Abstract
Pancreatic cancer (PC) is the fourth leading cause of cancer related deaths in the U.S., with a less than 6% five-year survival rate. Treatment is confounded by advanced stage of disease at presentation, frequent metastasis to distant organs at the time of diagnosis and resistance to conventional chemotherapy. In addition, the molecular pathogenesis of the disease is unclear. The extensive study of miRNAs over the past several years has revealed that miRNAs are frequently de-regulated in pancreatic cancer and contribute to the pathogenesis and aggressiveness of the disease. Several studies have tackled the practical difficulties in the application of miRNAs as viable therapeutic and diagnostic tools. Given that a single miRNA can affect a myriad of cellular processes, successful targeting of miRNAs as therapeutic agents could likely yield dramatic results. The current review attempts to summarize the advances in the field and assesses the prospects for miRNA profiling and targeting in aiding PC treatment.
Keywords: MiRNA, therapy, pancreatic cancer
INTRODUCTION
Pancreatic cancer is a highly lethal malignancy and the fourth leading cause of cancer related deaths in the U.S., with a less than 6% five-year survival rate [1]. The main reasons for the dismal prognosis of PC include the lack of understanding of its pathogenesis, a dearth of early diagnostic markers and molecular targets, and resistance to conventional therapy. Due to the deep abdominal location of the pancreas and the insidious nature of the disease, the clinical presentation of PC usually occurs at a late stage, by which time the disease has typically metastasized to local and distant organs in more than 85% of patients, rendering surgical and other medical interventions largely ineffective [1, 2]. Consequently, the mortality rate for the disease is nearly equal to its incidence. In light of these sobering facts, it is imperative to understand the molecular pathogenesis of PC so as to unearth early detection markers and molecular targets to design effective treatment regimens.
MicroRNAs (miRNAs)are non-coding RNAs (usually 18–24 nucleotides long) that are transcribed by RNA polymerase II and negatively regulate gene expression at the post-transcriptional level [3], either via degradation of mRNA (complete complementarity) [4] or inhibition (incomplete complementarity) of mRNA translation into protein [5]. They are phylogenetically conserved across species [6]. According to the miRBase 19 miRNA sequence database, more than 21,264 miRNA genes have been identified in 193 species and thus far, 1600 human and 855 mouse precursor miRNAs have been discovered. The miRNAs play an important role during development and normal physiological processes, like myoblast and cardiomyocyte proliferation and differentiation [7, 8], hematopoietic lineage differentiation [9], and cell fate specification.
Similarly, studies have shown that miRNA expression profiles are unique to each tumor type [10], are tissue specific [11] and are a better source of pathognomonic tumor data than expression profiles of mRNA [10]. Most miRNAs are located at the genomic regions associated with cancer such as fragile sites, or are adjacent to regions of loss of heterozygosity/amplifications. Thus, miRNAs represent a novel class of molecules that play an important role in gene regulation. Aberrant expression patterns of miRNAs have been reported in various cancers [12–19] and are associated with cancer pathogenesis [20–25], apoptosis [26, 27], and cell growth [28], thereby functioning as either tumor suppressors or oncogenes and contributing to tumor development and progression. Hence, these patterns may have diagnostic, prognostic and therapeutic values in several human malignancies. The present review focuses on the current status of miRNAs and their use for therapy of cancers, particularly, in pancreatic cancer.
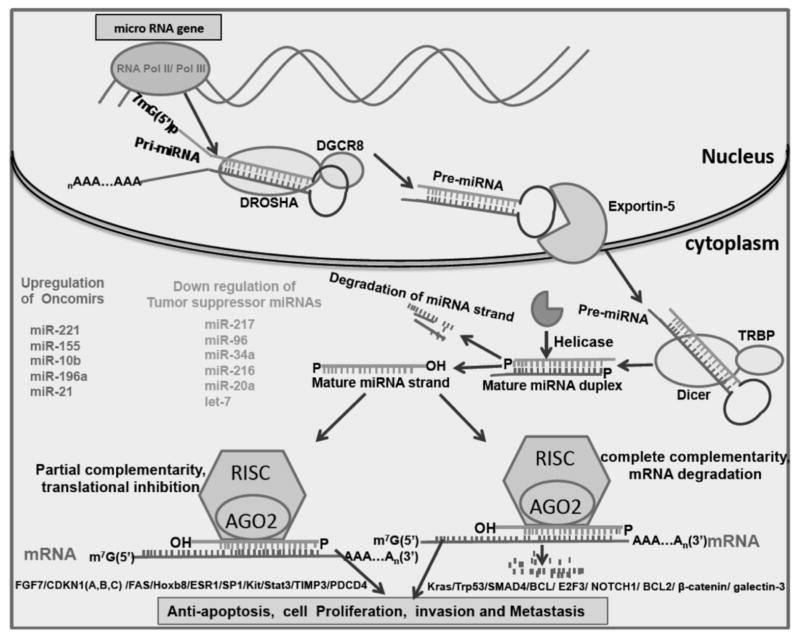
Biogenesis of miRNAs
The generation of mature miRNA (18–24 nucleotides) is a complex process involving a series of enzymatic reactions taking place both in the nucleus and cytoplasm [29] (Fig. 1). Initially, RNA polymerase II transcribes a long hairpin shaped precursor transcript called the pri-miRNA [3]. The pri-miRNA is trimmed into a ~ 75 nt long pre-miRNA by the ribonucleoprotein complex, Drosha/DGCR8 in the nucleus. The pre-miRNA is then transported to the cytoplasm by Exportin 5 [30], where the Dicer/TRBP complex removes the hairpin loop of the pre-miRNA. Subsequently, a RISC (RNA induced silencing complex) is formed, which removes one strand from the still-double stranded miRNA. The strand with the poorer 5′ end stability is removed, and the other is termed the “guide strand.” The RISC contains the argonaute protein or Ago, which, in humans, has 4 isoforms (1, 2, 3, and 4). The mRNA target is degraded only when the miRNA strand is bound to the Ago2-RISC [31]. The 2–8 bases at the 5′ end of the miRNA, termed the “seed” region, bind to the 3′ untranslated region of the target mRNA [3]. The extent of complementarity between the miRNA and its’ target mRNA also determines whether the target is degraded or its’ translation is inhibited. Since perfect complementarity with the target mRNA is not a pre-requisite for mRNA regulation, a single miRNA can modulate levels of several hundred target mRNAs (Fig. 1).
