Abstract
Mammalian protein production platforms have had a profound impact in many areas of basic and applied research, and an increasing number of blockbuster drugs are recombinant mammalian proteins. With global sales of these drugs exceeding US$120 billion per year, both industry and academic research groups continue to develop cost effective methods for producing mammalian proteins to support preclinical and clinical evaluations of potential therapeutics. While a wide range of platforms have been successfully exploited for laboratory use, the bulk of recent biologics have been produced in mammalian cell lines due to the requirement for post translational modification and the biosynthetic complexity of the target proteins. In this review we highlight the range of mammalian expression platforms available for recombinant protein production, as well as advances in technologies for the rapid and efficient selection of highly productive clones.
Keywords: protein production, mammalian expression, therapeutics, clonal selection
Introduction
The ability to produce mammalian proteins in recombinant systems has had a profound impact in many areas of basic and applied research, as well as the biotech sector. While academic research groups require recombinant mammalian proteins for functional analysis (e.g. cellular signaling pathways) and high resolution structure determination, the biotech sector has heavily invested in the production of protein therapeutics (i.e. biologics), as a relatively new and transformative approach to treating human diseases. An increasing number of blockbuster drugs are recombinant mammalian proteins and the United States Food and Drug Administration (FDA) has approved over 100 recombinant protein therapeutics to date [1]. With this success, both industry and academic research groups continue to develop cost effective methods for producing mammalian proteins to support pre-clinical and clinical evaluations of potential therapeutics.
The need for multi-milligram quantities of recombinant mammalian protein in the academic sector is, in part, driven by focused and team-based structural biology initiatives, such as the New York Structural Genomics Research Consortium (NYSGRC) [2] [http://www.nysgrc.org/psi3-cgi/index.cgi], as well as pre-clinical research. The screening of 10–20 constructs may be required to obtain the multi-milligrams yields needed for successful structure determination [3]. As our understanding of cellular signaling pathways grow, so does the need for structural characterization of the signaling components and more importantly, structures of the relevant multi-component protein assemblies. As the tools for producing these mammalian proteins improve so does the complexity and value of the structures determined; a recent report describing the highly sought after ternary complex of the Wnt pathway proteins, R-spondin 1, RNF43 and LGR5, highlights this point [4].
By far the greatest demand for recombinant mammalian proteins is for therapeutic development and applications. The production of recombinant proteins for biopharmaceutical use is a multi-billion dollar industry, with global sales over US$120 billion per year and an anticipated increase to US$150 billion by 2015 [5]. Of the top nine classes of biologic drugs sold in 2011, monoclonal antibodies (mAbs) command the highest sales (Figure 1) and this trend is growing [6].
Figure 1.
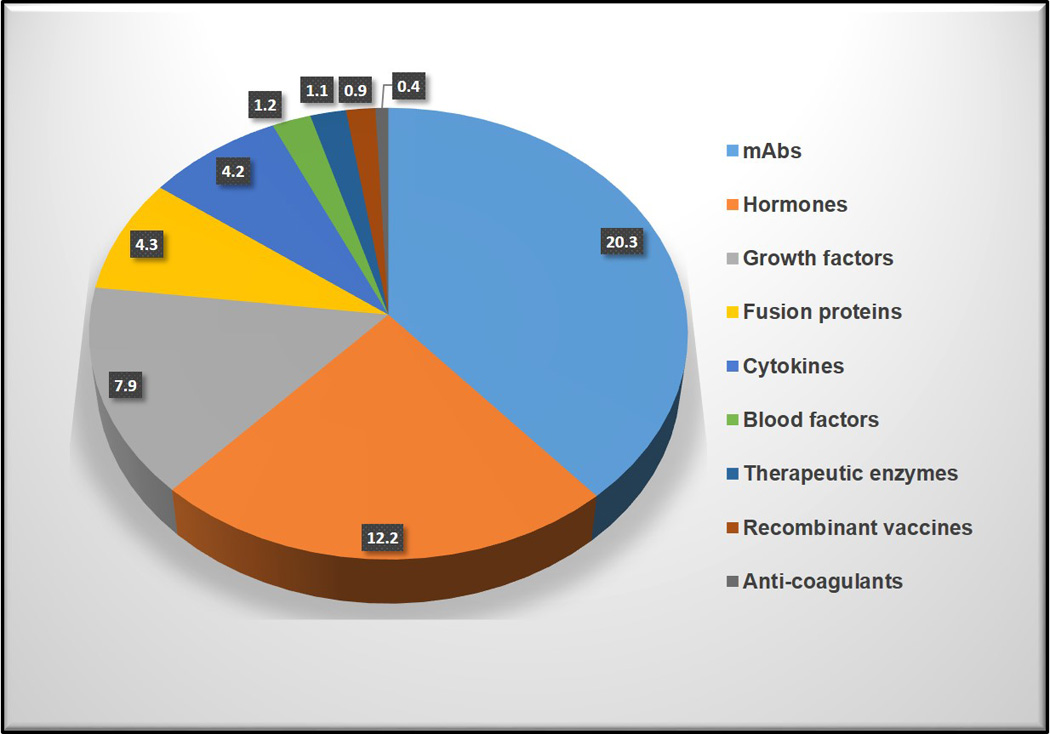
US sales of top nine classes of biologic drugs in 2011. Adapted from Aggarwal et. al. [6]
While Escherichia coli (E. coli), yeast and insect cell lines are still the preferred platforms for many protein expression groups, mammalian proteins often require mammalian cells for optimum yields and activity. While a wide range of platforms have been successfully exploited for laboratory use, the bulk of recent biologics have been produced in mammalian cell lines due to the requirement for post translational modification and the biosynthetic complexity of the target proteins [1]. In the case of monoclonal antibodies, Chinese Hamster Ovary (CHO) and NS0 (mouse myeloma) cells are the most commonly used lines [7,8]. With the substantial growth being realized in the area of biologics, there is a continuing demand for new and enhanced mammalian expression platforms. The goal of this review is to highlight the range of mammalian platforms available for recombinant protein production as well as advances in technologies for the rapid and efficient selection of highly productive clones.
Expression platforms
CHO
The workhorse of mammalian protein production (especially at industrial scale) is the CHO cell line, isolated by Theodore Puck in the late 1950’s [9]. Initially selected for radiation cytogenetic studies due to their low chromosome number (2n = 22), CHO cells have proven to be a hardy and reliable cell line in culture. Since the commercial introduction of human tissue plasminogen activator (tPA) as the first recombinant therapeutic protein produced from mammalian cells [10], the annual global revenue of products from CHO cells has increased to more than US$100 billion and continues to grow [11]. The wide spread success of the CHO platform is due to its unparalleled adaptability allowing for growth of these cells at high densities in suspension cultures and ease of adaptation to serum free conditions. There has been a great deal of improvement in the quality and availability of chemically defined, serum free media that is devoid of animal-derived materials. These tailored media are more cost effective, as they do not require supplementation with fetal calf serum; safer, as there is less risk of viral and prion contamination from bovine serum; and have simplified downstream processing requirements, as they contain fewer protein contaminants. In addition, a study in 1989 tested 44 human pathogens (including human immunodeficiency virus (HIV), influenza, polio, herpes and measles), and found the majority of them do not replicate in CHO cells, thus making CHOs ideal from a regulatory standpoint [12]. However, the adaptability of the CHO line also has its drawbacks, as each production target requires the selection of clones that exhibit the necessary phenotypic properties, including product quality/uniformity, doubling time and long term viability, under bioprocess conditions. Even when an appropriate CHO production clone has been identified, phenotypic drift (i.e. changes in the previously selected characteristics) is not uncommon and remains a challenge [13].
