Abstract
Progesterone receptors (PR) select and control genetic programs in the breast during normal mammary gland development, and progestin-driven processes contribute to the initiation and/or progression of breast cancer. Throughout the mammalian life span, progesterone exerts varying biological consequences on the mammary epithelial compartment, from brief proliferative spurts that occur with each luteal phase of the menstrual cycle to the massive expansion of the pregnant gland in preparation for lactation. These processes, while important developmentally, can become deregulated in breast cancer, thereby contributing to unchecked proliferation, increased survival, and invasive behaviors. Recently, our lab has focused on the molecular mechanisms, including phosphorylation events, by which PRs select specific target genes in response to progestins and other mitogenic hormonal signals (i.e. EGF, heregulin). Herein, we discuss the actions of cytoplasmic signaling molecules such as c-Src and mitogen-activated protein kinases as key mediators of PR promoter selectivity.
Keywords: Progesterone receptor (PR), Phosphorylation, Sumoylation, Promoter selectivity, Transcription
1. PR transcriptional activity is regulated by post-translational modifications
PR mediated gene expression occurs through multiple mechanisms, each of which is exquisitely controlled by a variety of extra- and intra-cellular processes. Classically, PRs act as ligand-activated transcription factors on promoters containing Progesterone Response Elements (PREs) as is the case for the c-myc gene (Moore et al., 1997). Alternatively, PR may alter gene expression non-classically, wherein the receptor tethers to other transcription factors such as SP1, AP1, or STATs (Owen et al., 1998; Proietti et al., 2005; Richer et al., 2002; Tseng et al., 2003), or initiates membrane proximal kinase cascades (c-Src, MAPK) to activate other transcription factor effectors such as members of the ETS family (Boonyaratanakornkit et al., 2001; Migliaccio et al., 1998). Each of these routes of gene regulation contributes to the biological response of the cell to progesterone.
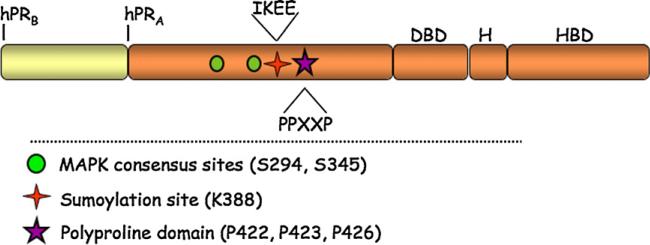
Recent data from our lab has shown that PR promoter selectivity is modulated by PR post-translational modifications. Steroid hormone receptors such as PR are phospho-proteins. Growth factor pathway signaling can stimulate activation of both ligand-bound and unliganded steroid hormone receptors through phosphorylation events (Kato, 2001; Labriola et al., 2003; Pierson-Mullany and Lange, 2004; Shen et al., 2001). PR contains 14 serine residues that are known to be phosphorylated by mitogenic protein kinases, including MAPK, Casein kinase II and CDK2 (Moore et al., 2007). These phosphorylation events can occur basally, in a hormone dependent manner, or upon kinase activation in response to peptide growth factors. Steroid hormone receptor phosphorylation has been shown to alter receptor transcriptional activation, stability, localization and protein complex formation (Lange, 2004). The specific functional consequences of phosphorylation on most PR serine residues have not been defined; however, a few sites have been well characterized. PR Ser294 (Fig. 1) is phosphorylated by the MAPK pathway in response to growth factor signaling such as EGF (Shen et al., 2001) and in response CDK2 activation (Daniel et al., 2007a). PR Ser294 phosphorylation also occurs rapidly in response to ligand binding (Shen et al., 2001) by a MAPK-independent pathway (Qiu and Lange, 2003). PR-B is robustly phosphorylated on Ser294 following progestin and growth factor treatment, while PR-A is only weakly regulated at this site (Clemm et al., 2000). Phosphorylation of PR Ser294 shuttles the receptor to the nucleus, and increases its transcriptional activity, while simultaneously decreasing its stability within the cell by targeting it for ubiquitinylation (Lange et al. 2000; Qiu and Lange, 2003). The ligand-dependent phosphorylation of Ser345 (Fig. 1) has also been shown to affect PR transcriptional synergy in the presence of progestin and activated MAPKs (Faivre et al., 2008; Lange et al., 2000). Ultimately, phosphorylation of PR serves to integrate signals from multiple pathways by modulating receptor promoter selectivity and changes in cell biology.