Fig. 1.
Biosynthesis of miRNAs and their role in pancreatic cancer. The RNA Pol II transcribes pri-miRNA, which is cleaved by DRO-SHA/DGCR8 complex to form pre-miRNA. Exportin-5 transports this pre-miRNA to the cytosol, where Dicer forms the mature single stranded miRNA. This strand then enters the RISC (RNA induced silencing complex) which suppresses the target mRNA either by translational repression (incomplete complementarity) or by target degradation (complete complemetarity).
miRNA BASED CLINICAL APPLICATIONS AND THERAPEUTIC STRATEGIES
miRNAs in Diagnosis, Classification of Tumors and as Prognostic Indicators
Over the last several years it has emerged that miRNA profiling can be used as a means to supplement traditional morphological and histological classification of tumor subtypes. It has been shown that miRNA profiling is superior to expression profiling of protein coding mRNAs for the purpose of determining a tumor sub-type as well as the tumor differentiation state and the developmental lineage of the tumor [10].
The profiling of miRNAs offers several advantages: miRNAs are highly stable in formalin-fixed tissues, plasma and serum miRNAs are stable [32], and even minute amounts (10ng) of total RNA are sufficient to obtain a miRNA expression profile [33]. Also, since the miRNA expression profile changes in virtually all tumors, a cohort of miRNAs for each tumor can be used to designate a good/poor prognosis. For instance, miR-210 has been shown to have a strong prognostic value in breast cancer, and its over-expression alone was sufficient to predict the poorer survival of patients [10, 34].
The levels of miRNA in the plasma, urine and feces of patients also reflect the presence of tumors [35]. Certain studies suggest that cells leach miRNA into the bloodstream via the passive process of necrosis [36]. On the contrary, some studies support the notion that plasma miRNAs are actively packaged in exosomes [37] and may even enter circulation as ‘free’ miRNAs bound to Argonaut proteins [38]. It has also been shown that the plasma miRNA profile does not reflect the one present in the tumor [39].
MiRNAs in Cancer Therapy
miRNAs target the 3′ UTR of target mRNAs causing either degradation of the mRNA (complete complementarity to target mRNA 3′ UTR region) or the inhibition of their translation (partial complementarity to target mRNA 3′ UTR region). Due to the aforementioned mechanism of action, each miRNA can thus target hundreds of mRNAs, thereby regulating several biological processes, including apoptosis, cell differentiation, proliferation and metabolism. Therefore, therapeutic strategies directed towards targeting of a single microRNA can yield dramatic results, affecting myriad cellular processes. Several studies have shown that therapeutic strategies can effectively target a specific miRNA, and they can broadly be classified as microRNA mimics and mi-croRNA antagonists.
MicroRNA antagonists
These miRNA antagonists are used in order to ablate miRNAs that display a gain of expression in the disease progression. Inhibition of such miRNAs can result in increased expression of mRNAs that are often tumor suppressors in cancer or may otherwise halt the progression of the disease. Most commonly, the antagonist comprises a single stranded oligonucleotide complementary to miRNA, with certain chemical modifications such as 2′-O-methyl-group-modified oligonucleotides and locked nucleic acid (LNA) modified oligonucleotides. These modifications ensure increased affinity for the target miRNA (not mRNAs) and that the target miRNA is sequestered within the RISC, preventing it from being processed.
Anti-miR Oligonucleotides
The 2′-O-methyl-group confers increased resistance to nucleases and increased binding affinity to RNAs [40]. The 2′-O-methyl-group modified oligonucleotides, 2′-fluoro/, 2′-O-methoxyethyl (2′-MOE)/and 2′-O-methyl nucleotides are modified at the 2′ position of the ribose sugar with the addition either a fluorine, methoxyethyl or a methyl group [41]. LNA is an RNA analogue where the ribose sugar is locked in a C3′-endo conformation due to a 2′-0, 4′-C methylene bridge [40]. Frequently referred to as ‘antagomirs’, these oligonucleotides are often conjugated to cholesterol to increase cellular intake. Intravenous administration of antagomirs against miR-122, a miRNA abundant in the liver and one that regulates the levels of cholesterol biosynthesis genes, resulted in a decrease in levels of the miRNA as well as a decrease in circulating cholesterol levels [42]. Several studies have indicated that antagomirs could be therapeutically useful in cardiac disease. An antagomir against miR-21 resulted in decreased cardiac fibrosis in a mouse model [43]. Likewise, administration of an antagomir against miR-133 caused reduced cardiac hypertrophy in vitro and in vivo, when administered to mice [44].
