Abstract
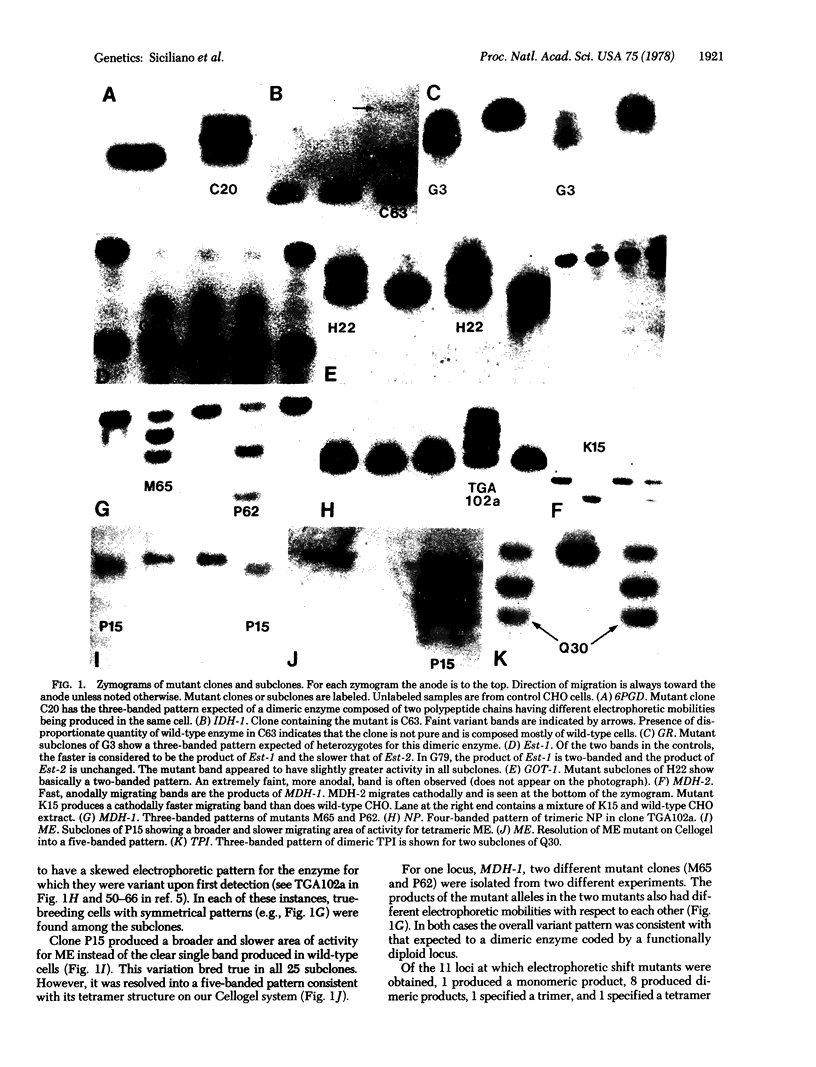
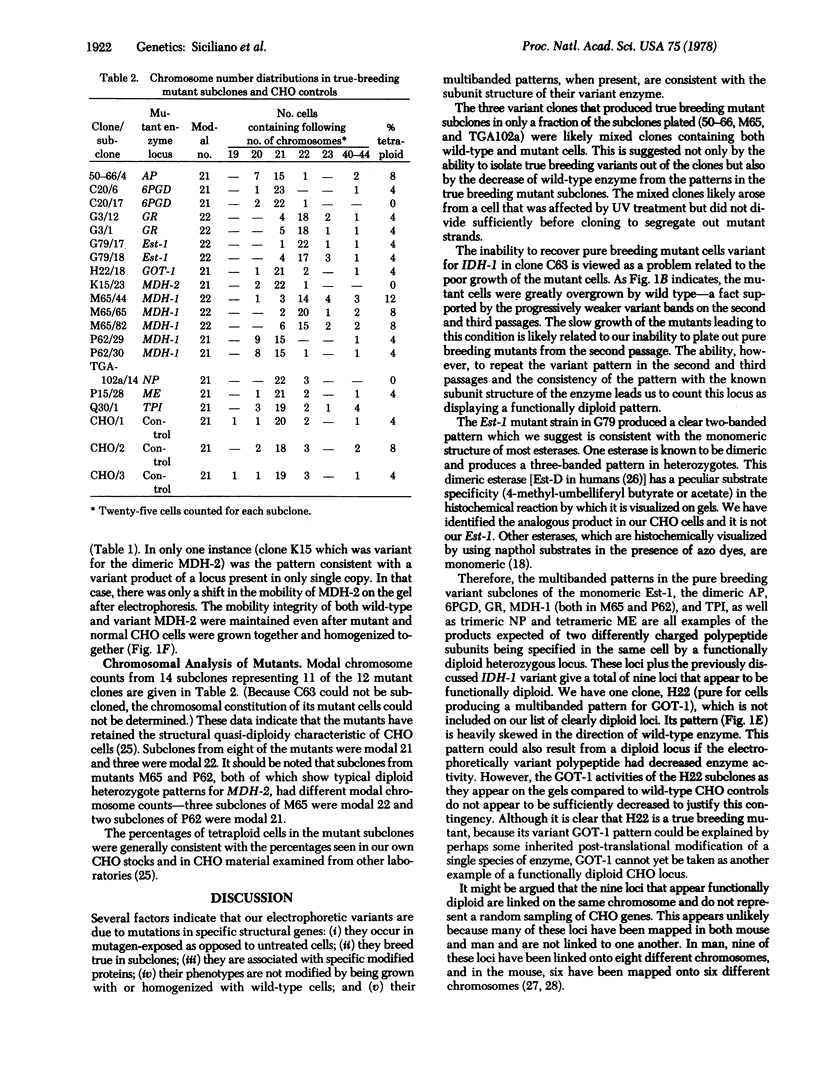
Electrophoretic shift mutants induced in Chinese hamster ovary (CHO) cells indicate that these cells not extensively functionally hemizygotic. Therefore, effective haploidy is unsatisfactory as a general theory to explain the frequency of recessive mutants in this cell line. CHO cells were screened for electrophoretic shift variants of enzymes coded by approximately 40 genetic loci. Clones isolated after exposure to ultraviolet radiation were examined by starch gel and Cellogel electrophoresis. Shift variants were recovered for enzymes representing 11 different loci. Variant clones were subcloned to demonstrate the heritability of the variations Mutants at nine loci produced multiple-banded patterns consistent with the patterns expected of genes at loci represented twice (diploid). Chromosome localization of these diploid loci in other mammalian species where they have been mapped, suggests that they represent a random sample of CHO genes. Chromosome analysis of mutant subclones indicated that the variation did not take place in tetraploid cells. The data indicate that the quasi-diploid CHO cells appear only as functionally hemizygous as would be expected of a slightly hypodiploid cell line derived from an organism in which the haploid number is 11.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chasin L. A. Mutations affecting adenine phosphoribosyl transferase activity in Chinese hamster cells. Cell. 1974 May;2(1):37–41. doi: 10.1016/0092-8674(74)90006-3. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Urlaub G. Chromosome-wide event accompanies the expression of recessive mutations in tetraploid cells. Science. 1975 Mar 21;187(4181):1091–1093. doi: 10.1126/science.1167702. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Giblett E. R. Polymorphism of soluble glutamic-pyruvic transaminase: a new genetic marker in man. Science. 1971 Jul 9;173(3992):148–149. doi: 10.1126/science.173.3992.148. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Omenn G. S. Genetic variation of the cytoplasmic and mitochondrial malic enzymes in the monkey Macaca nemestrina. Biochem Genet. 1972 Dec;7(3):289–301. doi: 10.1007/BF00484829. [DOI] [PubMed] [Google Scholar]
