Abstract
Objective
Over 90% of modified LDL in circulation is associated to specific antibodies circulating as part of immune complexes (IC); however, few studies have examined their relationship with cardiovascular disease.
Methods
We report the relationship between circulating concentrations of IC of oxidized LDL (oxLDL-IC), malondialdehyde-LDL (MDA-LDL-IC) and advanced glycation end products-LDL (AGE-LDL-IC) and progression of atherosclerosis over a 12 year period in 467 individuals with type 1 diabetes who participated in the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study. OxLDL-IC, AGE-LDL-IC and MDA-LDL-IC levels were measured at DCCT closeout. Internal carotid intima-medial thickness (IMT) was measured at EDIC follow-up years 1, 6 and 12.
Results
OxLDL-IC, AGE-LDL-IC and MDA-LDL-IC levels were significantly correlated with age, lipid levels, blood pressure levels and albumin excretion rates. Levels of oxLDL, AGE-LDL and MDA-LDL in isolated LDL-IC were highly inter-correlated (r=0.66 to 0.84, p<0.0001). After adjusting for cardiovascular risk factors individuals in the upper quartile of oxLDL-IC had a 2.98fold increased odds (CI: 1.34, 6.62) of having IMT ≥ 1.00 mm and had a 5.13-fold increased odds (CI: 1.98, 13.3) of having significant IMT progression, relative to those in the lowest quartile. Parallel odds ratios for AGE-LDL-IC were 2.95 (CI: 1.37, 6.34) and 3.50 (CI: 1.38, 8.86), while results for MDA-LDL-IC were 1.76 (0.87, 3.56) and 2.86 (1.20, 6.81).
Conclusion
Our study indicates that high levels of oxLDL-IC and AGE-LDL-IC are important predictors of carotid intima-medial thickening in patients with type 1 diabetes.
Keywords: modified LDL, subclinical atherosclerosis, carotid artery intima-media thickness, type 1 diabetes
Introduction
Many studies have demonstrated a relationship between modified LDL and the incidence of cardiovascular disease(1-9). Nonetheless, few studies have examined the relationship between the serum levels of modified LDL in immune complexes (mLDL-IC) and cardiovascular disease, even though over 90% of modified LDL in circulation is associated to specific antibodies, circulating as part of IC(10-11). Modified LDL when associated with the respective antibodies cannot be properly measured by standard immunoassays(5), which may explain why some studies have failed to find an association between levels of modified LDL and cardiovascular disease. In the past decade several studies have shown that LDL-IC are taken up by macrophages via Fcγ receptors(12) leading to marked intracellular accumulation of cholesterol esters and to the transformation of macrophages into foam cells, the hallmark of the atherosclerotic process(13-15).
The measurement of carotid artery intima-media thickness (IMT) by ultrasonography is an accepted noninvasive measure of subclinical atherosclerosis. Mean carotid artery IMT has been established as an early quantitative marker of generalized atherosclerosis because of its association with cardiovascular outcomes(16-17), cardiovascular risk factors (18-19), and atherosclerosis in other arterial beds (20-21). Mean carotid artery IMT can reflect a combination of arterial characteristics, including an early diffuse pre-atherosclerotic thickening of the carotid arteries, a single focal thickening of the carotid arteries that contributes disproportionately to the overall mean IMT measured across multiple sites, and at lower levels a non-atherosclerotic thickening that is an adaptive response to altered flow and shear and tensile stress on the arterial wall (22-23). In addition, variation in methodology across studies, with some studies including and other studies excluding sites of focal carotid artery plaque in their measurement, may alter the interpretation of mean carotid artery IMT. Moreover, carotid artery plaques occur more frequently and earlier in the internal carotid artery than in the common carotid artery(24). Hence, increased mean internal carotid artery (ICA) IMT measured at the site of maximal wall thickness likely reflects development of focal carotid artery plaques among older participants, whereas at younger ages it may reflect early diffuse pre-atherosclerotic thickening.