While enhancing clonal stability (i.e. product uniformity) is an area of intense study, the most significant improvements to CHO culture have resulted from the optimization of media, feeding strategies and downstream processing. These improvements have resulted in yields ranging between 2–6 g/L for antibody products [14]. A particular focus of product optimization in CHO cells is glycosylation, as it has been shown that variation in glycosylation patterns affects product stability and function, and non-natural glycoforms can be immunogenic [15–17]. This is particularly true of the terminal galactose-α-1,3-galactose (α-Gal) epitope, which has been demonstrated to be added to proteins produced in murine cell lines, and is capable of inducing an immune response in humans [18]. The importance of glycoform profiles in therapeutics has been highlighted by the adverse clinical effects associated with an induced IgE-mediated anaphylaxis response in patients treated with the commercial antibody Erbitux (cetuximab), which was manufactured in a murine myeloma cell line and possessed α-Gal epitopes [18,19]. It was thought that CHO cells lacked the biosynthetic machinery to produce the α-Gal epitope, but this assumption has been brought into question by reports that CHO cells can add the α-Gal antigen to recombinant products, as has been observed for Orencia (abatacept) [18]. Therefore, it is essential that CHO production clones be carefully monitored for product uniformity and safety in terms of glycosylation profiles.
The genomic variability of CHO cells and the fact that they are functionally hemizygous for many genes [20,21] also has certain advantages, as it has allowed for the isolation of mutant lines with deficiencies in metabolic enzymes. These mutants are dependent on certain nutrients for survival, making them ideal for the generation of producer lines by using the deficiency as a selection marker. Mutants of the enzyme dihydrofolate reductase (DHFR) were generated in this manner [22] and will be discussed below. To support further development of CHOs for protein production, a genome sequencing project was initiated in 2002 as a collaboration between the University of Minnesota and the Bioprocessing Technology Institute of Singapore (A*STAR). This collaboration led to two cDNA libraries being constructed from three CHO cell lines, grown under different conditions, corresponding to over 4,000 expressed sequence tags (ESTs) [23]. This initial study led to further sequencing efforts under the auspices of the Consortium on Chinese Hamster Ovary Cell Genomics in partnership with the Society for Biological Engineers (SBE), with the goal being the identification of genetic markers associated with high productivity [12].
While there is no doubt that CHO cells will continue to be used and developed for biopharmaceutical production, the push for generating more complex human proteins, with a better safety profile, has led to the further development of existing lines and the generation of novel human cell lines that we believe will be the platforms of the future.
HEK 293
Developed almost two decades after the CHO cell line, the 293 cell line was the first human line to be transformed using sheared adenovirus DNA fragments of the Ad5 serotype [24]. Transformation of the human embryonic kidney (HEK) cells by viral DNA was performed with a calcium transfection technique developed by Graham and van der Eb [25,26]. Since its development, the 293 cell line has become one of the most commonly used human cell lines for protein production. There have been many variants of the original line, such as 293N3S developed for suspension growth in bioreactors for adenovirus production [27] and the 293S line that was adapted to grow under serum free conditions [28]. In order to improve transient gene expression (discussed below), two additional lines were developed, the 293-T line expressing the SV40 large T-antigen [29] and the 293-E line expressing the Epstein-Barr virus EBNA1 protein [30]. The latter two lines support the episomal replication of plasmids containing either the SV40 origin of replication or the EBV oriP, respectively, thereby prolonging the expression of the target gene after transient transfection. While CHO cells have been the workhorse of recombinant protein production, the 293 line has grown in prominence with the realization that proteins produced in HEK cells are a closer match to naturally occurring human proteins in terms of post translational modification and function [31]. Improvements in culture processes has resulted in 293-based platforms capable of supporting antibody yields exceeding 1 g/L [32]. More recently the HKB11 line has been developed which is a hybrid between the HEK 293S line and a human B cell line, 2B8 (derived from a Burkitt’s lymphoma line) [33]. This line combines features of the parental lines, resulting in a system that is easy to transfect (a characteristic of 293s), with the capacity to secrete large amounts of protein (a characteristic of B cells). It’s utility has been amply demonstrated in the case of human coagulation factor VIII (which contains 25 potential N-linked glycosylation sites, 6 tyrosine sulfation sites and 7 disulfide bonds) with yields that are about 8-fold higher than the parental 293 line [34].
PER.C6
The PER.C6 (Crucell) line was originally generated from human retinoblast cells immortalized by transfection with an E1 minigene for the cost-effective production of safe, clinical-grade recombinant adenovirus vectors [35]. However this cell line also has several features that make it ideal for recombinant protein production: PER.C6 were generated in compliance with Good Laboratory Practice and has been extensively documented, the cell banks meet all pertinent United States and European Economic Community regulatory requirements and importantly (from a production standpoint), they can be grown in suspension to high cell densities (up to 1X107 cells/ml) in serum-free medium. These cells were shown to readily produce 300–500 mg/L of IgG without any media or feed optimization [36].
CAP/CAP-T
Similar to the PER.C6 line, CEVEC’s Amniocyte Production (CAP) cell line was originally developed for adenovirus production by transforming primary human amniocytes (obtained transabdominally by amniocentesis) using the E1 functions of Ad5 [37]. A variant of this line, CAP-T, was generated which constitutively expresses the SV40 large T antigen in order to make them suitable as a host for transient gene expression using plasmids carrying the SV40 ori; this line is capable of expressing and secreting previously difficult-to-express protein targets [38]. It is hypothesized that the stem cell-like origin of CAP cells may lead to a different or larger repertoire of processing enzymes and chaperones compared to more differentiated production cell lines. This hypothesis is supported by the example of the bone morphogenetic protein (BMP) antagonist protein which failed to be secreted from HEK 293 cells (where it was located in the insoluble fraction) but was successfully secreted into the medium by CAP cells. The yields of a few other test proteins (secreted embryonic alkaline phosphatase, SEAP and a kinase receptor extracellular domain) seem to indicate that the CAP-T cells are at least as good as the HEK 293-E cells, if not a little better, and superior to CHO cells [38].
Advances in gene delivery/integration
Transient transfection followed by drug selection has been the traditional method for DNA delivery and integration for creating producer lines. While successful, this is often a slow (4–6 months) and tedious process and the last decade has seen an increase in novel and sophisticated approaches for enhancing the efficiency of delivery and reducing the timeline for obtaining a producer cell line (Figure 2).