Fig. 1.
Progesterone receptor schematic. Progesterone receptors A and B contain a DNA binding domain (DBD), a hinge region (H), and a hormone-binding domain (HBD). The N-terminal region of PR contains the MAPK consensus sites Ser294 and Ser345, the sumoylation consensus site Lys388, and the polyproline rich domain.
Recently, phosphorylation of the glucocorticoid receptor (GR) has also been shown to alter gene selection and transcriptional activation. Blind and Garabedian (2008) demonstrated that GR molecules phosphorylated on specific serine residues differentially occupy endogenous target gene promoters. Using phospho-specific antibodies to three GR serines they showed by Chromatin-Immunoprecipitation (ChIP) assays that individual phospho-species displayed increased binding to certain promot ers while other phospho-species interacted minimally. Similarly, phosphorylation events are predicted to contribute to GR isoformspecific regulation of endogenous genes (Lu and Cidlowski, 2005, 2006). Clearly, steroid receptor phosphorylation is doing more than simply increasing or decreasing global receptor transcriptional activity; it may in fact be responsible for specifically localizing pools of modified receptors to select promoter types.
2. Liganded PR tethers to SP1 via MAPK-dependent Ser345 phosphorylation
Little is known about the mechanisms governing steroid receptor regulation of non-classical gene targets (non-HRE containing), in particular how rapid signaling events can alter promoter selectivity. Recently, Faivre et al. uncovered a mechanism by which rapid progesterone/PR initiated kinase signaling can alter the phosphorylation state of PR, thus targeting it to Sp1 sites for transcriptional activation (Faivre et al., 2008). Using T47D breast cancer cells expressing the PR-B isoform, Faivre et al. demonstrated that upon 10 min of progestin treatment PR was able to activate EGFR, c-Src, and downstream MAPK signaling leading to the phosphorylation of PR on Ser345 (Faivre et al., 2008). PR Ser345 is a proline-directed MAPK consensus site (PXXSP) located in the N-terminal region of the receptor (Fig. 1); phosphorylation of this site is entirely ligand dependent. However, inhibitors of EGFR, c-Src and MAPK activity blocked this progestin-induced phosphorylation event. Additionally, to show that progestin-initiated rapid cytoplasmic signaling was required for PR Ser345 phosphorylation the mPro mutant of PR-B was utilized. This mutant lacks the poly-proline motif shown to be required for PR interaction with the c-Src SH3 domain and progestin-induced c-Src activation (Boonyaratanakornkit et al., 2001). MPro PR-B cannot activate rapid signaling (i.e. to MAPK) and therefore failed to undergo Ser345 phosphorylation. Specificity for progestin-initiated signaling was further demonstrated by the use of EGF to activate MAPK; EGF stimulation induced phosphorylation of a different PR MAPK consensus site, Ser294, but was unable to induce PR Ser345 phosphorylation. PR rapid signaling, in this case, is thus functioning to prime PR (i.e. via Ser345 phosphorylation) for downstream genomic actions that require ligand-dependent PR/c-Src/MAPK complex formation and rapid signaling.