Recently, our group reported that mLDL-IC measured in baseline samples of the Diabetes Control and Complications trial (DCCT) cohort were strongly associated with progression and increased levels of carotid artery IMT 8-14 years later during the Epidemiology of Diabetes Interventions and Complication (EDIC) study (25). Importantly, the discriminatory power of oxLDL and AGE-LDL concentrations in isolated IC to predict high carotid artery IMT exceeded that of LDL-cholesterol, urinary albumin excretion rate (AER), and either systolic or diastolic blood pressure(25). At DCCT baseline, participants were young (27.1 ± 7.0 years, mean ±SD) and had relatively short duration diabetes (6.0 ± 4.2 years). Moreover, with the exception of having type 1 diabetes, DCCT participants had very few risk factors for cardiovascular disease.
In the current study we extend our findings with new longitudinal analyses. Levels of oxLDL, AGE-LDL and MDA-LDL in circulating IC measured at DCCT closeout (i.e., in 1993, 5 to 10 years after DCCT entry) were used to determine the odds for the subsequent development of increased carotid IMT. Internal and common carotid artery IMT levels 1, 6 and 12 years later (i.e., after entry into EDIC in 1994) were the primary outcomes of interest. At DCCT closeout relative to baseline, participants were not only older and with longer duration of diabetes, but also had higher LDL-cholesterol and blood pressure levels. Moreover, for the current analyses IMT information was available across three time points as compared to two in our previous analysis. This extends the follow-up time from the initial measurement of carotid artery IMT from 5 to 11 years. In addition, we also consider LDL and HDL particle concentration as potential confounders of the association between mLDL-IC and cardiovascular disease. In summary, we aimed to determine the predictive value of modified LDL-IC levels for progression of atherosclerosis and increased carotid artery IMT at later stages of the atherosclerotic process, and compare them to conventional cardiovascular risk factors.
Material and Methods
This study was performed on a subgroup of 467 subjects from the DCCT/EDIC cohort. The DCCT cohort included 1,441 patients who at study entry (1984-89) were 13-39 years of age and had Type 1 diabetes for 1-15 years (26). At DCCT entry, none of the patients had hypertension or dyslipidemia, and therefore were not on lipid-lowering or anti-hypertensive therapy.
The DCCT cohort was randomly assigned to intensive or conventional diabetes therapy and followed for an average of 6.5 years. In 1994, after intensive therapy had been demonstrated to have major beneficial effects on microvascular complications, the interventional phase of the study was stopped and the observational phase (EDIC) was initiated (27). During the ongoing EDIC observational phase, the patients have been under the care of their personal physicians and encouraged to practice intensive diabetes therapy.
Of the 1,441 DCCT participants, 1375 of the 1425 surviving members volunteered to participate in the EDIC observational follow up study; 905 of these individuals had blood collected longitudinally as part of a sub-study on biomarkers of vascular disease. From these 905 subjects, 517 patients were selected to participate in the sub-study of modified LDL-IC. In the selection of these 517 patients, those with abnormal albuminuria, increased Early Treatment Diabetic Retinopathy Study (ETDRS) score (≥10), and elevated carotid atherosclerosis (≥25% stenosis at a lesion) were oversampled (i.e. all available participants were sampled); resulting in 157 of the 517 patients having one of these three endpoints and 361 of the patients having none of these endpoints. The 361 were selected as a simple random sample of the remaining study participants. Of the 517 with modified LDL-IC measured 467 had IMT measured during EDIC (28). The DCCT and EDIC studies were approved by the Institutional Review Board of all participating DCCT/EDIC centers and all participants provided written informed consent.
Assessment of Carotid Intima-Media thickness
Carotid ultrasonography was first performed 1-2 years after initiation of EDIC (EDIC Year 1) and repeated at EDIC Year 6 and EDIC Year 12. The measurement of IMT in the DCCT/EDIC cohort has been described in detail (29-30). In brief, a single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries (CCA) and three longitudinal views in different imaging planes of each internal carotid artery (ICA) were obtained by certified technicians at the clinical centers, recorded on S-VHS tapes and read in a central unit (Tufts Medical Center, Boston, MA) by two readers masked to participant characteristics. The maximum IMT (mm) of the CCA was defined as the mean of the maximum IMT for near and far walls on both right and left sides. The maximum IMT of the ICA was defined in the same way, and the results of the three scans (i.e., anterior, lateral and posterior views of both sides) were averaged.
