control of emotional processing develops throughout adolescence into early adulthood. This makes it specifically interesting to investigate adolescents. Although these studies also indicate the need to compare different age groups during adolescence, in the present study we were mainly interested in vulnerability markers for the development of anxiety disorders and thus chose a target group of 14-year-olds that is considered to be ideal for the analysis of the neurophysiological, genetic, and behavioral correlates of early risk factors for adolescent mental health (Brown et al, 2000). To our knowledge, only one study directly compared adolescents and adults during pavlovian conditioning (Lau et al, 2011). This comparison is important because regions involved in aversive pavlovian conditioning such as the amygdala, mature early, whereas prefrontal regions mature late. This may promote differences between adolescents and adults based on maturity and should be interesting in the development of anxiety disorders. As noted above, Lau et al (2011) showed that adolescents could verbally discriminate between safety and threat cues, but adults formed more differentiated threat/safety boundaries than adolescents. Adolescents showed a markedly smaller difference in their rated fear to the CS+ vs CS− than adults. On the basis of these findings, Lau et al (2011) suggested that adolescents have less distinct verbal categories for stimuli labeled as fear or safety than adults in accordance with their hypothesis that maturity of the PFC brings better categorization skills. This was also supported by an increased activation in early maturing brain regions during threat/safety discrimination in adolescents. In addition, in their study the formation of threat/safety categories in adults, but not in adolescents, was positively correlated with DLPFC activity and the DLPFC responded more to safety than threat cues. In our adolescent sample, we did not find a significant correlation between CS+/− differentiation in the ratings of valence or arousal or the responses to CS+ or CS− and DLPFC activation but we did not have an adult comparison group.
Although most studies observed an association of neuroticism and subcortical–cortical connectivity during tasks involving the processing of aversive emotional materials (see, Cremers et al, 2010), neuroticism is also directly associated with brain changes in the amygdala and prefrontal regions, independent of a task (see, Ormel et al, 2013). This leads to the question to what extent the brain changes observed here are underlying neuroticism and to what extent neuroticism actually modulates the brain changes. If neuroticism is by itself related to altered brain structure/function, the present findings would be mediated by these pre-existing neural alterations. However, such interpretations are speculative, as it is not clear how the degree of neuroticism affects such brain changes.
This study has several limitations. We used the NEO-FFI in our 14-year-old sample, which is recommended for adolescents of 16 years and above. Roth (2002) investigated the applicability of the NEO-FFI—German version to adolescents aged 14–16 and found satisfactory reliability and factorial validity except for one scale ‘agreeableness'. In a sample of 1236 adolescents, they showed a mean neuroticism score of 20.67 (1.72; corrected for the number of items), which is comparable with our results (M=20.59 (1.716 corrected)). These results are also in line with studies investigating neuroticism in adults (eg, Canli et al, 2001). The study of Roth indicates the applicability of the NEO-FFI in a sample of adolescents for research purposes. The groups of participants showing high vs low neuroticism values were too small to employ PPI analyses within these groups. Furthermore, these results are limited to a sample of healthy adolescents. Future studies should investigate the differences between healthy participants with a low neuroticism level and those participants showing an increased neuroticism level (eg, participants suffering from anxiety disorder) to substantiate our results.
In most animal studies, the hippocampus was divided into a dorsal part, which corresponds to the human posterior hippocampus, and a ventral part, which corresponds to the human anterior hippocampus (see, Fanselow and Dong, 2010). Fanselow and Dong (2010) suggested that the dorsal/posterior part is primarily involved in the cognitive process of learning and memory, whereas the ventral/anterior part is associated with motivational and emotional behavior. Furthermore, Satpute et al (2012) proposed that both the anterior and the posterior division of the hippocampus are associated with different forms of anxiety. Whereas state anxiety is related to activity in the anterior part, trait anxiety is related to the posterior part. In our study, ROI analyses were performed using the mask of the hippocampus of the WFU pickatlas (Maldjian et al, 2003). This did not allow a clear distinction between the anterior and posterior hippocampus. However, in relation to other studies that investigated the subdivisions of the hippocampus in humans (eg, Benetti et al, 2009), the coordinates of our hippocampus activation can be assigned to the posterior part. This is in line with the assumption that the posterior part is primarily involved in the cognitive process of learning and memory (Fanselow and Dong, 2010). Further analyses should investigate the functional connectivity between the right amygdala and the subdivisions of the hippocampus. The interpretation of our results is also restricted by the inherent limitations of functional connectivity measures. PPI analyses indicate the correlation between the amygdala time series and all other voxels in the brain. Therefore, the results do not imply a causal relationship between the regions involved and it is not possible to assess the direction of the effects. In the present study, we used a classical preprocessing approach with 6 motion parameters as implemented in SPM8. This is a very reliable approach, commonly used in a large number of studies including PPI analyses, however, in functional connectivity analyses, specifically on resting-state data, (micro-)motion was shown to affect the results (see, Fair et al, 2012). Thus, we used follow-up analyses to check whether our findings remained significant after using a more individualized assessment of motion and a denoising procedure (see Supplementary Material). As a result of this more restrictive analysis, the coupling of the amygdala and DLPFC, VMPFC and hippocampus remained significant, whereas the coupling of the amygdala and ACC did not (see Supplementary Table 3). This underlines that motion can differently affect functional imaging analyses and that not only resting state but also stimulation-related fMRI data can be affected. Thus, a careful control of motion is essential for fMRI data.