LNA-antimiRs can form very strong duplexes with complementary RNAs. Due to their very strong affinity for their target miRNAs, a very short sequence of LNA modified oligo, complementary only to the seed region of the miRNA is sufficient to cause effective targeting of the miRNA [45]. An LNA-antimiR against miR-122 was seen to protect chimpanzees chronically infected with Hepatitis C against liver pathology and viremia [46].
The major disadvantage of anti-miR oligonucleotides is that they may affect endogenous RNA species other than the intended target miRNA. The effective in vivo delivery is hampered by the possibility of off-target effects in organ systems other than the intended organ. However, this challenge could be overcome by conjugating the anti-miR oligonucleotide with ligands for target organ specific cell surface receptors. Despite these disadvantages, anti-miR oligonucleotides remain a promising option for effectively targeting endogenous oncogenic miRNAs.
Small Molecules
Small molecules are low molecular weight compounds that can bind nucleic acids, including endogenous miRNAs, resulting in diminished cellular miRNA. A high throughput screening of a small molecule library using a luciferase reporter system in HeLa cells showed that two small molecules antagonised miR-21 expression, while one molecule enhanced miR-21 efficacy [47]. Two small molecules that inhibited miR-122 function were also identified. Also, small molecule inhibitors of miR-122 were also shown to decrease the replication of the Hepatitis C virus in human liver cells [48]. A recent study used a molecular beacon based method to screen effective inhibitors of miRNA function, and found that out of the 14 aminoglycosides screened; five were able to antagonize miR-27a function [49]. The inhibitors identified interfered with Dicer function.
Thus, as the technology for the delivery and targeting of miRNAs develops, small molecule inhibitors of miRNAs may, in the future, be deployed as viable treatment strategies. However, most small molecule inhibitors have only been studied in vitro, and much needs to be ascertained with regard to effective delivery and effects of these inhibitors in vivo.
miRNA Sponges
Sponges, like other miRNA antagonists, contain complementary binding sites to a specific miRNA. However, these are produced by a transgene. A ‘sponge’, ‘eraser’ or ‘decoy’ comprises a vector that expresses miRNA target sequences, thereby preventing that miRNA from regulating its endogenous targets and ‘soaking’ the miRNA up. Since sponges typically have binding sites complementary to only the seed region of a miRNA, they can target an entire family of miRNAs [40]. They act in the manner of scavengers, binding miRNAs, thereby preventing them from regulating their normal mRNA targets.
Typically, the miRNA sponge is expressed downstream of a luciferase or green fluorescent protein governed by a strong promoter. The level of expression of the reporter gene can be used to monitor the degree of miRNA scavenging by the miRNA sponge. The miRNA sponge sequences are imperfectly complementary to the target mRNA sequence since perfect complementarity will induce immediate degradation of the sponge, making the miRNA available for suppression of other targets. However, imperfect complementarity will result in a longer binding of the miRNA to the target sequence, preventing the miRNA from suppressing other targets. Most miRNA sponges have 4–10 miRNA binding sites, each separated by a few nucleotides [50].
A limitation of the miRNA sponge method is the necessity of calibrating the concentration of the sponge to the amount of miRNA present in the cell. Cells with high endogenous miRNA levels will need higher doses, which may not be physiologically achievable. In addition, testing the efficacy of a sponge mediated miRNA ablation is not as straightforward as with genetic knock-out of a miRNA. As with other methods of miRNA antagonists, in vivo delivery remains a challenge, with concerns of off-target effects being major obstacles.
Agents that Increase miRNA Function
miRNA Mimics
The goal of using miRNA mimics is to generate a synthetic 18–22 nucleotide oligonucleotide that is identical to the endogenous miRNA and targets the same mRNAs. However, studies have shown that a two-stranded oligonucleotide is 100–1000 fold more effective than a single-stranded mimic [51]. Therefore, a miRNA oligonucleotide mimic comprises an RNA duplex, with one strand, dubbed the “guide” strand which is identical to the miRNA being mimicked and a “passenger” strand that is either partly or fully complementary to the guide strand. The 3′ end of the passenger strand is usually modified with cholesterol in order to increase cellular uptake [40]. Since the miRNA mimic will also affect non-target tissue when administered, be susceptible to nuclease degradation and be targeted by the innate immune system, this method has its limitations with regard to its therapeutic applications.