To examine the consequences of PR Ser345 phosphorylation on PR gene regulation, Faivre et al. generated a S345A mutant that cannot undergo phosphorylation at this site (Faivre et al., 2008). This mutant (S345A) was tested on a variety of progestin responsive promoters. S345A PR-B transcriptional activity was comparable to wt PR-B on classical promoters such as 2xPRE-luciferase reporter constructs and the Serum and Glucocorticoid Receptor Kinase (SGK) endogenous promoter. However, S345A PR-B was unable to activate transcription on non-classical (non-PRE containing) progestin responsive promoters such as p21 and EGFR, both of which contain numerous Sp1 sites (Hudson et al., 1990; Ishii et al., 1985; Owen et al., 1998). Upon further examination, wt PR-B, but not S345A or mPro, was able to interact with Sp1 in co-immunoprecipitation experiments, and capable of tethering to Sp1 sites in the p21 promoter as measured in ChIP assays. PR Ser345 phosphorylation therefore functions to target PR to specific progestin-regulated promoters that contain Sp1 sites (i.e. p21 and EGFR) via PR/Sp1 tethering (Fig. 2). Progestin responsive genes regulated by PR tethering are predicted to be a large cohort of known PR target genes (Jacobsen et al., 2005; Richer et al., 2002) and thus critical for progestin mediated biology in the breast; these studies highlight the essential role of protein kinases in PR transcriptional activation and promoter selectivity.
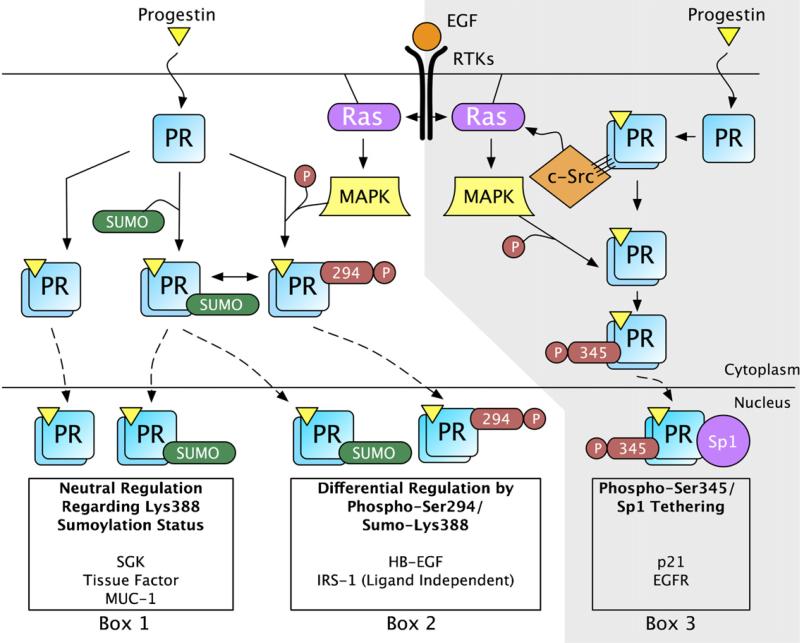
Fig. 2.
Progestins and growth factors initiate signaling cascades that regulate PR post-translational modifications resulting in promoter selectivity. (Left; unshaded) In response to progestin binding, a subset of PR is sumoylated on Lys388. In response to growth factor treatment (e.g. EGF), MAPK signaling is activated and PR is phosphorylated at Ser294. PR phosphorylation at Ser294 inhibits PR sumoylation at Lys388. These signaling mechanisms provide three different post-translationally modified populations of PR that can activate gene transcription at selected promoters. The transcriptional regulation of SGK, Tissue Factor, and MUC1 are similarly regulated regardless of the PR Lys388 sumoylation status (Box 1). When PR is phosphorylated at Ser294 (not sumoylated at Lys388), HB-EGF expression is elevated compared to sumoylated PR (Box 2). In the absence of ligand, IRS-1 expression is higher when regulated by phosphorylated PR, compared to sumoylated PR (Box 2). (Right; shaded) Additionally, progestin treatment results in PR-mediated activation of c-Src through direct interaction of PR's polyproline motif with c-Src's SH3 domain (Boonyaratanakornkit et al., 2001). Progestin-induced c-Src activation results in MAPK signaling and phosphorylation at PR Ser345 (Faivre et al., 2008). Ser345 phosphorylated PR can tether to Sp1 and activate transcription at promoters with Sp1 binding sites like p21 and EGFR (Box 3). Many additional genes are likely to be differentially regulated by post-translationally modified PR transcription factors.