Figure 2.
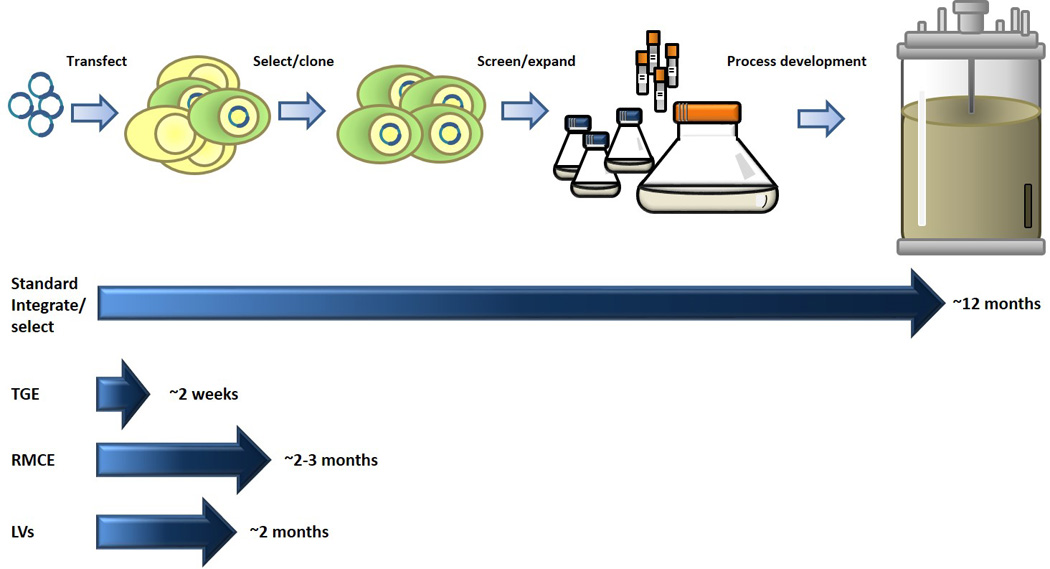
Timelines for gene delivery and production methods. Standard protocol for generating stable producer lines and scaling up to reactor size shown on top. This process is compared to transient gene expression (TGE), Recombinase mediated cassette exchange (RMCE) and Lentivirus driven integration (LVs).
Transient gene expression (TGE)
One strategy to accelerate the protein production process is to eliminate the drug selection step by performing transient transfections at scale. Using this approach, clonal cell lines are not necessary as cells are directly cultured (and transfected) at the production scale and therefore do not need to be cultivated for long periods of time. The use of suspension adapted HEK and CHO lines, along with optimized commercial media, as well as the use of inexpensive transfection agents (such as calcium phosphate and polyethylenimine, PEI) has made large scale transient transfection an attractive and successful approach (reviewed in [39,40]). Transfection at scale implies culture volumes ranging from several hundred milliliters in shake flasks or square bottles [41] to several hundred liters in stirred tank bioreactors, orbital shakers [42] or Wave™ reactors [43].
One of the drawbacks of this approach is the need for multi-milligram quantities of recombinant plasmid DNA; however, obtaining decigram quantities of plasmid DNA from a few liters of E. coli is fairly routine [39]. It should be noted that the quality of the DNA used for transfection can be an important factor; while the quality may not be of critical importance for most TGE experiments, it can be essential for the production of clinical grade proteins where endotoxin and other contaminants need to be minimized or eliminated [44,45].
As for the transfection reagent, 25 kDa linear PEI is the most commonly used in TGE approaches and has a long and successful track record [46–48]. While most transfections are performed at cell densities of 1X105–1X106 cells/ml, recent reports have shown enhanced production with high density transfection. For example, CHO cells transfected at 4X106 cells/ml produced up to 250 mg of antibody at scale in 14 days [49] and HEK 293 cells transfected at densities as high as 2X107 cells/ml resulted in a doubling of product titer [50]. Of note, the latter study involved direct transfection, in which DNA and PEI were added directly to the culture without a priori complex formation, resulting in improved ease of handling and reduced transfection variability.
Once a transient production cell line has been established, several strategies are available for maximizing protein expression. These approaches, while useful in stable producer lines, are especially important in a transient setting where the culture period is generally limited to 10–14 days. One method involves the over-expression of anti-apoptotic proteins, such as bcl-2 family members [51–53] and Bcl-x(L) [54,55], which leads to higher viability and improved yields. Another approach is to induce cell cycle arrest thereby improving productivity; this strategy can be accomplished either chemically using anti-mitotic agents (such as hydroxyurea, nocodazole, colchicine, paclitaxel or vinblastine) [56] or genetically by targeting cell cycle inhibitors such as p18, p21 or p27Kip1 [39]. Growth factors such as acidic Fibroblast Growth Factor (aFGF) or recombinant insulin-like growth factor (LR3-IGF) can also increase cell densities and improve yield. DNA methyltransferase inhibitors and histone deacetylase inhibitors such as valproic acid and sodium butyrate have also been shown to improve recombinant protein yields in transient settings [57]. Ultimately, a combination of factors may be necessary to achieve the desired enhancement, such as those utilized in the ‘XLG protocol’ that involves over-expression of p18 and p21, expression of aFGF and addition of Valproic Acid. These modifications result in antibody production that exceeds 1 g/L in HEK 293-E cells, a 27-fold improvement over standard approaches [32].
Recombinase Mediated Cassette Exchange (RMCE)
Integration of exogenous DNA into a host chromosome is for the most part a random event. As a result, most integrations are unproductive as only a small proportion of a cell’s genome (~0.1%) is actively transcribed. Therefore, if the recombinant construct can be targeted to an active and stable region (hotspot) of the genome then even a single integration at such a favorable site can lead to higher production yields than multiple integrations at sub-optimal regions of the genome [58]. This idea led to the development of RMCE, initially as a means of developing transgenic mouse models (reviewed in [59]), but more recently for the generation of stable producer lines for recombinant proteins. In brief, this strategy involves the development of a master cell line that contains a single integration of a reporter gene at a hotspot flanked by integration target sequences such as Flp recognition target sites (FRT) or loxp sites (for the Cre system). The gene of interest can then be introduced into this line in the presence of the appropriate recombinase leading to the exchange of the reporter gene with the target gene, “Flp-out” or “Cre-out” [60]. The use of adeno- or non-integrating lentivirus has also been shown to facilitate this reaction [61,62]. While this system promises to improve the speed and efficiency with which producer lines can be generated, the actual improvement in yields reported to date has only been moderate [63].