miRNA DELIVERY SYSTEMS
Lipid Based Delivery Systems
Liposomes have been used for the delivery of conventional drugs as well as synthetic miRNA-based drugs. Liposomes have a small diameter of <100nm, which allows for a high drug to lipid ratio [52]. Lipid-based mimics have been shown to have increased cellular uptake, as well as being able to better evade the innate immune system. Additionally, liposomes have a high circulation lifetime and can penetrate the tumor in high concentrations. They are administered intravenously or intra-tumorally; however, they penetrate all tissues equally. Thus, they may have deleterious effects in non-target tissues. A lipid based delivery system was used to deliver a synthetic miR-34a mimic systemically via intravenous injections in a mouse model of non-small cell lung cancer and did not affect liver or kidney enzyme levels or trigger an immune response [53]. A recent study overcame the problem of miRNA-based therapy affecting non-target tissue via a targeted miR-34a expression plasmid (T-VISA-miR-34a) that used the human telomerase reverse transcriptase (hTERT) promoter that is active solely in cancerous tissue and repressed in benign tumors or normal tissues [54]. When the miR34a expression plasmid was delivered via liposomal complexes in an orthotopic mouse model of breast cancer, there was a significant reduction of tumor growth, without any effects on normal tissues [54]. Thus, in the future, miRNA-based therapeutic agents may be directly delivered to the tumor without any deleterious effects on non-cancerous organs.
Viral Delivery
Adeno-associated viruses (AAV) are often used for delivering miRNAs. Tissue-specific promoters can be used to ensure efficient delivery to the organ of interest. Also, AAVs possess different serotypes with differing affinity for various organs, further aiding in the delivery of miRNAs to a specific location. In a mouse model of liver cancer, AAV mediated delivery of miR-26a hindered cancer progression [55].
EMERGING miRNAs IN CANCER THERAPEUTICS
‘Miravirsen’, a drug containing antagomir targeting miR-122 has currently undergone phase 2a clinical trials, and the results show a significant protective effect on patients with Hepatitis C [56]. The tumor suppressor miR-34a is also another miRNA that is headed for clinical trials for the treatment of various cancers [57]. The following are some miR-NAs that hold promise as therapeutic agents in cancer.
Let-7
Let-7 plays a role in the larval development of C. elegans, as well as in lung and breast cancer in humans. Let 7 is a tumor suppressor miRNA and its levels decrease during the progression of most cancers. There is a decrease in Let-7 levels in laryngeal, pancreatic, colon, lung and breast cancer in humans [58]. Target mRNAs include hRAS, kRAS, HMGA2, LIN28 and NF2 [58]. Studies in laryngeal cancer cells lines have shown that transfection with Let-7a mimics or Let-7a precursor miRNA caused reduced proliferation and growth [59]. Also, mice with an activated kRAS transgene, when given Let-7 intra-nasally via an adenovirus, have stunted lung tumor development in comparison to control mice expressing only the kRAS transgene [60]. Thus, Let-7 administration may be a viable therapeutic strategy for lung cancer. In pancreatic cancer, Let-7 expression is reduced and restoration of Let-7 in pancreatic cancer cells with plasmid based Let-7 resulted in decreased cellular proliferation [61]. However, intra-tumoral administration of the plasmid and implantation of Capan-1 cells stably overexpressing Let-7 microRNA did not alter tumor growth/proliferation [61].
miR-21
Since it is frequently over-expressed in a variety of cancers such as breast [62] and colorectal [63] cancer, miR-21 is an oncogenic miRNA. It is located on chromosome 17 and regulates CDKN1A, PTEN, PDCD4 and several other tumor suppressors [64–66]. Suppression of miR-21 using an LNA-based antagonist administered via an intra-peritoneal injection resulted in a decrease in autoimmune splenomegaly in mice with systemic lupus erythematosus [67]. This miRNA can also be used as a diagnostic and prognostic marker in colorectal cancer, where elevated levels of miR-21 are seen in the stool of patients [68]. Also, increased serum miR-21 is related to treatment responsiveness in patients with pancreatic and lung cancers [69, 70]. In lung cancer, mutations in the EGFR are more frequent in non-smokers with the disease [71]. This mutation confers an increased responsiveness to tyrosine kinase inhibitor based therapy. It has recently been shown that there is an increased expression of miR-21 in these patients, and that the in vitro targeting of miR-21 using antagomirs sensitizes cancer cells to tyrosine kinase inhibitor based therapy [71].
miR-26a
This miRNA has been found to be down-regulated in hepatocellular carcinoma [72], nasopharyngeal carcinoma [73], lymphomas [74], and breast cancer [75]. Targets of this miRNA include the histone-lysine N-methly transferase, EZH2 [73]and PTEN [76]. Restoration of this miRNA in liver cancer cells and in vivo using an AAV based delivery method in a mouse model of liver cancer resulted in a reduction in cell proliferation, and cell cycle arrest, which was attributed to the down-regulation of cyclins D2 and E2 [55]. Also, the mice displayed stunted disease progression However; miR-26a expression is elevated in high grade glioma [77] and cholangiocarcinoma, suggesting that the miRNA can also act as an oncogene depending on cellular context. Indeed, it was found to be one of the five miRNAs that independently promoted acute T lymphoblastic leukemia [76].