- Davidson R. G., Cortner J. A. Genetic variant of human erythrocyte malate dehydrogenase. Nature. 1967 Aug 12;215(5102):761–762. doi: 10.1038/215761a0. [DOI] [PubMed] [Google Scholar]
- Davidson R. G., Cortner J. A. Mitochondrial malate dehydrogenase: a new genetic polymorphism in man. Science. 1967 Sep 29;157(3796):1569–1571. doi: 10.1126/science.157.3796.1569. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Inherited variants of human nucleoside phosphorylase. Ann Hum Genet. 1971 May;34(4):395–408. doi: 10.1111/j.1469-1809.1971.tb00252.x. [DOI] [PubMed] [Google Scholar]
- HENDERSON N. S. ISOZYMES OF ISOCITRATE DEHYDROGENASE: SUBUNIT STRUCTURE AND INTRACELLULAR LOCATION. J Exp Zool. 1965 Apr;158:263–273. doi: 10.1002/jez.1401580303. [DOI] [PubMed] [Google Scholar]
- HENNING U., YANOFSKY C. An electrophoretic study of mutationally altered A proteins of the tryptophan synthetase of Escherichia coli. J Mol Biol. 1963 Jan;6:16–21. doi: 10.1016/s0022-2836(63)80077-7. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Mestriner M. A., Cortner J., Harris H. Esterase D: a new human polymorphism. Ann Hum Genet. 1973 Oct;37(2):119–137. doi: 10.1111/j.1469-1809.1973.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Humphrey R. M., Sedita B. A., Meyn R. E. Recovery of Chinese hamster cells from ultra-violet irradiation damage. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(1):61–69. doi: 10.1080/09553007014550821. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Markert C. L. The molecular basis for isozymes. Ann N Y Acad Sci. 1968 Jun 14;151(1):14–40. doi: 10.1111/j.1749-6632.1968.tb11876.x. [DOI] [PubMed] [Google Scholar]
- McCOY T. A., MAXWELL M., KRUSE P. F., Jr Amino acid requirements of the Novikoff hepatoma in vitro. Proc Soc Exp Biol Med. 1959 Jan;100(1):115–118. doi: 10.3181/00379727-100-24542. [DOI] [PubMed] [Google Scholar]