Viral delivery
Lentiviral vectors (LVs) derived from human immunodeficiency virus type-1 (HIV-1) have a long history of efficient gene delivery in gene therapy applications [64]. Given the efficiency with which LVs are capable of transducing a broad range of cell types, it is not surprising that LVs have been recently used as a gene delivery tool for protein production. The ‘Daedalus’ system has shown promising results for the rapid generation of stable producer lines in HEK 293 Freestyle cells (Invitrogen), with yields of 20–100 mg/L reported for a variety of secreted proteins without the need for clonal selection [65]. LVs have also been used in the CHO platform where certain clones were shown to produce as much as 200 mg/L of the human tumor necrosis factor receptor-Fc fusion protein [66]. One of the drawbacks of the LV system is the modest packaging size of the lentivirus capsid (~10 kilobases), which may limit the maximum size of the recombinant protein being expressed.
Enhancement of protein production using UCOE, SAR and MAR elements
Major factors contributing to the loss of productivity over time are the positional effects that an integrated gene experiences in the context of the host genome. To overcome the effects of silencing and the associated loss in protein yield, several new locus control elements have been developed, including scaffold or matrix attachment regions (S/MARs) and Ubiquitous Chromatin Opening Elements (UCOEs) [67]. S/MARs are DNA sequences which serve as attachment points to the nuclear matrix, thus maintaining a transcriptionally active chromatin structure. These sequences have also been shown to enhance DNA demethylation, making genes more accessible to the transcriptional machinery [68,69]. MARs have also been shown to serve as binding sites for transcription factors like CCCTC binding factor (CTCF) and nuclear matrix proteins (NMP) that enhance gene expression [70–72]. The β-interferon SAR, human β-globin MAR and chicken lysozyme MAR have been shown to promote stable protein expression (as long as 6 months) and increase the occurrence of high producing clones, thereby reducing the need for clonal selection [73–76].
UCOEs (Merck Millipore) are methylation-free CpG-rich sequences that help maintain chromatin in an ‘open’ conformation, resulting in significantly reduced silencing. These sequences enhance protein expression over extended periods of time in in vitro transfection experiments [77,78], as well as in gene therapy settings [79,80]. While effective UCOE elements tend to be in the 2–4 kb range, it is notable that the Daedalus system uses the smallest UCOE sequence described to date (0.7 kb), with activity that is equivalent to the larger fragments [65]. The use of UCOEs in producer line development has shown a 3–6 fold improvement in protein yield over several months of culture [65,81].
Advances in selection methodology
The traditional methods of integration followed by drug selection for generating stable producer lines can be tedious and time consuming, often taking several months. Further, increasing the dose of selection agent in order to select for higher producing clones can lead to a loss of successful clones due to the slower growth of these cells at high drug concentration. As a result, there have been considerable efforts to increase the speed and efficiency of selection methods and to enhance the final producer yields.
Destabilized selection markers
An attractive alternative to increasing selection pressure by increasing the concentration of the drug is to attenuate the activity of the selection marker so that high producer lines are still efficiently selected at lower doses of the drug. The hope is to reduce the undesirable side-effects of the drug (e.g. slow cell growth) while maintaining sufficient selection pressure to obtain highly producing clones. This strategy is exemplified by the introduction of mutations into the neomycin phosphotransferase II selection marker (Neo), which results in reduced affinity for the selection drug neomycin. This approach led to an improvement in monoclonal antibody production from between 6 to 17 fold [67,82,83]. Alternatively, mutation of the Neo selection gene can be avoided by placing the gene under the control of a weak promoter (Herpes simplex virus thymidine kinase), which reduces transcription and availability of Neo [84]. Another approach is to re-engineer the resistance sequence to contain the least preferred codons of the host organism (i.e., ‘de-optimization’) in order to lower translational efficiency and reduce the cellular concentration of the resistance protein (e.g., dihydrofolate reductase; DHFR) [85].
One of the most popular selection systems in current use is methotrexate (MTX) amplification with DHFR-deficient CHO cells [12,14,63,67,86]. The DHFR-deficient CHO cells (CHO-DG44) were created by gamma-ray induced mutation of CHO cells and require the presence of glycine, hypoxanthine and thymidine to survive [22,87]. Once the cells are transfected with the gene of interest (linked to the DHFR selection gene), hypoxanthine and thymidine are withdrawn from the culture medium to select for the successful transfectants. Further selection pressure is introduced by the addition of MTX to the culture which acts as a competitive inhibitor of DHFR and promotes MTX amplification, selecting for cells with higher copy numbers (and therefore productivity) of the DHFR gene and associated target gene [88]. In order to weaken the selection marker, and therefore increase stringency, Yap and colleagues investigated destabilizing the mRNA, as well as the DHFR protein [89]. Their strategy was to introduce short AU rich elements (AREs) into the mRNA which constitutively destabilizes the mRNA. In a further modification, the murine ornithine decarboxylase (MODC) PEST sequence was added to the dhfr gene, resulting in higher turnover of the protein. The authors tested this strategy with the expression of interferon gamma (IFNγ) and found that 1.7-, 6.6- and 13.3-fold improvements in specific IFNγ productivities were obtained with the application of ARE, MODC PEST, and both ARE and MODC PEST, respectively. In an attempt to more closely link the gene of interest and selection marker (thereby preventing the possibility of gene fragmentation and loss of the target gene), Lam and colleagues introduced the dhfr gene directly downstream of the gene of interest using an Internal Ribosome Entry Sequence (IRES) [90]. This strategy allowed for both genes to be translated off of the same RNA; in addition, the stringency of selection was further increased by adding the PEST sequence to the dhfr gene.
The glutamine synthetase (GS) system is another method of gene amplification, which has been successfully used with GS-negative NS0 cells [91], as well as GS-expressing CHO cells [92]. The CHO cells, containing an endogenous GS gene, need to be treated with the GS enzyme inhibitor methionine sulfoximine (MSX) to suppress the activity of endogenous GS. Increasing levels of MSX can then be used to select for clones with a high copy number of the selection gene and flanking target gene. This method has been used to drive target gene copy number in CHO cells to as many as 200 copies [93]. A fortuitous advantage of this system is that these cells do not require glutamine supplementation in the media, and as a result the culture does not accumulate high levels of toxic ammonia. The GS system has been recently enhanced with the development of a GS-knockout CHO line (CHOK1SV) that led to a significant increase in selection stringency and a six-fold improvement in the efficiency of identifying similar numbers of highly productive cell lines (compared to the standard protocol) for a given recombinant monoclonal antibody [94].
Fluorescence-Activated Cell Sorting (FACS)-Based Screening
Flow cytometry is a powerful technology that allows for the rapid, objective and sensitive multiparametric analysis of cells [95]. In addition to its analytical capabilities, flow cytometry also allows for the separation of the analyzed cells. In a flow cytometer cells pass in a single stream, one by one, in front of a laser. The laser then interrogates the stream of cells for different morphologic, structural and fluorescent properties of the cell. At present as many as a dozen or more of these characteristics can be acquired for each cell passing through the cytometer, providing a wealth of information regarding the health and various other user-defined parameters of each cell. While most cytometers are devoted solely to analysis, many can also sort cells based on characteristics that the user would like to obtain, making the sorting of transfection positive cells (detected by an associated fluorescent reporter) routine. Recently, high-throughput cytometers, such as the HyperCyt (Intellicyt), have dramatically increased the speed with which cell populations can be interrogated for user-defined characteristics [96,97]. Flow cytometers can be used to sort cells at the single-cell level, depositing ~1 cell/well of a 96-well plate, or can be used for bulk sorting, such as the top 10% of Green Fluorescent Protein reporter positive cells; allowing for the creation of clonal or oligoclonal populations in a matter of hours.