miR-29
The miR-29 family of miRNAs can function as either tumor suppressors or oncogenes. For instance, miR-29a and miR-29b are down-regulated in mantle cell lymphoma [78]. These miRNAs have been proposed to target several cell cycle regulators and oncogenes such as MCL-1 (induced myeloid leukemia cell differentiation protein), CDK6, dedifferentiation factors (KLF4, Kruppel-like factor 4), and long noncoding RNAs (MEG3, maternally expressed 3). Over-expression of miR-29 induces apoptosis thereby reducing tumorigenecity in lung cancer and acute myeloid leukemia (AML) xenografts [79]. However, in vivo over-expression of miR-29 in immature and mature B-cells resulted in a propensity for these mice to develop B-cell CLL, suggesting that this miRNA can act as an oncogene or tumor suppressor depending on the cellular context [79, 80]. A recent study has shown that miR-29 can cause a global alteration in methylation by regulating the levels of DNA methyl transferases 3A and B, thereby de-repressing tumor suppressors like FHIT (fragile histidine triad protein) and WWOX (WW domain containing oxidoreductase) [81].
miR-34
The miR-34 (comprising miR-34a, b, and c) family of miRNAs is transcriptionally induced by p53 and p38-MAPK pathways in response to DNA damage [33]. This family of miRNAs is responsible for the translational repression of the Myc oncogene. Myc normally causes the transcription of the oncogenic miR-17–92 miRNA cluster. Additional targets include NOTCH1, BCL2, E2F3, VEGFA, SIRT1, CCND1 and CDK6 [82]. The miR-34 family of miRNAs is frequently deregulated (reduced) in lung, breast, prostate and liver cancers [58]. Recent studies in a mouse model of non-small cell lung carcinoma have shown that systemic or local administration of a chemically synthesized miR-34a molecule via a lipid-based delivery system resulted in attenuation of tumor growth [53]. There were negligible side effects on other organ systems, suggesting that the miRNA is well tolerated.
PROSPECTS FOR miRNA-BASED THERAPY IN PANCREATIC CANCER
Pancreatic cancer is characterized by aggressive growth and the presence of metastases at the time of diagnosis. Surgically resectable tumors represent only a small percentage of pancreatic cancer cases. The disease has a grim five-year survival rate of only 6% [1]. Therefore, better treatment strategies are sorely needed and early detection is of critical importance.
As mentioned previously, miRNA profiling has been shown to be effective in differentiating normal tissue from cancer, distinguishing between tumor sub-types as well as being predictive of the chemotherapy outcome in various cancers. Bloomston et al. (2007) used a microRNA microarray to demonstrate that PDAC (pancreatic ductal adenocarcinoma) can be distinguished from chronic pancreatitis and normal pancreas [12]. It was shown that PDAC samples show an increase in 21 miRNAS and a decrease in 4 miR-NAs in comparison to normal pancreatic tissue, and that this difference in expression enabled differentiation between the two with 90% accuracy. Also, the over-expression of 15 miRNAs and down-regulation of 8 miRNAs was sufficient to distinguish between PDAC and chronic pancreatitis. Six miRNAs were found to be predictive of a better prognosis (beyond two years): miR30a-3p, miR-105, miR-127, miR-187, miR-452, and miR-518a-2 in patients with lymph node positive disease. It has also been shown that 79% of PDACs show an increase in miR-21 expression, a miRNA that down-regulates PTEN [83]. Over-expression of miR-21 was found to be predictive of a worse prognosis. Patients with PDAC show increased levels of miR-21 in the serum [84]. Chemo-resistant cell lines of pancreatic cancer display an over-expression of miR-21 [84]. A recent study suggested that a panel of five miRNAs in formalin-fixed paraffin embedded (FFPE) pancreatic cancer tissue were predictive of overall prognosis A high expression of miR-212 and miR-675 and a low expression of miR-148a, miR-187, and let-7g were independently predictive of a poorer overall survival in patients that had undergone surgical resection [85].
miRNAs as Pancreatic Cancer Diagnostic Tools
Endoscopic ultrasound guided fine needle aspiration is often used for diagnosis and staging of pancreatic ductal adenocarcinoma. A study of miRNAs present in fine needle aspirates showed that miR-196a and miR-217 aided in the diagnosis of PDAC [86] and in distinguishing it from chronic pancreatitis. The cystic fluid from intra-papillary cystic neoplasms (IPMNs) was also studied and found to contain miR-155, which is being examined as a potential biomarker for the disease [87]. A recent study suggested that plasma miR-221 is elevated in PC in comparison to benign non-cancerous conditions, and that elevated levels of plasma miR-221 could be used as a diagnostic tool. In addition, it was noted that elevated plasma miR-221 was predictive of distant metastasis and the non-resectable status of the tumor [88].
As mentioned previously, miRNAs are frequently present in bodily fluids of cancer patients. A plasma microRNA profile for pancreatic cancer, comprising miR-21, miR-210, miR-155 and miR-196a has been proposed [89]. While the actual diagnosis of pancreatic cancer is most often done by traditional methods, such as biopsies of tissues and ultrasound guided fine needle aspiration, the detection of miR-NAs in the plasma/serum could represent a non-invasive method of determining the extent of metastasis and general prognosis of the patient.
miRNAs as Pancreatic Cancer Therapeutic Tools
While the current treatment of PDAC patients consists of a variable combination of surgery, chemotherapy and radiation, several studies have shown that targeting miRNAs commonly dysregulated in pancreatic cancer sensitizes cells to chemotherapeutic drugs such as gemcitabine. For instance, a study showed that treatment of a panel of pancreatic cancer cell lines with anti-sense oligos targeting miR-21 and miR-221 resulted in increased apoptosis of cells. When treated with Gemcitabine, a commonly used chemotherapeutic drug for PDAC, the cells with anti- miR-21 and anti-miR-221 displayed increased sensitivity to the effects of the drug [90].