The above pathway (PR-induced rapid c-Src/MAPK activation) provides a concrete example of the ability of PR to integrate rapid membrane proximal signaling with its genomic actions; progesterone initiated kinase cascades directly phosphorylate PR in signaling complexes, causing it to tether to Sp1, and thus localize to specific Sp1-regulated promoters (Fig. 2). Therefore, PR rapid signaling provides a feed-forward mechanism for activating highly specific PR mediated transcriptional events. These data illustrate that individual phosphorylation events are able to direct PR to specific promoters and dictate the genetic program to be utilized in the presence of progestins, ultimately affecting biological outcomes. For example, PR-B initiated rapid signaling has previously been shown to be critical for progestin induced S-phase entry of breast cancer cells (Skildum et al., 2005). Faivre et al. questioned whether the downstream PR phosphorylation on Ser345, and subsequent Sp1 tethering events, were required for this biological response (Faivre et al., 2008). Blockade of EGFR, c-Src, MAPK and Sp1 via their respective inhibitors abolished progestin induced S-phase entry of T47D cells expressing wt PR-B as measured by flow cytometry. These data demonstrate that a specific PR phosphorylation (Ser345) mediates PR promoter selection of genes that are directly responsible for the initiation of cell cycle progression in breast cancer cells.
Further exploration of the ability of PR Ser345 phosphorylation to alter PR gene selectivity is warranted. In addition to Sp1, Ser345 or other PR phosphorylation sites, may alter PR tethering to progestin responsive genes that contain functional AP1 (Tseng et al., 2003) and/or STAT (Proietti et al., 2005; Richer et al., 2002) sites. Additional phosphorylation or post-translational modification events are predicted to contribute to the specificity of regulation of complex tethering mechanisms in the context of selected endogenous gene promoters.
3. Phospho-regulation of PR sumoylation alters promoter selectivity
Recently, we found that PR phosphorylation at Ser294 also contributes to differential hormone responsiveness and gene transcription, in part by directing promoter selectivity through a mechanism of PR sumoylation/desumoylation (Daniel et al., 2007a). Growth factor receptor tyrosine kinase activation (e.g. via EGF) (Qiu and Lange, 2003) or progestin binding to PRs activates multiple signaling pathways, in part resulting in direct PR Ser294 phosphorylation (Shen et al., 2001; Skildum et al., 2005). In early studies by Qiu et al., T47D breast cancer cells were pre-treated with EGF for 15–30 min and then treated with increasing concentrations of progestin (Qiu and Lange, 2003). The EGF pre-treated cells displayed heightened PR transcriptional activity in response to a range of progestin treatment (10−8 to 10−12 M R5020) relative to growth factor naive cells. Cells expressing phospho-mutant S294A PR remained insensitive to EGF pretreatment (Daniel et al., 2007b; Qiu and Lange, 2003). These data suggested that when PR Ser294 is phosphorylated, receptors are transcriptionally hypersensitive to low progestin concentrations relative to non-phosphorylated receptors. Notably, S294A mutant PR often exhibit impaired transcriptional responses when expressed stably (i.e. at levels comparable to endogenous PRs) (Shen et al., 2001). However, transcriptional defects are overcome when these receptors are expressed at high concentrations (i.e. in transient assays) (Qiu and Lange, 2003). Together, these data suggest that PR Ser294 phosphorylation, in response to progestins and/or growth factor signaling, functions to attenuate a repressive modification of PR at PRE-driven promoters, perhaps mediated by a limiting factor(s).