A non-invasive technique termed ‘cold capture’ has recently been developed to detect the amount of recombinant protein being secreted by a producer line. In this approach cells are incubated on ice (4 °C) to slow the secretion process and a fluorescent antibody is used for the FACS-based detection of recombinant proteins ‘trapped’ on the cell surface [98]. The top FACS-isolated clones can show a five- to seven-fold increase in yield. Further work showed that the same process can be performed at room temperature (21 °C) and that use of phycoerythrin (PE) as a fluorophore for detecting the secreted protein was superior to fluorescein isothiocyanate (FITC) in terms of stability of the signal [99].
An alternate non-invasive strategy is the use of FITC-labeled methotrexate (F-MTX) to bind an intracellular DHFR selection marker [100]. This strategy allowed for the rapid isolation of gene amplified cells from a heterogeneous pool.
Summary
Given the rapid pace at which new cell lines, media formulations, gene delivery and clonal selection techniques are emerging, the production of proteins for basic research and pharmaceutical use will continue to benefit from enhanced efficiencies and reduced costs. While transient transfection has experienced a revival due to optimization of large scale and high density transfection techniques, alternate methods of gene delivery such as RMCE and viral platforms have increased the options available. When combined with sophisticated selection methods, these approaches readily enable the screening of large numbers of target variants for expression or the production of gram quantities of a therapeutic target. The advent of novel human production cell lines continues to push the boundaries of the targets that can be expressed, their quality and total yields. It would not be surprising if the reliance on CHO cells for therapeutic production starts to wane as pharmaceutical companies invest in and embrace these new cell lines; it is estimated that there are already more than a dozen PER.C6-based products in Phase I/II clinical trials [101].
Despite the increasing number of protein production platforms available for use, there is no ‘magic bullet’ capable of meeting all challenges. The idiosyncratic behavior of each protein and the eventual downstream applications, along with cost and ease of use, drive the decision towards one platform or another. The large-scale NYSGRC structure and function discovery program uses a combination of platforms (E. coli, insect and mammalian cells) to produce target proteins. While most groups cannot or will not take such a broad approach to protein production, the options within a given platform are continuously expanding. Therefore, a limitation of protein production, at the moment, seems to be associated with the complexity of targets that can be imagined rather than production techniques available.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grants GM094662, GM094665 and CA013330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhu J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012;30:1158–1170. doi: 10.1016/j.biotechadv.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Almo SC, Garforth SJ, Hillerich BS, Love JD, Seidel RD, Burley SK. Protein production from the structural genomics perspective: achievements and future needs. Curr. Opin. Struct. Biol. 2013:1–10. doi: 10.1016/j.sbi.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savitsky P, Bray J, Cooper CDO, Marsden BD, Mahajan P, Burgess-Brown N a, Gileadi O. Highthroughput production of human proteins for crystallization: the SGC experience. J. Struct. Biol. 2010;172:3–13. doi: 10.1016/j.jsb.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P-H, Chen X, Lin Z, Fang D, He X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013:1345–1350. doi: 10.1101/gad.219915.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler M, Meneses-Acosta a. Recent advances in technology supporting biopharmaceutical production from mammalian cells. Appl. Microbiol. Biotechnol. 2012;96:885–894. doi: 10.1007/s00253-012-4451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal SR. What’s fueling the biotech engine-2011 to 2012. Nat. Biotechnol. 2012;30:1191–1197. doi: 10.1038/nbt.2437. [DOI] [PubMed] [Google Scholar]
- 7.Birch JR, Onakunle Y. Biopharmaceutical proteins: opportunities and challenges. Methods Mol. Biol. 2005;308:1–16. doi: 10.1385/1-59259-922-2:001. [DOI] [PubMed] [Google Scholar]
- 8.Chu L, Robinson DK. Industrial choices for protein production by large-scale cell culture. Curr. Opin. Biotechnol. 2001:180–187. doi: 10.1016/s0958-1669(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 9.Puck T, Cieciura S, Robinson A. Genetics of somatic mammalian cells III. Long-term cultivation of euploid cells from human and animal subjects. J. Exp. Med. 1958 doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deschênes I, Finkle CD, Winocour PD. Effective use of BCH-2763, a new potent injectable direct thrombin inhibitor, in combination with tissue plasminogen activator (tPA) in a rat arterial thrombolysis model. Thromb. Haemost. 1998;80:186–191. [PubMed] [Google Scholar]
- 11.Jadhav V, Hackl M, Druz A, Shridhar S, Chung C-Y, Heffner KM, Kreil DP, Betenbaugh M, Shiloach J, Barron N, Grillari J, Borth N. CHO microRNA engineering is growing up: Recent successes and future challenges. Biotechnol. Adv. 2013 doi: 10.1016/j.biotechadv.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayapal K, Wlaschin K. Recombinant protein therapeutics from CHO cells-20 years and counting. Chem. Eng. Prog. 2007;103:40–47. [Google Scholar]
- 13.Jadhav V, Hackl M, Druz A, Shridhar S, Chung C-Y, Heffner KM, Kreil DP, Betenbaugh M, Shiloach J, Barron N, Grillari J, Borth N. CHO microRNA engineering is growing up: Recent successes and future challenges. Biotechnol. Adv. 2013 doi: 10.1016/j.biotechadv.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 15.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 16.Beck A, Wagner-Rousset E. Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr. Pharm. Biotechnol. 2008;9:482–501. doi: 10.2174/138920108786786411. [DOI] [PubMed] [Google Scholar]
- 17.Costa AR, Rodrigues ME, Henriques M, Oliveira R, Azeredo J. Glycosylation: impact, control and improvement during therapeutic protein production. Crit. Rev. Biotechnol. 2013;8551:1–19. doi: 10.3109/07388551.2013.793649. [DOI] [PubMed] [Google Scholar]