Assessment of Computed Tomography of Coronary Artery Calcification (CAC)
Coronary artery computed tomography (CT) was performed at EDIC 7–9 year. CT was performed using a C-150 cardiac-gated electron beam. All participants were scanned twice over calibration phantoms of known physical calcium concentration. Scans were read centrally at the Harbor-UCLA (University of California, Los Angeles) Research and Education Institute (Torrance, CA) to identify and quantify CAC, calibrated according to the readings of the phantom using the method of Agatston et al. The average score from the two scans was used in the analysis.
Measurement of AGE-LDL, oxLDL and MDA-LDL in human circulating immune complexes
Serum samples were obtained after an overnight fast during the DCCT closeout examination and stored at -80°C. We measured oxLDL, MDA-LDL and AGE-LDL by first precipitating circulating immune complexes from serum and then fractionating these IC by protein G affinity chromatography, separating the predominant IgG antibody from modified LDL, as previously described (31-32). The reactivity of modified LDL separated from LDL-IC with antibodies specific for different LDL modifications (oxLDL, MDA-LDL and AGE-LDL) was then assayed with capture assays developed in our laboratory (33). The characteristics of the antibodies used in the assay and the specificity and reproducibility of the capture assays have been previously reported (11, 33). Coefficients of variation for 50 samples measured in two separate assays were 5.2% for oxLDL, 0.5% for MDA-LDL, and 8.3% for AGE-LDL. The development of standards for calibration of the oxLDL, MDA-LDL, and AGE-LDL assays, as well as sensitivity, reproducibility, and recovery data for the capture assays have been reported elsewhere (33). The levels of the different LDL modifications in human circulating IC were expressed in function of the amount of apolipoprotein B contained in the IC, and the final values given as the concentration per mL of serum.
Other procedures
Demographic and clinical characteristics of the subjects were collected at the closeout of the DCCT study. At that time, each participant underwent a standardized physical examination and laboratory testing including hemoglobin A1c (27, 34), fasting lipid profiles, blood pressure and 4-hour urine collections for measurement of AER and creatinine clearance. Study mean hemoglobin A1c (HbA1c) was calculated as the weighted mean from DCCT-baseline through EDIC year 12 (last IMT measurement). Additionally, lipoprotein subclasses were measured by LipoScience, Inc., (Raleigh, NC) using NMR spectroscopy on 1295 samples obtained at the DCCT examination prior to the closeout examination, of which 383 had immune complex data available from the sub-study on biomarkers of vascular disease (∼1 year between NMR and IC measures) as well as IMT measurements at EDIC years 1 and 12. The methodologies to measure conventional CVD risk factors (27, 35) and NMR lipoprotein subclasses(36) have been described elsewhere. At DCCT baseline, participants were grouped into one of two cohorts based on their retinopathy and renal status and duration of type 1 diabetes (37).
Statistical Analysis
Prospective analyses were carried out in which the levels of oxLDL, AGE-LDL and MDA-LDL in LDL-IC, measured at DCCT closeout, functioned as a biomarker for an individual's levels of LDL, degree of oxidative stress and immune response. Internal and common IMT levels 1-12 years later (EDIC years 1, 6 and 12) were the primary outcomes of interest. Online Figure 1 is a schematic that depicts timing of biomarker sample collection and outcomes. All modified LDL values were standardized to mg of Apolipoprotein-B per L of serum and are expressed as mg/L.
Prior to analysis, each IC was categorized into quartiles which were used as the primary independent variables of interest. Means and proportions were determined for participant demographic and clinical characteristics at DCCT closeout stratified by oxLDL-IC quartile. Trends across quartile were tested using an F-statistic obtained from a generalized linear model. Spearman rank order correlation coefficients were determined between modified LDL levels and cardiovascular risk factors of interest.