In sum, the present study showed in a sample of healthy 14-year-old adolescents that participants higher in neuroticism display stronger amygdala-PFC (VMPFC, DLPFC, and ACC) and amygdala–hippocampus connectivity. This highlights a close association of neuroticism and the connectivity of brain regions related to emotional processing and the consolidation of learned fear associations. This could indicate enhanced vulnerability for anxiety disorders in highly neurotic participants, which is especially important in adolescence, because adolescence represents a vulnerable time for the onset of mental disorders, particularly anxiety disorders.
FUNDING AND DISCLOSURE
GJB has received honoraria for teaching from General Electric, and receives consultancy payments from IXICO. JG has received research funding from the German Federal Ministry of Education and Research, research funding from AstraZeneca, Eli Lilly & Co, Janssen-Cilag, and Bristol-Myers Squibb, and speaker fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb. TB served in an advisory or consultancy role for Bristol-Myers-Sqibb, Develco Pharma, Lilly, Medice, Novartis, Shire, and Viforpharma. He received conference attendance support and conference support, and received speaker's fee from Lilly, Janssen McNeil, Medice, Novartis, Shire, and UCB. He has been involved in clinical trials conducted by Lilly, Shire, and Novartis. The present work is unrelated to the above grants and relationships. AS received research funding from the German Federal Ministry of Education and Research, the European Commission (FP6) and Lundbeck, and speaker Honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibib, Eli Lilly & Co, Pfizer, Lundbeck, Wyeth, and UCB. AS was a consultant for Actelion. Educational grants were given by the Stifterverband für die Deutsche Eissenschaft, the Berlin Brandenburgische Akademie der Wissenschaften, the Boehringer Ingelheim Fonds, the Eli Lilly International Foundation, Janssen-Cilag, Pfizer, and Eli Lilly & Co. AH has received research funding from the German Research Foundation and the Bernstein Center for Computational Neuroscience Berlin (German Federal Ministry of Education and Research), Eli Lilly & Company, Janssen-Cilag, and Bristol-Myers Squibb. AH has received Speaker Honoraria from Janssen-Cilag, Johnson & Johnson, Lilly, Pfizer, and Servier.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
ACKNOWLEDGEMENTS
Support for this study was provided by the IMAGEN project, which receives research funding from the European Community's Sixth Framework Program (LSHM-CT-2007–037286) and the Deutsche Forschungsgemeinschaft (SFB636/C1). This manuscript reflects the views of the authors only, and the Community is not liable for any use that may be made of the information contained therein. Psytools software (Delosis, London, UK) was used to conduct the behavioral characterization via its internet-based platform. The assessment battery of questionnaires and cognitive tasks was self-administered both in participants' homes and at the neuroimaging facilities.