- 18.Bosques CJ, Collins BE, Meador JW, Sarvaiya H, Murphy JL, Dellorusso G, Bulik D a, Hsu I-H, Washburn N, Sipsey SF, Myette JR, Raman R, Shriver Z, Sasisekharan R, Venkataraman G. Chinese hamster ovary cells can produce galactose-α-1,3-galactose antigens on proteins. Nat. Biotechnol. 2010;28:1153–1156. doi: 10.1038/nbt1110-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold DF, Misbah S a. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha- 1,3-galactose. N. EnglJMed. 2008;358:2735. doi: 10.1056/NEJMc080834. author reply 2735-6. [DOI] [PubMed] [Google Scholar]
- 20.Chasin LA, Urlaub G. Chromosome-wide event accompanies the expression of recessive mutations in tetraploid cells. Science. 1975;187:1091–1093. doi: 10.1126/science.1167702. [DOI] [PubMed] [Google Scholar]
- 21.Simon AE, Taylor MW, Bradley WE, Thompson LH. Model involving gene inactivation in the generation of autosomal recessive mutants in mammalian cells in culture. Mol. Cell. Biol. 1982;2:1126–1133. doi: 10.1128/mcb.2.9.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urlaub G, Chasin L a. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc. Natl. Acad. SciUSA. 1980;77:4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wlaschin KF, Nissom PM, Gatti M, de L, Ong PF, Arleen S, Tan KS, Rink A, Cham B, Wong K, Yap M, Hu W-S. EST sequencing for gene discovery in Chinese hamster ovary cells. Biotechnol. Bioeng. 2005;91:592–606. doi: 10.1002/bit.20511. [DOI] [PubMed] [Google Scholar]
- 24.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 25.Graham FL, van der Eb AJ. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;54:536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- 26.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 27.Graham FL. Growth of 293 cells in suspension culture. J. Gen. Virol. 1987;68(Pt 3):937–940. doi: 10.1099/0022-1317-68-3-937. [DOI] [PubMed] [Google Scholar]
- 28.Garnier A, Côté J, Nadeau I, Kamen A, Massie B. Scale-up of the adenovirus expression system for the production of recombinant protein in human 293S cells. Cytotechnology. 1994;15:145–155. doi: 10.1007/BF00762389. [DOI] [PubMed] [Google Scholar]
- 29.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:e9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today. 2010;15:773–780. doi: 10.1016/j.drudis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Backliwal G, Hildinger M, Chenuet S, Wulhfard S, De Jesus M, Wurm FM. Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res. 2008;36:e96. doi: 10.1093/nar/gkn423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho MS, Yee H, Chan S. Establishment of a human somatic hybrid cell line for recombinant protein production. J. Biomed. Sci. 2002;9:631–638. doi: 10.1159/000067294. [DOI] [PubMed] [Google Scholar]
- 34.Mei B, Chen Y, Chen J, Pan CQ, Murphy JE. Expression of human coagulation factor VIII in a human hybrid cell line, HKB11. Mol. Biotechnol. 2006;34:165–178. doi: 10.1385/MB:34:2:165. [DOI] [PubMed] [Google Scholar]
- 35.Fallaux F, Bout A. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- 36.Jones D, Kroos N, Anema R, van Montfort B, Vooys A, van der Kraats S, van der Helm E, Smits S, Schouten J, Brouwer K, Lagerwerf F, van Berkel P, Opstelten D-J, Logtenberg T, Bout A. High-level expression of recombinant IgG in the human cell line per.c6. Biotechnol. Prog. 2003;19:163–168. doi: 10.1021/bp025574h. [DOI] [PubMed] [Google Scholar]
- 37.Schiedner G, Hertel S, Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum. Gene Ther. 2000;11:2105–2116. doi: 10.1089/104303400750001417. [DOI] [PubMed] [Google Scholar]
- 38.Fischer S, Charara N, Gerber A, Wölfel J, Schiedner G, Voedisch B, Geisse S. Transient recombinant protein expression in a human amniocyte cell line: the CAP-T® cell system. Biotechnol. Bioeng. 2012;109:2250–2261. doi: 10.1002/bit.24514. [DOI] [PubMed] [Google Scholar]
- 39.Geisse S. Reflections on more than 10 years of TGE approaches. Protein Expr. Purif. 2009;64:99–107. doi: 10.1016/j.pep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol. Lett. 2007;29:677–684. doi: 10.1007/s10529-006-9297-y. [DOI] [PubMed] [Google Scholar]
- 41.Muller N, Girard P, Hacker DL, Jordan M, Wurm FM. Orbital shaker technology for the cultivation of mammalian cells in suspension. Biotechnol. Bioeng. 2005;89:400–406. doi: 10.1002/bit.20358. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Stettler M, Reif O, Kocourek A, Dejesus M, Hacker DL, Wurm FM. Shaken helical track bioreactors: Providing oxygen to high-density cultures of mammalian cells at volumes up to 1000 L by surface aeration with air. N. Biotechnol. 2008;25:68–75. doi: 10.1016/j.nbt.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Geisse S, Henke M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J. Struct. Funct. Genomics. 2005;6:165–170. doi: 10.1007/s10969-005-2826-4. [DOI] [PubMed] [Google Scholar]
- 44.Schmid G, Schlaeger EJ, Wipf B. Non-GMP plasmid production for transient transfection in bioreactors. Cytotechnology. 2001;35:157–164. doi: 10.1023/A:1013148203049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozkov A, Larsson B, Gillström S, Björnestedt R, Schmidt SR. Large-scale production of endotoxin-free plasmids for transient expression in mammalian cell culture. Biotechnol. Bioeng. 2008;99:557–566. doi: 10.1002/bit.21603. [DOI] [PubMed] [Google Scholar]
- 46.Haldankar R, Li D, Saremi Z, Baikalov C, Deshpande R. Serum-free suspension large-scale transient transfection of CHO cells in WAVE bioreactors. Mol. Biotechnol. 2006;34:191–199. doi: 10.1385/mb:34:2:191. [DOI] [PubMed] [Google Scholar]
- 47.Geisse S, Jordan M, Wurm FM. Large-scale transient expression of therapeutic proteins in mammalian cells. Methods Mol. Biol. 2005;308:87–98. doi: 10.1385/1-59259-922-2:087. [DOI] [PubMed] [Google Scholar]
- 48.Liu C, Dalby B, Chen W, Kilzer JM, Chiou HC. Transient transfection factors for high-level recombinant protein production in suspension cultured mammalian cells. Mol. Biotechnol. 2008;39:141–153. doi: 10.1007/s12033-008-9051-x. [DOI] [PubMed] [Google Scholar]
- 49.Rajendra Y, Kiseljak D, Baldi L, Hacker DL, Wurm FM. A simple high-yielding process for transient gene expression in CHO cells. J. Biotechnol. 2011;153:22–26. doi: 10.1016/j.jbiotec.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Backliwal G, Hildinger M, Hasija V. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. 2008;99:721–727. doi: 10.1002/bit.21596. [DOI] [PubMed] [Google Scholar]