The expression of miR-21 has also been shown to be linked to a poorer prognosis and gemcitabine resistance [83, 84]. When pre-miR-21 was transfected into pancreatic cancer cells that were subsequently treated with gemcitabine, miR -21 was shown to hinder the anti-proliferative and anti-apoptotic actions of the drug [91]. Studies of 82 paraffin-embedded, formalin-fixed patient samples post-resection showed that low miR-21 expression levels corresponded to better response to adjuvant chemotherapy [92]. Another miRNA, miR-181b, was implicated in sensitizing PC cells to gemcitabine therapy. The chemo-resistant cell lines (SW1990/GR and CFPAC-1/GR) when transfected with lentiviral vectors containing miR-181b mimics showed increased sensitivity to the drug. Similar findings were obtained when PC cell lines expressing the vector were implanted as xenografts in nude mice. The increased repression of Bcl-2 by miR-181b was cited as the reason for this increased chemosensitivity [93].
Other miRNAs that could potentially be targeted/administered therapeutically include miR-200a, which is an oncogenic miRNA and targets E-Cadherin [94], the tumor suppressor miRNA 20a, which targets the STAT3 transcription factor [95]. When miR-20a was over-expressed in Panc-1 and BXPC3 pancreatic cancer cell lines there was a decreased proliferation and metastasis both in vivo and in vitro [95].
kRAS is a critical tumor initiating oncogene, coding for a small GTPase involved in signaling, and is mutated in greater than 90% of all cases of pancreatic cancer [96]. A tumor suppressor miRNA, miR-96 regulates the expression of kRAS, and is significantly decreased in PDAC tissue in comparison to normal tissue [97]. In addition, it has been demonstrated that ectopic expression of miR-96 using a synthetic miRNA precursor inhibited kRAS expression and reduced cellular proliferation and invasion both in vitro and in vivo [97]. Given the importance of the kRAS gene in the pathogenesis of PC, ectopic overexpression of this miRNA may yield promising results.
As summarized above, there is dysregulation of a number of miRNAs in pancreatic cancer. Therefore, therapy that aims to inhibit/re-introduce miRNAs that are up-regulated/down-regulated could turn out to be viable therapeutic strategies. For example, miR-10a has been reported to be up-regulated in metastatic pancreatic cancer [12]. Also, miR-10a has been shown to be a target of retinoic acid, and retinoic acid receptor antagonists cause miR-10a suppression, thereby repressing metastasis [98]. Two miRNAs, miR-224 and miR-486 were also shown to aid in PC metastasis, in part, by targeting CD40, a tumor necrosis factor receptor family member. The expression of these miRNAs was greater in highly metastatic cancer than in non-invasive PC [99]. MiR-34, which is a p53 target and is reduced in pancreatic cancer, was over-expressed using lentiviral constructs and miR-34 mimics in p53 mutant cell lines BXPC3 and MiaPACA-2. This resulted in decreased cell growth, cell cycle arrest at G1 and G2/M and sensitization to chemotherapy [100].
The tumor suppressor miRNA miR-146a, which decreases in PDAC, was shown to regulate EGFR and NF-ΚB expression [101]. The treatment of pancreatic cancer cells with the naturally derived compounds 3, 3′-diinodolylmethane or isoflavone resulted in a restoration of miR-146a expression and the consequent reduction in EGFR and NF-ΚB levels. This study demonstrated the promise of naturally derived compounds in miRNA based pancreatic cancer therapy.
CONCLUSIONS
Much has been discovered about the numerous miRNAs that are aberrantly expressed in pancreatic cancer. A number of them could prove to be effective drug targets; however, effective targeting of the affected organ and minimizing off-target effects of the miRNA-based therapeutic agent represent formidable challenges. Some of the other promising miRNAs for targeted therapy, including the cellular processes that they affect, have been summarized in Table 1. In the future, a miRNA panel could be used for diagnostic and prognostic purposes in pancreatic cancer. Additionally, miRNA targeted therapy could be used to supplement conventional chemotherapy. Issues such as miRNA toxicity to non-target tissues and possible side effects in humans will be challenges that the field will have to overcome.