Transcriptional repression of target genes is often mediated by sumoylated transcription factors; many transcription factors are modified by SUMO attachment in a phosphorylation-dependent manner (e.g. Elk-1, c-Fos, AIB1) (Bossis et al., 2005; Wu et al., 2006; Yang et al., 2003). SUMO (small ubiquitin-like modifier) is a small (~10 kD) protein that is reversibly attached to lysine residues of target proteins, often altering protein–protein interactions, subcellular localization, stability, and/or transcriptional activity (reviewed in Geiss-Friedlander and Melchior, 2007). Sumoylation is a dynamic post-translational event that requires an enzymatic cascade, similar to ubiquitinylation, in which SUMO molecules are processed and attached to target proteins facilitated by Ubc9 and E3 ligases. SUMO-1 is ubiquitously expressed in mammalian tissues (Melchior, 2000). Notably, the expression of SENPs, the enzymes responsible for desumoylation, is hormone-dependent in prostate (Cheng et al., 2006) and mammary epithelial cells (unpublished data).
In response to progestin treatment, a subset of PR molecules are rapidly sumoylated at Lys388 (Abdel-Hafiz et al., 2002) (Fig. 1). The transcriptional consequences of sumoylated PR were recently investigated by creating a mutant PR that cannot be sumoylated at Lys388 (K388R) (Abdel-Hafiz et al., 2002). In transient assays conducted in HeLa cells, as well as in subsequent studies using T47D cells stably expressing SUMO-deficient or wt PR-B, the transcriptional activity of SUMO-deficient PR-B was elevated at least 10-fold compared to wt PR-B (Abdel-Hafiz et al., 2002; Daniel et al., 2007a). Additionally, in breast cancer cells, sub-physiological concentrations of progestin (10−11 M R5020) activated SUMO-deficient (20-fold) but not wt PR-B (Daniel et al., 2007a). Thus, K388R PR-B is transcriptionally hyperactive relative to wt PR-B, while phospho-mutant S294A PR-B functions as a weak transcription factor; sumoylation at PR Lys388 results in transcriptional repression and phosphorylation (Ser294) antagonizes this effect. These findings led Daniel et al. to predict that PR Ser294 phosphorylation negatively regulates sumoylation at PR Lys388 (Daniel et al., 2007a). In in vitro SUMO assays, PR-B was rapidly sumoylated in response to both progestin and antiprogestin (Chauchereau et al., 2003; Daniel et al., 2007a). In further studies, cells treated with EGF prior to ligand exhibited increased ERK1/2 activation, phosphorylation of Ser294, and decreased PR-B sumoylation (Daniel et al., 2007a). EGF naive cells were not persistently phosphorylated at Ser294 and retained PR-B sumoylation in the presence of ligand. Daniel et al. provided additional data showing that forced phosphorylation at PR Ser294, by expression of constitutively active MEK1-R4F or CDK2-TY, blocked ligand-induced PR sumoylation, creating a hyper-active receptor; Ser294-dephosphorylated PR remained heavily sumoylated (Daniel et al., 2007a). Thus, dynamic changes in PR sumoylation/desumoylation provide a phosphorylation-dependent mechanism for regulation of PR transcriptional de-repression. Recently, Zhang et al. (2007) demonstrated the negative regulation of PR Lys388 sumoylation by CUEDC2, a protein that promoted ubiquitination at this site and PR degradation.
As measured in 2xPRE-luciferase reporter assays, sumoylated (S294A) PR are transcriptionally repressed relative to SUMO-deficient (i.e. phosphorylated wt or K388R) receptors. However, on endogenous promoters, PR transcriptional activity is differentially sensitive to Ser294 phosphorylation/Lys388 desumoylation. For example, the endogenous PR target gene, HB-EGF, was up-regulated 5-fold by sumo-deficient K388R PR-B compared to wt PR-B, following progestin treatment (Daniel et al., 2007a). In addition, the transcriptional activity of wt PR-B was further elevated by the addition of SENP-1, a PR-desumoylating enzyme. Interestingly, IRS-1 expression was insensitive to progestin, but dependent upon PR-B expression and Ser294 phosphorylation (Qiu and Lange, 2003). Similarly, this gene is up-regulated in breast cancer cells stably expressing sumo-deficient PR (unpublished observation). In contrast, other endogenous classical PR target genes, such as Tissue Factor (TF, F3) (Kato et al., 2005), MUC1 (Brayman et al., 2006) and SGK (Jeong et al., 2005), are up-regulated similarly by both wt PR-B and sumo-deficient K388R PR-B (Daniel et al., 2007a and unpublished observations). These data indicate that the degree of PR-B Ser294 phosphorylation or Lys388 sumoylation can dramatically affect transcriptional activation at a subset of endogenous PR-regulated promoters while having no effect on others (Fig. 2).