- McKusick V. A., Ruddle F. H. The status of the gene map of the human chromosomes. Science. 1977 Apr 22;196(4288):390–405. doi: 10.1126/science.850784. [DOI] [PubMed] [Google Scholar]
- Nichols E. A., Ruddle F. H. Polymorphism and linkage of glutathione reductase in Mus musculus. Biochem Genet. 1975 Jun;13(5-6):323–329. doi: 10.1007/BF00485817. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr C. W. Erythrocyte phosphogluconate dehydrogenase polymorphism. Nature. 1966 Apr 30;210(5035):487–489. doi: 10.1038/210487a0. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinson H., Meienhofer M. C., Dreyfus J. C. A new isozyme of triose phosphate isomerase specific to hominoids. J Mol Evol. 1973 Nov 27;2(4):243–250. doi: 10.1007/BF01654093. [DOI] [PubMed] [Google Scholar]
- SHAW C. R. THE USE OF GENETIC VARIATION IN THE ANALYSIS OF ISOZYME STRUCTURE. Brookhaven Symp Biol. 1964 Dec;17:117–130. [PubMed] [Google Scholar]
- Shaws T. B., Lalley P. A. Control of lysosomal acid phosphatase expression in man-mouse cell hybrids. Biochem Genet. 1974 Feb;11(2):121–139. doi: 10.1007/BF00485769. [DOI] [PubMed] [Google Scholar]
- Siciliano M. J., Bordelon M. R., Kohler P. O. Expression of human adenosine deaminase after fusion of adenosine deaminase-deficient cells with mouse fibroblasts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):936–940. doi: 10.1073/pnas.75.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Waldren C. A. Isolation of UV-sensitive variants of CHO-K1 by nylon cloth replica plating. Somatic Cell Genet. 1977 Jul;3(4):431–440. doi: 10.1007/BF01542971. [DOI] [PubMed] [Google Scholar]
- TJIO J. H., PUCK T. T. Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture. J Exp Med. 1958 Aug 1;108(2):259–268. doi: 10.1084/jem.108.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack J. E., Sharp M. Comparative autosomal linkage in mammals: genetics of esterases in Mus musculus and Rattus norvegicus. Genetics. 1976 Apr;82(4):665–675. doi: 10.1093/genetics/82.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]