- 51.Figueroa B, Sauerwald TM, Mastrangelo a J, Hardwick JM, Betenbaugh MJ. Comparison of Bcl-2 to a Bcl-2 deletion mutant for mammalian cells exposed to culture insults. Biotechnol. Bioeng. 2001;73:211–222. doi: 10.1002/bit.1053. [DOI] [PubMed] [Google Scholar]
- 52.Tey BT, Singh RP, Piredda L, Piacentini M, Al-Rubeai M. Influence of bcl-2 on cell death during the cultivation of a Chinese hamster ovary cell line expressing a chimeric antibody. Biotechnol. Bioeng. 2000;68:31–43. doi: 10.1002/(sici)1097-0290(20000405)68:1<31::aid-bit4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 53.Kim NS, Lee GM. Response of recombinant Chinese hamster ovary cells to hyperosmotic pressure: effect of Bcl-2 overexpression. J. Biotechnol. 2002;95:237–248. doi: 10.1016/s0168-1656(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 54.Majors BS, Betenbaugh MJ, Pederson NE, Chiang GG. Enhancement of transient gene expression and culture viability using Chinese hamster ovary cells overexpressing Bcl-x(L) Biotechnol. Bioeng. 2008;101:567–578. doi: 10.1002/bit.21917. [DOI] [PubMed] [Google Scholar]
- 55.Lee SK, Lee GM. Development of apoptosis-resistant dihydrofolate reductase-deficient Chinese hamster ovary cell line. Biotechnol. Bioeng. 2003;82:872–876. doi: 10.1002/bit.10633. [DOI] [PubMed] [Google Scholar]
- 56.Tait AS, Brown CJ, Galbraith DJ, Hines MJ, Hoare M, Birch JR, James DC. Transient production of recombinant proteins by Chinese hamster ovary cells using polyethyleneimine/DNA complexes in combination with microtubule disrupting anti-mitotic agents. Biotechnol. Bioeng. 2004;88:707–721. doi: 10.1002/bit.20265. [DOI] [PubMed] [Google Scholar]
- 57.Geisse S. Transient expression technologies: past, present, and future. Methods Mol. Biol. (Clifton, NJ) 2012;899 doi: 10.1007/978-1-61779-921-1_13. [DOI] [PubMed] [Google Scholar]
- 58.Wirth M, Bode J, Zettlmeissl G, Hauser H. Isolation of overproducing recombinant mammalian cell lines by a fast and simple selection procedure. Gene. 1988;73:419–426. doi: 10.1016/0378-1119(88)90506-9. [DOI] [PubMed] [Google Scholar]
- 59.Turan S, Galla M, Ernst E, Qiao J, Voelkel C, Schiedlmeier B, Zehe C, Bode J. Recombinasemediated cassette exchange (RMCE): traditional concepts and current challenges. J. Mol. Biol. 2011;407:193–221. doi: 10.1016/j.jmb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Turan S, Kuehle J, Schambach A, Baum C, Bode J. Multiplexing RMCE: versatile extensions of the Flp-recombinase-mediated cassette-exchange technology. J. Mol. Biol. 2010;402:52–69. doi: 10.1016/j.jmb.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 61.Sorrell D a, Robinson CJ, Smith J-A, Kolb AF. Recombinase mediated cassette exchange into genomic targets using an adenovirus vector. Nucleic Acids Res. 2010;38:e123. doi: 10.1093/nar/gkq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres R, García A, Payá M, Ramirez JC. Non-integrative lentivirus drives high-frequency cremediated cassette exchange in human cells. PLoS One. 2011;6:e19794. doi: 10.1371/journal.pone.0019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agrawal V. Strategies for Rapid Production of Therapeutic Proteins in Mammalian Cells. Bioprocess Int. 2012;10 [Google Scholar]
- 64.Wiznerowicz M, Trono D. Harnessing HIV for therapy, basic research and biotechnology. Trends Biotechnol. 2005;23:42–47. doi: 10.1016/j.tibtech.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Bandaranayake AD, Correnti C, Ryu BY, Brault M, Strong RK, Rawlings DJ. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic Acids Res. 2011;39:e143. doi: 10.1093/nar/gkr706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oberbek A, Matasci M, Hacker DL, Wurm FM. Generation of stable, high-producing CHO cell lines by lentiviral vector-mediated gene transfer in serum-free suspension culture. Biotechnol. Bioeng. 2010;41 doi: 10.1002/bit.22968. [DOI] [PubMed] [Google Scholar]
- 67.Lai T, Yang Y, Ng S. Advances in Mammalian Cell Line Development Technologies for Recombinant Protein Production. Pharmaceuticals. 2013;6:579–603. doi: 10.3390/ph6050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu B, Benjamin D, Zheng Y, Angliker H, Thiry S, Siegmann M, Jost JP. Overexpression of 5- methylcytosine DNA glycosylase in human embryonic kidney cells EcR293 demethylates the promoter of a hormone-regulated reporter gene. Proc. Natl. Acad. SciUSA. 2001;98:5031–5036. doi: 10.1073/pnas.091097298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jost JP, Oakeley EJ, Zhu B, Benjamin D, Thiry S, Siegmann M, Jost YC. 5-Methylcytosine DNA glycosylase participates in the genome-wide loss of DNA methylation occurring during mouse myoblast differentiation. Nucleic Acids Res. 2001;29:4452–4461. doi: 10.1093/nar/29.21.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bidwell JP, Torrungruang K, Alvarez M, Rhodes SJ, Shah R, Jones DR, Charoonpatrapong K, Hock JM, Watt AJ. Involvement of the nuclear matrix in the control of skeletal genes: the NMP1 (YY1), NMP2 (Cbfa1), and NMP4 (Nmp4/CIZ) transcription factors. Crit. Rev. Eukaryot. Gene Expr. 2001;11:279–297. [PubMed] [Google Scholar]
- 71.Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- 72.Girod P-A, Nguyen D-Q, Calabrese D, Puttini S, Grandjean M, Martinet D, Regamey A, Saugy D, Beckmann JS, Bucher P, Mermod N. Genome-wide prediction of matrix attachment regions that increase gene expression in mammalian cells. Nat. Methods. 2007;4:747–753. doi: 10.1038/nmeth1076. [DOI] [PubMed] [Google Scholar]
- 73.Girod P-A, Zahn-Zabal M, Mermod N. Use of the chicken lysozyme 5’ matrix attachment region to generate high producer CHO cell lines. Biotechnol. Bioeng. 2005;91:1–11. doi: 10.1002/bit.20563. [DOI] [PubMed] [Google Scholar]
- 74.Kim J Do, Yoon Y, Hwang H-Y, Park JS, Yu S, Lee J, Baek K, Yoon J. Efficient selection of stable chinese hamster ovary (CHO) cell lines for expression of recombinant proteins by using human interferon beta SAR element. Biotechnol. Prog. 21:933–937. doi: 10.1021/bp049598v. [DOI] [PubMed] [Google Scholar]