The basis for this selectivity is unknown, but likely involves the SUMO-dependent recognition of complex sequences within PRE-containing promoter regions, and their associated proteins, that are not easily modeled using traditional PR reporter genes. For example, reporter-gene assays demonstrated that promoter composition dramatically affects transcriptional regulation by sumoylation of glucocorticoid receptors (GR), which exert transcriptional repression of artificial promoters containing 2–4 tandem, but not a single, GRE element (Holmstrom et al., 2003). Similarly, differences in promoter composition of endogenous PR target genes may contribute to their recognition by sumoylated PR. The HB-EGF promoter contains at least 22 PRE half-sites (Daniel et al., 2007a) and is quite sensitive to SUMO-deficient PR (Daniel et al., 2007a). The MUC1 promoter, containing few functional PRE half-sites (Brayman et al., 2006), and the SGK promoter, containing only one non-canonical GRE (Itani et al., 2002) and multiple PRE half-sites, appear “blind” to sumoylated PR (Daniel et al., 2007a and unpublished observations). Studies on the regulation of PR sumoylation and mechanisms of transcriptional repression at selected endogenous promoters are currently underway.
Differential transcriptional regulation by epigenetically modified PRs at endogenous target promoters is functionally significant with regard to understanding PR biochemistry. However, it is important to connect PR gene selectivity to biologically relevant consequences in breast cancer cells. To model anchorage-independent cell growth, Daniel et al. utilized T47D breast cancer cells stably expressing wt, phospho-mutant S294A or sumo-deficient K388R PR-B grown in soft agar (Daniel et al., 2007a). Cells expressing wt and K388R PR-B had similar levels of anchorage independent growth when treated with 10−8 M R5020. However, the cells expressing K388R PR-B responded to sub-physiological progestin concentrations as shown by significantly more anchorage-independent cell growth, relative to cells expressing wt PR-B; cells expressing S294A PR-B failed to develop significantly increased colony numbers. This suggests that PR-B promoter selectivity, regulated by a balance between the degree of PR Ser294 phosphorylation (i.e. desumoylated) and Lys388 sumoylation (dephosphorylated), can dramatically alter genetic expression programs that confer changes in anchorage-independent breast cancer cell growth.
4. Regulation of PR by phosphorylation in breast cancer
Progestins increase breast cancer risk when taken as part of hormone replacement therapy (Beral, 2003; Chlebowski et al., 2003) and progestins have also been shown to modify mammary epithelial progenitor cell numbers (Horwitz et al., 2008). These studies implicate PR as a potential driver of breast cancer progression. The data reviewed herein suggest that PR transcriptional activity in breast cancer is regulated by phosphorylation events initiated by both growth factor pathways and/or PR ligands. High intracellular kinase activity is common in breast cancers (Tsutsui et al., 2002). PR is thus predicted to be heavily phosphorylated, under-sumoylated, and hyperactive on selected promoters. Phosphorylated PRs are able to drive S phase entry (Faivre et al., 2008; Skildum et al., 2005) and anchorage-independent growth (Daniel et al., 2007a,b; Faivre and Lange, 2007) of breast cancer cells in response to low progestin concentrations. These hyperactive receptors are likely destabilized due to increased phosphorylation-dependent ubiquitinylation (Lange et al., 2000) and may therefore be difficult to detect in breast tissues by current clinical methods. We caution that rapidly turning over, phosphorylated PRs may contribute to breast cancer phenotypes in tumors categorized as PR low or null.