Inverse probability weighted logistic regression was used to model the odds ratios associated with increased ICA IMT in the presence of uneven sampling)(38). In the selection of patients for the proposed study all individuals with abnormal albuminuria, an ETDRS score ≥10, or ≥25% stenosis at a carotid lesion were sampled, while a simple random sample was used to select remaining study participants. The odds associated with being in the upper versus lower measurements of ICA IMT (i.e., upper quintile versus lower four quintiles) at EDIC years 6 and 12 for increases in mLDL-IC was assessed followed by the odds associated with high progression of ICA IMT from EDIC year 1 to EDIC year 12 (i.e., high progression being defined as being in the upper quintile of ICA IMT change).
Additionally, ICA IMT and CCA IMT values were categorized as clinically elevated (≥ 1.0 mm and ≥ 0.75 mm, respectively) versus normal at all available time points (EDIC years 1, 6, and 12). A Cochran-Armitage test for trend was used to assess the overall ordered association between elevated values of IMT and CAC with quartiles of oxidized LDL-IC. A repeated measures logistic regression model using the methods of generalized estimating equations (GEE) was applied to assess the overall effect of increases of mLDL-IC on IMT levels during the 12 years of EDIC IMT follow up. Working correlation structures were independently compared and the final model structure was chosen using the quasi-likelihood under the independence model criterion statistic. Odds ratios and asymptotic 95% confidence intervals were computed for all analysis. Three models were used to evaluate each association of interest; model 1 adjusted for DCCT treatment assignment, age, gender, primary retinopathy cohort, diabetes duration, natural logarithm of AER, HbA1c at DCCT closeout and IMT ultrasound reader; model 2 additionally adjusted for LDL-cholesterol, HDL-cholesterol and systolic blood pressure at DCCT closeout; smoking status at enrollment; ACE/ARB use, any statin use through EDIC year 12, and DCCT/EDIC study mean HbA1c rather than DCCT closeout HbA1c. Finally, model 3 was assessed on the subsample with the NMR determined LDL and HDL particle concentration rather than cholesterol levels and additional covariates as stated for model 2. Additionally, the concordance statistic (c-statistic; an approximation to the area under the receiver-operating curve (ROC AUC)) was used to compare the discriminatory power of various multivariate logistic regression models. Reported p-values are two-sided with a type I error rate for significance of α = 0.05. All analyses were performed using SAS v. 9.3 (SAS Institute, Cary, NC, USA, 2011).
Results
At DCCT closeout, the mean age of the study population was 34.2 ± 6.8 years, the mean duration of diabetes was 12.5 ± 5.1 years, 242 (51.8 %) of the 467 subjects studied were males and 46% were assigned to the DCCT intensive treatment group. Comparing DCCT closeout characteristics of these 467 subjects with the remaining DCCT cohort, duration of diabetes was longer and AER was higher. Blood pressure, lipid, and HbA1c as well as age, gender, drinking and smoking status were similar in those included and excluded in this study's subcohort.
At DCCT closeout, the levels of oxLDL, AGE-LDL and MDA-LDL in isolated LDL-IC were significantly correlated with age, lipid levels, blood pressure levels and albumin excretion rates. Diabetes duration was significantly correlated with oxLDL and AGE-LDL in isolated IC, but not with MDA-LDL in IC (Online Table 1). Correlations of modified LDL-IC with LDL-cholesterol (r=0.24 to 0.32, p<0.001), LDL-particle concentration (r=0.20 to 0.26, p<0.001), HDL-cholesterol (r=-0.12 to -0.21, p<0.001 to p=0.011) and HDL-particle concentration (r=-0.10 to -0.16, p<0.001 to p=0.038) were of moderate magnitude. The levels of oxLDL, AGE-LDL and MDA-LDL in isolated LDL-IC were all highly inter-correlated (r=0.66 to 0.84, p<0.0001). Across quartiles of oxLDL in isolated IC, duration of diabetes and smoking status were similar, while percent male, age, HbA1c, albumin excretion rate, LDL-cholesterol, LDL-particle concentration, systolic and diastolic blood pressure levels increased (Table 1). HDL-cholesterol and HDL-particle concentration decreased across quartiles of oxLDL in isolated IC.