Table 1.
miRNAs as Potential Therapeutic Targets in Pancreatic Cancer
| miRNA | Tumor suppressor/oncogenic | Predicted target genes | Cellular processes affected | References |
|---|---|---|---|---|
| miR 21 | Oncogenic | CDK6, PDCD4, CDKN1A, FAS, IL6R, SOCS5, APAF1, NFlB, TPM1 | Apoptosis, cellular proliferation, migratory and invasive properties | [91] |
| miR-221 | Oncogenic | CDKN1B, CDKN1C, KIT | Cell migration and proliferation | [90] |
| miR-155 | Oncogenic | AGTR1, APC, ARID2, BACH1, CEBPB, CYR61, DET1, EDN1, ETS1, FADD, FGF7, FOXO3 | Cell migration | [87] |
| miR-196a | Oncogenic | NRAS, HOXB8, HMGA2, ANNEXIN A1 | Cell proliferation and differentiation | [102] |
| Let 7 | Tumor suppressor | LIN28, HRAS, KRAS, HMGA2, TRIM71, NF2 | Cellular proliferation | [61] |
| miR-34a | Tumor suppressor | NOTCH1, BCL2, E2F3, VEGFA, SIRT1, CCND1, CDK6 | Apoptosis, cellular proliferation | [100] |
| miR-217 | Tumor suppressor | KRAS, SIRT1, PTEN | Proliferation, survival and invasion | [103] |
| miR-216a | Tumor suppressor | PTEN, CDC42, CD44, SIRT1 | Tumorigenecity | [104, 105] |
Acknowledgments
FINANCIAL STATEMENT
The authors on this work are supported, in part, by grants from the National Institutes of Health (TMEN U54 CA163120, EDRN UO1 CA111294, RO1 CA131944, RO1 CA133774, SPORE P50 CA127297 and RO1 CA78590).
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Matsuno S, Egawa S, Fukuyama S, et al. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219–30. doi: 10.1097/00006676-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–40. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Carthew RW. Gene regulation by microRNAs. Curr Opin Genet Dev. 2006;16:203–8. doi: 10.1016/j.gde.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Pasquinelli AE, Reinhart BJ, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 7.Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–20. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 9.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Schug J, McKenna LB, et al. Tissue-specific regulation of mouse microRNA genes in endoderm-derived tissues. Nucleic Acids Res. 2011;39:454–63. doi: 10.1093/nar/gkq782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 13.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA. 2004;101:11755–60. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–54. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer. 2004;39:167–9. doi: 10.1002/gcc.10316. [DOI] [PubMed] [Google Scholar]
- 18.Michael MZ, O’ Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–91. [PubMed] [Google Scholar]
- 19.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 20.Caldas C, Brenton JD. Sizing up miRNAs as cancer genes. Nat Med. 2005;11:712–4. doi: 10.1038/nm0705-712. [DOI] [PubMed] [Google Scholar]
- 21.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 22.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 23.McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13:253–8. doi: 10.1016/s1044-579x(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer PS. Cancer genomics: small RNAs with big impacts. Nature. 2005;435:745–6. doi: 10.1038/435745a. [DOI] [PubMed] [Google Scholar]
- 25.Olson P, Lu J, Zhang H, et al. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–65. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 30.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–6. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SK, Pal BM, Girschick HJ, Bhadra U. MicroRNAs--micro in size but macro in function. FEBS J. 2008;275:4929–44. doi: 10.1111/j.1742-4658.2008.06624.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. microRNAs in cancer management. Lancet Oncol. 2012;13:e249–e258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 34.Rothe F, Ignatiadis M, Chaboteaux C, et al. Global microRNA expression profiling identifies MiR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–7. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 38.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pigati L, Yaddanapudi SC, Iyengar R, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;20:5, e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery RL, van RE. Therapeutic advances in MicroRNA targeting. J Cardiovasc Pharmacol. 2011;57:1–7. doi: 10.1097/FJC.0b013e3181f603d0. [DOI] [PubMed] [Google Scholar]
- 41.Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3:1–3. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 43.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 44.Care A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 45.Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S. Inhibition of microRNA function by antimiR oligonucleotides. Silence. 2012;3:1–3. doi: 10.1186/1758-907X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gumireddy K, Young DD, Xiong X, et al. Small-molecule inhibitors of microrna miR-21 function. Angew Chem Int Ed Engl. 2008;47:7482–4. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young DD, Connelly CM, Grohmann C, Deiters A. Small molecule modifiers of microRNA miR-122 function for the treatment of hepatitis C virus infection and hepatocellular carcinoma. J Am Chem Soc. 2010;132:7976–81. doi: 10.1021/ja910275u. [DOI] [PubMed] [Google Scholar]
- 49.Bose D, Jayaraj GG, Kumar S, Maiti S. A Molecular-Beacon-Based Screen for Small Molecule Inhibitors of miRNA Maturation. ACS Chem Biol. 2013;8(5):930–8. doi: 10.1021/cb300650y. [DOI] [PubMed] [Google Scholar]
- 50.Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–50. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–6. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang V, Wu W. MicroRNA-based therapeutics for cancer. BioDrugs. 2009;23:15–23. doi: 10.2165/00063030-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 53.Trang P, Wiggins JF, Daige CL, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–22. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Xie X, Luo J, et al. Targeted expression of miR-34a using the T-VISA system suppresses breast cancer cell growth and invasion. Mol Ther. 2012;20:2326–34. doi: 10.1038/mt.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV Infection by Targeting MicroRNA. N Engl J Med. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 57.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrocca F, Lieberman J. Micromanipulating cancer: microRNA-based therapeutics? RNA Biol. 2009;6:335–40. doi: 10.4161/rna.6.3.9013. [DOI] [PubMed] [Google Scholar]
- 59.Long XB, Sun GB, Hu S, et al. Let-7a microRNA functions as a potential tumor suppressor in human laryngeal cancer. Oncol Rep. 2009;22:1189–95. doi: 10.3892/or_00000554. [DOI] [PubMed] [Google Scholar]