5. Conclusions
Collectively, the available data indicate that phosphorylation of steroid hormone receptors serves to shuttle subsets of the pool of differentially modified receptor species in the cell to different promoters instead of the previous notion of fine tuning global receptor activity at “uniform” (i.e. model reporter gene) promoters. This is to suggest that receptors phosphorylated on Ser345 bind Sp1 and localize to promoters such as p21 or EGFR, while receptors phosphorylated on Ser294 may be shuttled more efficiently to separate promoters including that of HB-EGF (Fig. 2). Still other promoters are insensitive to these modifications, such as MUC1, SGK, and Tissue Factor. In order to determine the specific transcriptional consequences of phosphorylation at these endogenous regulatory sites we must move away from experiments using generic Hormone Response Element (HRE) reporters and examine endogenous gene targets for changes in subsets of hormone responsive genes. For example, liganded S345A PR-B mutant receptors are comparable to wt PR-B receptors in 2xPRE-luciferase reporter assays, while unresponsive to progestins on a subset of non-classical progestin responsive genes (i.e. Sp1-containing endogenous promoter contexts). Alternatively, when stably expressed in breast cancer cells, S294A PR-B receptors are weak transcription factors on transiently introduced 2xPRE-luciferase constructs, yet these mutant receptors can activate transcription from the endogenous SGK promoter (unpublished observation).
Phosphorylation-dependent regulation of PR promoter selectivity provides a mechanism for cells to integrate a variety of external signals and select gene programs accordingly. In this manner, phospho-PRs act as sensors for growth factor-initiated pathways and couple them to hormonal controls. For example, in breast cancer models, progesterone alone elicits a weaker growth response in soft agar relative to progesterone plus EGF; the effects of EGF in this assay are abolished by mutation of PR Ser294 (Daniel et al., 2007b). Thus, PR is functioning as a node of integration for EGFR signaling. Progesterone-initiated rapid signaling events, growth factor signaling inputs, and PR mediated genomic actions presumably converge to produce precisely timed proliferation in the mammary gland. Any and all of these pathways can be corrupted in breast cancer. Targeting them collectively using selective protein kinase inhibitors and antiprogestins is likely to be a productive strategy.
Acknowledgments
Studies on the role of progesterone and breast cancer were funded by NIH/NCI grants (to C.A. Lange) R01 CA123763 (formerly R01 DK53825) and R21 CA116790.
References
- Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J. Biol. Chem. 2002;277:33950–33956. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J. Steroid Biochem. Mol. Biol. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Bossis G, Malnou CE, Farras R, Andermarcher E, Hipskind R, Rodriguez M, Schmidt D, Muller S, Jariel-Encontre I, Piechaczyk M. Down-regulation of c-Fos/c-Jun AP-1 dimer activity by sumoylation. Mol. Cell. Biol. 2005;25:6964–6979. doi: 10.1128/MCB.25.16.6964-6979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol. Endocrinol. 2006;20:2278–2291. doi: 10.1210/me.2005-0343. [DOI] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauchereau A, Amazit L, Quesne M, Guiochon-Mantel A, Milgrom E. Sumoylation of the progesterone receptor and of the steroid receptor coactivator SRC-1. J. Biol. Chem. 2003;278:12335–12343. doi: 10.1074/jbc.M207148200. [DOI] [PubMed] [Google Scholar]
- Cheng J, Bawa T, Lee P, Gong L, Yeh ET. Role of desumoylation in the development of prostate cancer. Neoplasia. 2006;8:667–676. doi: 10.1593/neo.06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy post-menopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol. Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol. Endocrinol. 2007a;21:2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007b;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol. Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol. Cell. Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat. Rev. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15758–15763. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5774–5779. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LG, Thompson KL, Xu J, Gill GN. Identification and characterization of a regulated promoter element in the epidermal growth factor receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1990;87:7536–7540. doi: 10.1073/pnas.87.19.