Table 1.
Demographics and Clinical characteristics [means ± standard deviation or proportions (n)] at DCCT closeout stratified by quartile of oxLDL in LDL-IC (n = 465 with Year 12 ICA IMT).
| oxLDL in LDL-IC Quartiles (cut-points, mg/L) | |||||
|---|---|---|---|---|---|
| 1st Quartile (0.37-34.1) | 2nd Quartile (34.5-70.4) | 3rd Quartile (70.5-125.9) | 4th Quartile (126-1182) | Trend P | |
| Age (yrs) | 33 ± 6.4 | 34 ± 7.2 | 35 ± 6.6 | 35 ± 6.7 | 0.009 |
| Male | 42% (50) | 52% (63) | 51% (57) | 63% (72) | 0.003 |
| DCCT Intensive Treatment | 43% (51) | 56% (68) | 50% (55) | 36% (41) | 0.179 |
| Primary Retinopathy Cohort | 45% (54) | 48% (59) | 50% (55) | 37% (43) | 0.269 |
| Duration of T1DM (yrs) | 12 ± 5.0 | 12 ± 5.0 | 13 ± 5.3 | 13 ± 4.9 | 0.081 |
| AER (mg/24 hours)† | 9.3 ± 2.4 | 12.2 ± 2.8 | 11.8 ± 3.0 | 17.1 ± 4.5 | <0.001 |
| HbA1c (%) | 8.2 ± 1.4 | 8.3 ± 1.7 | 8.4 ± 1.8 | 8.8 ± 1.7 | 0.027 |
| LDL-Cholesterol (mg/dL) | 99 ± 24 | 113 ± 28 | 115 ± 29 | 125 ± 32 | <0.001 |
| HDL-Cholesterol (mg/dL) | 55 ± 14 | 51 ± 12 | 50 ± 11 | 49 ± 12 | <0.001 |
| LDL-Particle Concentration (nmol/L) | 912 ± 353 | 1029 ± 405 | 1051 ± 385 | 1158 ± 384 | <0.001 |
| HDL-Particle Concentration (ìmol/L) | 36 ± 7 | 35 ± 7 | 35 ± 6 | 33 ± 6 | <0.001 |
| Systolic BP (mmHg) | 113 ± 11 | 116 ± 10 | 117 ± 13 | 119 ± 11 | <0.001 |
| Diastolic BP (mmHg) | 72 ± 7.8 | 73 ± 8.6 | 76 ± 8.5 | 77 ± 7.9 | <0.001 |
| Current Smoker | 18% (21) | 24% (29) | 22% (24) | 13% (15) | 0.327 |
AER, albumin excretion rate;
Unadjusted;
Due to non-normal distributions geometric means are presented.
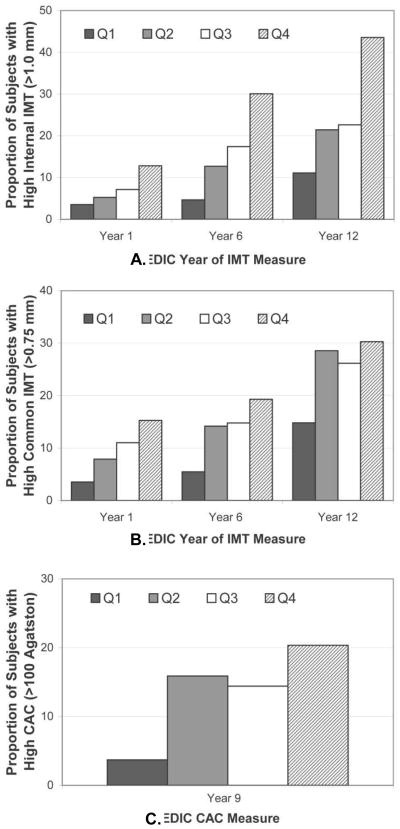
The prevalence of ICA-IMT >1.0 mm increased from EDIC Year 1 through EDIC Year 12; moreover, within each EDIC Year in which carotid artery IMT was assessed, the prevalence of having ICA-IMT >1.0 mm increased across the quartiles of oxLDL-IC (Figure 1). Results for CCA-IMT>0.75 mm were similar. The prevalence of levels of CCA-IMT>0.75 mm increased each time that carotid artery IMT assessment was performed; however, while prevalence of having CCA-IMT > 0.75 mm increased across quartiles of oxLDL-IC at EDIC years 1 and 6, by EDIC Year 12 the prevalence of CCA-IMT >0.75 mm was similarly high in the upper three quartiles of oxLDL-IC. Results for coronary artery calcium were similar to those of CCA IMT at EDIC year 12 in that the proportion of participants with CAC >100 Agatston was similarly high