- 60.Esquela-Kerscher A, Trang P, Wiggins JF, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–64. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 61.Torrisani J, Bournet B, du Rieu MC, et al. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum Gene Ther. 2009;20:831–44. doi: 10.1089/hum.2008.134. [DOI] [PubMed] [Google Scholar]
- 62.Iorio MV, Casalini P, Tagliabue E, Menard S, Croce CM. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur J Cancer. 2008;44:2753–9. doi: 10.1016/j.ejca.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 63.Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–36. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 65.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J Biol Chem. 2007;282:14328–36. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 66.Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garchow BG, Bartulos EO, Leung YT, et al. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3:605–15. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu CW, Ng SS, Dong YJ, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–45. doi: 10.1136/gut.2011.239236. [DOI] [PubMed] [Google Scholar]
- 69.Gao W, Lu X, Liu L, et al. MiRNA-21: a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol Ther. 2012;13:330–40. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 70.Liu R, Chen X, Du Y, et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem. 2012;58:610–8. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 71.Seike M, Goto A, Okano T, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci USA. 2009;106:12085–90. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–47. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu J, He ML, Wang L, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–33. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 74.Sander S, Bullinger L, Wirth T. Repressing the repressor: a new mode of MYC action in lymphomagenesis. Cell Cycle. 2009;8:556–9. doi: 10.4161/cc.8.4.7599. [DOI] [PubMed] [Google Scholar]
- 75.Liu XX, Li XJ, Zhang B, et al. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett. 2011;585:1363–7. doi: 10.1016/j.febslet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 76.Mavrakis KJ, Van Der Meulen J, Wolfe AL, et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL) Nat Genet. 2011;43:673–8. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huse JT, Brennan C, Hambardzumyan D, et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–37. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao JJ, Lin J, Lwin T, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–9. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Santanam U, Zanesi N, Efanov A, et al. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci USA. 2010;107:12210–5. doi: 10.1073/pnas.1007186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han YC, Park CY, Bhagat G, et al. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–10. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rokhlin OW, Scheinker VS, Taghiyev AF, et al. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biol Ther. 2008;7:1288–96. doi: 10.4161/cbt.7.8.6284. [DOI] [PubMed] [Google Scholar]
- 83.Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–6. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali S, Almhanna K, Chen W, Philip PA, Sarkar FH. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res. 2010;3:28–47. [PMC free article] [PubMed] [Google Scholar]
- 85.Schultz NA, Andersen KK, Roslind A, et al. Prognostic microRNAs in cancer tissue from patients operated for pancreatic cancer--five microRNAs in a prognostic index. World J Surg. 2012;36:2699–707. doi: 10.1007/s00268-012-1705-y. [DOI] [PubMed] [Google Scholar]
- 86.Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009;8:340–6. doi: 10.4161/cbt.8.4.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi T, Komatsu S, Ichikawa D, et al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108:361–9. doi: 10.1038/bjc.2012.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang J, Chen J, Chang P, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2009;2:807–13. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–9. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 91.Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther. 2009;8:1067–74. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 92.Hwang JH, Voortman J, Giovannetti E, et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010;5:e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai B, An Y, Lv N, et al. miRNA-181b increases the sensitivity of pancreatic ductal adenocarcinoma cells to gemcitabine in vitro and in nude mice by targeting BCL-2. Oncol Rep. 2013;29:1769–76. doi: 10.3892/or.2013.2297. [DOI] [PubMed] [Google Scholar]
- 94.Li A, Omura N, Hong SM, et al. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–37. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan H, Wu J, Liu W, et al. MicroRNA-20a overexpression inhibited proliferation and metastasis of pancreatic carcinoma cells. Hum Gene Ther. 2010;21:1723–34. doi: 10.1089/hum.2010.061. [DOI] [PubMed] [Google Scholar]
- 96.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 97.Yu S, Lu Z, Liu C, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–25. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 98.Weiss FU, Marques IJ, Woltering JM, et al. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009;137:2136–45. doi: 10.1053/j.gastro.2009.08.065. [DOI] [PubMed] [Google Scholar]
- 99.Mees ST, Mardin WA, Sielker S, et al. Involvement of CD40 targeting miR-224 and miR-486 on the progression of pancreatic ductal adenocarcinomas. Ann Surg Oncol. 2009;16:2339–50. doi: 10.1245/s10434-009-0531-4. [DOI] [PubMed] [Google Scholar]
- 100.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, Vandenboom TG, Wang Z, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–95. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen C, Zhang Y, Zhang L, Weakley SM, Yao Q. MicroRNA-196: critical roles and clinical applications in development and cancer. J Cell Mol Med. 2011;15:14–23. doi: 10.1111/j.1582-4934.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao WG, Yu SN, Lu ZH, et al. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31:1726–33. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
- 104.Hou B, Jian Z, Chen S, et al. Expression of miR-216a in pancreatic cancer and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1628–31. [PubMed] [Google Scholar]
- 105.Park JY, Helm J, Coppola D, et al. MicroRNAs in pancreatic ductal adenocarcinoma. World J Gastroenterol. 2011;17:817–27. doi: 10.3748/wjg.v17.i7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]