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Xu YH, Stratton RH, Roe BA, Merlino GT, Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1985;82:4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail PM, Amato P, Soyal SM, DeMayo FJ, Conneely OM, O'Malley BW, Lydon JP. Progesterone involvement in breast development and tumorigenesis—as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids. 2003;68:779–787. doi: 10.1016/s0039-128x(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Itani OA, Liu KZ, Cornish KL, Campbell JR, Thomas CP. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5′-flanking region. Am. J. Physiol. 2002;283:E971–E979. doi: 10.1152/ajpendo.00021.2002. [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Schittone SA, Richer JK, Horwitz KB. Progesterone-independent effects of human progesterone receptors (PRs) in estrogen receptor-positive breast cancer: PR isoform-specific gene regulation and tumor biology. Mol. Endocrinol. 2005;19:574–587. doi: 10.1210/me.2004-0287. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–3505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- Kato S. Estrogen receptor-mediated cross-talk with growth factor signaling pathways. Breast Cancer. 2001;8:3–9. doi: 10.1007/BF02967472. [DOI] [PubMed] [Google Scholar]
- Kato S, Pinto M, Carvajal A, Espinoza N, Monso C, Sadarangani A, Villalon M, Brosens JJ, White JO, Richer JK, et al. Progesterone increases tissue factor gene expression, procoagulant activity, and invasion in the breast cancer cell line ZR-75-1. J. Clin. Endocrinol. Metab. 2005;90:1181–1188. doi: 10.1210/jc.2004-0857. [DOI] [PubMed] [Google Scholar]
- Labriola L, Salatino M, Proietti CJ, Pecci A, Coso OA, Kornblihtt AR, Char-reau EH, Elizalde PV. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol. Cell. Biol. 2003;23:1095–1111. doi: 10.1128/MCB.23.3.1095-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CA. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol. Endocrinol. 2004;18:269–278. doi: 10.1210/me.2003-0331. [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16:301–307. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Melchior F. SUMO—nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MR, Zhou JL, Blankenship KA, Strobl JS, Edwards DP, Gentry RN. A sequence in the 5′ flanking region confers progestin responsiveness on the human c-myc gene. J. Steroid Biochem. Mol. Biol. 1997;62:243–252. doi: 10.1016/s0960-0760(97)00036-8. [DOI] [PubMed] [Google Scholar]
- Moore NL, Narayanan R, Weigel NL. Cyclin dependent kinase 2 and the regulation of human progesterone receptor activity. Steroids. 2007;72:202–209. doi: 10.1016/j.steroids.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J. Biol. Chem. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol. Cell. Biol. 2004;24:10542–10557. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, et al. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol. Cell. Biol. 2005;25:4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Lange CA. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J. Steroid Biochem. Mol. Biol. 2003;85:147–157. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol. Cell. Biol. 2001;21:6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skildum A, Faivre E, Lange CA. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol. Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- Tseng L, Tang M, Wang Z, Mazella J. Progesterone receptor (hPR) upregulates the fibronectin promoter activity in human decidual fibroblasts. DNA Cell Biol. 2003;22:633–640. doi: 10.1089/104454903770238102. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res. Treat. 2002;71:67–75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- Wu H, Sun L, Zhang Y, Chen Y, Shi B, Li R, Wang Y, Liang J, Fan D, Wu G, et al. Coordinated regulation of AIB1 transcriptional activity by sumoylation and phosphorylation. J. Biol. Chem. 2006;281:21848–21856. doi: 10.1074/jbc.M603772200. [DOI] [PubMed] [Google Scholar]
- Yang SH, Jaffray E, Hay RT, Sharrocks AD. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol. Cell. 2003;12:63–74. doi: 10.1016/s1097-2765(03)00265-x. [DOI] [PubMed] [Google Scholar]
- Zhang PJ, Zhao J, Li HY, Man JH, He K, Zhou T, Pan X, Li AL, Gong WL, Jin BF, et al. CUE domain containing 2 regulates degradation of progesterone receptor by ubiquitin-proteasome. EMBO J. 2007;26:1831–1842. doi: 10.1038/sj.emboj.7601602. [DOI] [PMC free article] [PubMed] [Google Scholar]