in the upper three quartiles of oxLDL-IC. Focusing on mean ICA IMT levels as the outcome and stratifying by oxLDL quartiles, ICA IMT levels increased across oxLDL quartiles at EDIC year 1 (Linear Trend Test; P = 0.026), EDIC year 6 (Linear Trend Test; P < 0.001) and EDIC year 12 (Linear Trend Test; P < 0.001) after adjusting for treatment group, retinopathy cohort, age, sex, diabetes duration, HbA1c, logarithm of AER and ultrasonography equipment (Online Figure 2). Similar findings were observed across AGE-LDL quartiles, while slightly weaker findings were observed across MDA-LDL quartiles.
Figure 1.
Unadjusted proportion of subjects with high internal carotid artery IMT, high common carotid artery IMT and high coronary artery calcium during EDIC by quartile of oxLDL in isolated LDL-IC. Oxidized LDL-IC Quartile ranges: 1st 0.37-34.1; 2nd 34.5-70.4; 3rd 70.5-125.9; 4th 126.0-1182.
Panel A: ICA IMT ≥ 1.00 mm; Cochran-Armitage Trend Test: Year 1 (Z = -2.76; P = 0.006); Year 6 (Z = -5.14; P < 0.001); Year 12 (Z = -5.31; P < 0.001);
Panel B: CCA IMT ≥ 0.75 mm; Cochran-Armitage Trend Test: Year 1 (Z = -3.15; P = 0.002); Year 6 (Z = -2.91; P = 0.004); Year 12 (Z = -2.35; P = 0.019);
Panel C: CAC >100 Agatston at EDIC Year 9; Cochran-Armitage Trend Test: OxLDL-IC (Z = - 3.31; P < 0.001); AGE LDL-IC (Z = -2.97; P = 0.003); MDA LDL-IC (Z = -2.85; P = 0.004).
Logistic regression was used to examine the ability of the concentrations of oxLDL in isolated IC to predict high ICA IMT at EDIC Year 6 [i.e., being in the upper quintile as compared to the lower 4 quintiles of ICA IMT (high IMT ≥0.809 mm)] and at EDIC Year 12 (i.e., high IMT ≥1.07 mm) (Table 2). Individuals in the highest quartile of oxLDL in isolated IC had a 4-fold increased odds [3.99 (95% CI: 1.60, 9.40)] of having high versus normal ICA IMT at EDIC year 12 relative to those in the lowest quartile of oxLDL, after controlling for DCCT treatment group, retinopathy cohort, age, sex, diabetes duration, hemoglobin A1c, logarithm of AER and reader ID (model 1). Additionally adjusting for study mean HbA1c, LDL-cholesterol, HDL-cholesterol, systolic blood pressure, ACE/ARB use, statin use and smoking status (model 2) attenuated the odds ratios slightly to 3.52 (95% CI: 1.36, 9.10). Adjusting for LDL and HDL particle concentration rather than cholesterol level (model 3) modified the odds ratio only slightly to 4.18 (95% CI: 1.48, 11.8).
Table 2.