- 75.Kim J-M, Kim J-S, Park D-H, Kang HS, Yoon J, Baek K, Yoon Y. Improved recombinant gene expression in CHO cells using matrix attachment regions. J. Biotechnol. 2004;107:95–105. doi: 10.1016/j.jbiotec.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 76.Zahn-Zabal M, Kobr M, Girod P a, Imhof M, Chatellard P, de Jesus M, Wurm F, Mermod N. Development of stable cell lines for production or regulated expression using matrix attachment regions. J. Biotechnol. 2001;87:29–42. doi: 10.1016/s0168-1656(00)00423-5. [DOI] [PubMed] [Google Scholar]
- 77.Antoniou M, Harland L, Mustoe T, Williams S, Holdstock J, Yague E, Mulcahy T, Griffiths M, Edwards S, Ioannou P, Mountain A, Crombie R. Transgenes encompassing dual-promoter CpG islands from the human TBP and HNRPA2B1 loci are resistant to heterochromatin-mediated silencing. Genomics. 2003;82:269–279. doi: 10.1016/s0888-7543(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 78.Williams S, Mustoe T, Mulcahy T, Griffiths M, Simpson D, Antoniou M, Irvine A, Mountain A, Crombie R. CpG-island fragments from the HNRPA2B1/CBX3 genomic locus reduce silencing and enhance transgene expression from the hCMV promoter/enhancer in mammalian cells. BMC Biotechnol. 2005;5:17. doi: 10.1186/1472-6750-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Schambach A, Sinclair J, Kinnon C, Gaspar HB, Antoniou M, Thrasher AJ. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang F, Frost AR, Blundell MP, Bales O, Antoniou MN, Thrasher AJ. A ubiquitous chromatin opening element (UCOE) confers resistance to DNA methylation-mediated silencing of lentiviral vectors. Mol. Ther. 2010;18:1640–1649. doi: 10.1038/mt.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye J, Alvin K, Latif H, Hsu A, Parikh V, Whitmer T, Tellers M, de la Cruz Edmonds MC, Ly J, Salmon P, Markusen JF. Rapid protein production using CHO stable transfection pools. Biotechnol. Prog. 2010;26:1431–1437. doi: 10.1002/btpr.469. [DOI] [PubMed] [Google Scholar]
- 82.Sautter K, Enenkel B. Selection of high-producing CHO cells using NPT selection marker with reduced enzyme activity. Biotechnol. Bioeng. 2005;89:530–538. doi: 10.1002/bit.20374. [DOI] [PubMed] [Google Scholar]
- 83.Ho SCL, Bardor M, Feng H, Mariati, Tong YW, Song Z, Yap MGS, Yang Y. IRES-mediated Tricistronic vectors for enhancing generation of high monoclonal antibody expressing CHO cell lines. J. Biotechnol. 2012;157:130–139. doi: 10.1016/j.jbiotec.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 84.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 85.Westwood AD, Rowe DA. Clarke HRG Improved recombinant protein yield using a codon deoptimized DHFR selectable marker in a CHEF1 expression plasmid. Biotechnol. Prog. 26:1558–1566. doi: 10.1002/btpr.491. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R, Shen W. Monoclonal antibody expression in Mammalian cells. Antib. Eng. 2012;907:341–358. doi: 10.1007/978-1-61779-974-7_20. [DOI] [PubMed] [Google Scholar]
- 87.Urlaub G, Käs E, Carothers AM, Chasin LA. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983;33:405–412. doi: 10.1016/0092-8674(83)90422-1. [DOI] [PubMed] [Google Scholar]
- 88.Kaufman RJ, Sharp PA. Amplification and expression of sequences cotransfected with a modular dihydrofolate reductase complementary dna gene. J. Mol. Biol. 1982;159:601–621. doi: 10.1016/0022-2836(82)90103-6. [DOI] [PubMed] [Google Scholar]
- 89.Ng SK, Wang DIC, Yap MGS. Application of destabilizing sequences on selection marker for improved recombinant protein productivity in CHO-DG44. Metab. Eng. 2007;9:304–316. doi: 10.1016/j.ymben.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Ng SK, Tan TRM, Wang Y, Ng D, Goh L-T, Bardor M, Wong VVT, Lam KP. Production of Functional Soluble Dectin-1 Glycoprotein Using an IRES-Linked Destabilized-Dihydrofolate Reductase Expression Vector. PLoS One. 2012;7:e52785. doi: 10.1371/journal.pone.0052785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnes LM, Bentley CM, Dickson a J. Advances in animal cell recombinant protein production: GS-NS0 expression system. Cytotechnology. 2000;32:109–123. doi: 10.1023/A:1008170710003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pu H, Cashion LM, Kretschmer PJ, Liu Z. Rapid establishment of high-producing cell lines using dicistronic vectors with glutamine synthetase as the selection marker. Mol. Biotechnol. 1998;10:17–25. doi: 10.1007/BF02745860. [DOI] [PubMed] [Google Scholar]
- 93.Kingston RE, Kaufman RJ, Bebbington CR, Rolfe MR. Amplification using CHO cell expression vectors. Chapter 16. Curr. Protoc. Mol. Biol. 2002 doi: 10.1002/0471142727.mb1623s60. Unit 16.23. [DOI] [PubMed] [Google Scholar]
- 94.Fan L, Kadura I, Krebs LE, Hatfield CC, Shaw MM, Frye CC. Improving the efficiency of CHO cell line generation using glutamine synthetase gene knockout cells. Biotechnol. Bioeng. 2012;109:1007–1015. doi: 10.1002/bit.24365. [DOI] [PubMed] [Google Scholar]
- 95.Orfao a, Ruiz-Arguelles a. General concepts about cell sorting techniques. Clin. Biochem. 1996;29:5–9. doi: 10.1016/0009-9120(95)02017-9. [DOI] [PubMed] [Google Scholar]
- 96.Young SM, Bologa C, Prossnitz ER, Oprea TI, Sklar L a, Edwards BS. High-throughput screening with HyperCyt flow cytometry to detect small molecule formylpeptide receptor ligands. J. Biomol. Screen. 2005;10:374–382. doi: 10.1177/1087057105274532. [DOI] [PubMed] [Google Scholar]
- 97.Ibrahim SF, van den Engh G. High-speed cell sorting: fundamentals and recent advances. Curr. Opin. Biotechnol. 2003;14:5–12. doi: 10.1016/s0958-1669(02)00009-5. [DOI] [PubMed] [Google Scholar]
- 98.Song M, Raphaelli K, Jones ML, Aliabadi-Zadeh K, Leung KM, Crowley D, Hughes B, Mahler S, Gray PP, Huang EP, Chin DY. Clonal selection of high producing, stably transfected HEK293 cell lines utilizing modified, high-throughput FACS screening. J. Chem. Technol. Biotechnol. 2011;86:935–941. [Google Scholar]
- 99.Pichler J, Hesse F, Wieser M, Kunert R, Galosy SS, Mott JE, Borth N. A study on the temperature dependency and time course of the cold capture antibody secretion assay. J. Biotechnol. 2009;141:80–83. doi: 10.1016/j.jbiotec.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Yoshikawa T, Nakanishi F, Ogura Y, Oi D, Omasa T, Katakura Y, Kishimoto M, Suga KI. Flow cytometry: an improved method for the selection of highly productive gene-amplified CHO cells using flow cytometry. Biotechnol. Bioeng. 2001;74:435–442. doi: 10.1002/bit.1134. [DOI] [PubMed] [Google Scholar]
- 101.Durocher Y, Butler M. Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotechnol. 2009;20:700–707. doi: 10.1016/j.copbio.2009.10.008. [DOI] [PubMed] [Google Scholar]