Supplementary Material
References
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci. 2011;14:1468–1474. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- Benetti S, Mechelli A, Picchioni M, Broome M, Williams S, McGuire P. Functional integration between the posterior hippocampus and prefrontal cortex is impaired in both first episode schizophrenia and the at risk mental state. Brain. 2009;132:2426–2436. doi: 10.1093/brain/awp098. [DOI] [PubMed] [Google Scholar]
- Bradley M, Lang P.1999International affective digitized sounds (IADS)ednUniversity of Florida: Gainesville, FL, USA [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. Brain activation to emotional words in depressed vs healthy subjects. Neuroreport. 2004;15:2585–2588. doi: 10.1097/00001756-200412030-00005. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on Brain reactivity to emotional stimuli. Behav Neurosci. 2001;115:33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Compas BE, Orosan PG, Grant KE. Adolescent stress and coping: implications for psychopathology during adolescence. J Adolesc. 1993;16:331–349. doi: 10.1006/jado.1993.1028. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. Stability and change in personality assessment: the revised NEO personality inventory in the year 2000. J Pers Assess. 1997;68:86–94. doi: 10.1207/s15327752jpa6801_7. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Lindsey Bergman R, Naliboff B, Lipp OV, Negoro H, et al. Is aversive learning a marker of risk for anxiety disorders in children. Behav Res Ther. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Nazarian M, Mineka S, Zinbarg RE, Griffith JW. Does neuroticism in adolescents moderate contextual and explicit threat cue modulation of the startle reflex. Biol Psychiatry. 2009;65:220–226. doi: 10.1016/j.biopsych.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Wolitzky-Taylor KB, Mineka S, Zinbarg R, Waters AM, Vrshek-Schallhorn S, et al. Elevated responding to safe conditions as a specific risk factor for anxiety versus depressive disorders: evidence from a longitudinal investigation. J Abnorm Psychol. 2012;121:315–324. doi: 10.1037/a0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJ, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49:963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Ekman P. Emotion in the Human Face. Cambridge University Press: Cambridge, UK; 1982. [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iver S, Bathula D, Mills KL, Dosenbach NU. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 2012;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures. Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and well-being assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- Haas BW, Constable RT, Canli T. Stop the sadness: neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. Neuroimage. 2008;42:385–392. doi: 10.1016/j.neuroimage.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Kim SH, Hamann S. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cogn Affect. Behav Neurosci. 2009;9:1–15. doi: 10.3758/CABN.9.1.1. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Verosky SC, Miyakawa A, Knight RT, D'Esposito M. The influence of personality on neural mechanisms of observational fear and reward learning. Neuropsychologia. 2008;46:2709–2724. doi: 10.1016/j.neuropsychologia.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5:307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B. Predicting anxiety and depression from personality: is there a synergistic effect of neuroticism and extraversion. J Abnorm Psychol. 2000;109:145–149. doi: 10.1037//0021-843x.109.1.145. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011a;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb. Cortex. 2011b;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, et al. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci USA. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liu CC, Crone NE, Franaszczuk PJ, Cheng DT, Schretlen DS, Lenz FA. Fear conditioning is associated with dynamic directed functional interactions between and within the human amygdala, hippocampus, and frontal lobe. Neuroscience. 2011;189:359–369. doi: 10.1016/j.neuroscience.2011.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, Blechert J, Philipsen A, van Elst LT, Kalisch R, Tüscher O. Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression. PLoS One. 2012;7:e50120. doi: 10.1371/journal.pone.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci USA. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol. 2013;591:2381–2391. doi: 10.1113/jphysiol.2012.248575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. Am J Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Neumann DL, Waters AM, Westbury HR. The use of an unpleasant sound as the unconditional stimulus in aversive Pavlovian conditioning experiments that involve children and adolescent participants. Behav Res Methods. 2008;40:622–625. doi: 10.3758/brm.40.2.622. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK. Modeling of activation data in the BrainMap database: detection of outliers. Hum Brain Mapp. 2002;15:146–156. doi: 10.1002/hbm.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Ormel J, Bastiaansen A, Riese H, Bos EH, Servaas M, Ellenbogen M, et al. The biological and psychological basis of neuroticism: Current status and future directions. Neurosci and Biobehav Reviews. 2013;37:59–72. doi: 10.1016/j.neubiorev.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2012;60:523–529. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Überprüfung der Anwendbarkeit des NEO-Fünf-Faktoren Inventars (NEO-FFI) bei Jugendlichen im Alter zwischen 14 und 16 Jahren. Diagnostica. 2002;48:59–67. [Google Scholar]
- Satpute AB, Mumford JA, Naliboff BD, Poldrack RA. Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion. 2012;12:58–68. doi: 10.1037/a0026517. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M. Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain. 2005;1:6. doi: 10.1186/1744-8069-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg YJ, Deyoung CG, Hirsh JB. Gender differences in personality across the ten aspects of the big five. Front Psychol. 2011;2:178. doi: 10.3389/fpsyg.2011.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, et al. Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex. 2006;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci Lett. 2006;406:194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