Adjusted* Odds Ratio (and 95% confidence interval) from logistic† and GEE‡ regression models for quartiles of oxLDL-IC in relation to elevated internal carotid IMT and IMT progression.
| 6 Year ICA IMT† | 12 Year ICA IMT† | IMT Progression Year 1 to 12† | Elevated ICA IMT‡ | |
|---|---|---|---|---|
| Model 1* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.65 (0.74-3.66) | 1.35 (0.57-3.19) | 1.66 (0.70-3.95) | 1.94 (0.91-4.14) |
| Quartile 3 | 1.53 (0.67-3.49) | 2.09 (0.84-5.24) | 1.89 (0.75-4.78) | 2.13 (0.99-4.57) |
| Quartile 4 | 2.72 (1.24-5.97) | 3.99 (1.69-9.40) | 4.93 (2.08-11.7) | 4.78 (2.30-9.91) |
| ROC AUC | 0.748 | 0.813 | 0.797 | --- |
| Model 2* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.18 (0.52-2.67) | 1.10 (0.44-2.74) | 1.55 (0.59-4.03) | 1.32 (0.61-2.89) |
| Quartile 3 | 1.13 (0.49-2.61) | 1.89 (0.74-4.87) | 1.94 (0.74-5.07) | 1.66 (0.78-3.55) |
| Quartile 4 | 1.79 (0.78-4.12) | 3.52 (1.36-9.10) | 5.13 (1.98-13.3) | 2.98 (1.34-6.62) |
| ROC AUC | 0.778 | 0.849 | 0.833 | --- |
| Model 3* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.69 (0.71-4.02) | 1.05 (0.38-2.90) | 1.47 (0.51-4.25) | 1.31 (0.56-3.05) |
| Quartile 3 | 1.52 (0.62-3.75) | 1.71 (0.60-4.85) | 2.06 (0.72-5.88) | 1.73 (0.74-4.03) |
| Quartile 4 | 3.04 (1.24-7.49) | 4.18 (1.48-11.8) | 5.89 (2.12-16.4) | 3.66 (1.52-8.79) |
| ROC AUC | 0.785 | 0.862 | 0.845 | --- |
Model 1 is adjusted for age, gender, primary retinopathy cohort, diabetes duration, natural log of AER, IMT reader ID and closeout HbA1c; Model 2 is additionally adjusted for LDL-C, HDL-C, SBP at DCCT closeout; smoking status at enrollment; ACE/ARB use and statin use through EDIC year 12 and study mean HbA1c rather than closeout HbA1c; Model 3 is adjusted for LDL and HDL mean particle concentration rather than LDL and HDL cholesterol levels.
For the outcome ICA IMT progression from EDIC Year 1 to 12 [i.e., being in the upper quintile as compared to the lower 4 quintiles of ICA IMT progression (high progression ≥ 0.37 mm)] results were slightly stronger for each model (Table 2). Respective odds ratios were 4.93 (95% CI: 2.08, 11.7), 5.13 (95% CI: 1.98, 13.3) and 5.89 (95% CI: 2.12, 16.4). Finally, results from the GEE model which considered elevated ICA IMT (i.e., ICA IMT ≥1.00 mm) across the three time points also indicated a strong graded association (Table 2) .
Parallel analyses for AGE-LDL-IC resulted in an odds ratio of 1.96 (95% CI: 0.80, 4.79) for EDIC Year 12 ICA IMT, an odds ratio of 3.50 (95 % CI: 1.38, 8.86) for ICA IMT progression and an odds ratio of 2.95 (95% CI: 1.37, 6.34) for elevated ICA IMT after adjustment for standard cardiovascular risk factors (Table 3; Model 2). Adjusting for LDL and HDL particle concentration rather than LDL and HDL cholesterol concentration resulted in respective odds ratios of 2.19 (95% CI: 0.80, 5.96), 3.61 (95% CI: 1.34, 9.68) and 3.07 (95% CI: 1.34, 7.06). Similarly, parallel analyses for MDA-LDL-IC resulted in an odds ratio of 3.03 (95% CI: 1.25, 7.34) for EDIC Year 12 ICA IMT, an odds ratio of 2.86 (95 % CI: 1.20, 6.81) for ICA IMT progression and an odds ratio of 1.76 (95% CI: 0.87, 3.56) for elevated ICA IMT after adjustment for standard cardiovascular risk factors (Table 4). Adjusting for LDL and HDL particle concentration rather than cholesterol concentration resulted in respective odds ratios of 3.10 (95% CI: 1.17, 8.24), 3.03 (95% CI: 1.21, 7.62) and 2.40 (95% CI: 1.10, 5.22).
Table 3.
Adjusted* Odds Ratio (and 95% confidence interval) from logistic† and GEE‡ regression models for quartiles of AGE-LDL-IC in relation to elevated internal carotid IMT and IMT progression.
| 6 Year ICA IMT† | 12 Year ICA IMT† | IMT Progression Year 1 to 12† | Elevated ICA IMT‡ | |
|---|---|---|---|---|
| Model 1* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.43 (0.60-3.41) | 1.38 (0.60-3.21) | 1.49 (0.62-3.59) | 1.54 (0.73-3.24) |
| Quartile 3 | 1.95 (0.85-4.46) | 1.81 (0.77-4.24) | 2.20 (0.92-5.24) | 2.48 (1.22-5.06) |
| Quartile 4 | 2.95 (1.33-6.55) | 2.82 (1.25-6.39) | 4.03 (1.72-9.45) | 4.14 (2.06-8.31) |
| ROC AUC | 0.754 | 0.805 | 0.792 | |
| Model 2* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.11 (0.45-2.71) | 1.11 (0.46-2.69) | 1.27 (0.50-3.20) | 1.34 (0.62-2.93) |
| Quartile 3 | 1.65 (0.72-3.78) | 1.65 (0.71-3.82) | 2.15 (0.88-5.21) | 2.04 (0.98-4.24) |
| Quartile 4 | 2.05 (0.88-4.74) | 1.96 (0.80-4.79) | 3.50 (1.38-8.86) | 2.95 (1.37-6.34) |
| ROC AUC | 0.784 | 0.839 | 0.826 | |
| Model 3* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 1.26 (0.49-3.26) | 0.87 (0.32-2.40) | 0.98 (0.35-2.81) | 1.21 (0.51-2.87) |
| Quartile 3 | 1.80 (0.72-4.47) | 1.64 (0.63-4.27) | 1.87 (0.72-4.89) | 1.82 (0.82-4.03) |
| Quartile 4 | 2.63 (1.05-6.55) | 2.19 (0.80-5.96) | 3.61 (1.34-9.68) | 3.07 (1.34-7.06) |
| ROC AUC | 0.784 | 0.854 | 0.841 |
Models are as specified in Table 2.
Table 4.
Adjusted* Odds Ratio (and 95% confidence interval) from logistic† and GEE‡ regression models for quartiles of MDA-LDL-IC in relation to elevated internal carotid IMT and IMT progression
| 6 Year ICA IMT† | 12 Year ICA IMT† | IMT Progression Year 1 to 12† | Elevated ICA IMT‡ | |
|---|---|---|---|---|
| Model 1* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 2.88 (1.29-6.40) | 1.59 (0.69-3.64) | 1.14 (0.49-2.64) | 1.33 (0.66-2.60) |
| Quartile 3 | 1.58 (0.69-3.63) | 1.50 (0.64-3.51) | 1.62 (0.72-3.64) | 1.29 (0.64-2.60) |
| Quartile 4 | 2.71 (1.28-5.76) | 2.82 (1.26-6.31) | 2.58 (1.18-5.64) | 2.43 (1.26-4.68) |
| ROC AUC | 0.744 | 0.802 | 0.772 | |
| Model 2* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 2.55 (1.12-5.82) | 1.42 (0.59-3.40) | 1.00 (0.40-2.53) | 0.89 (0.43-1.83) |
| Quartile 3 | 1.31 (0.58-2.97) | 1.24 (0.52-2.99) | 1.40 (0.60-3.27) | 0.84 (0.40-1.76) |
| Quartile 4 | 2.24 (1.05-4.79) | 3.03 (1.25-7.34) | 2.86 (1.20-6.81) | 1.76 (0.87-3.56) |
| ROC AUC | 0.784 | 0.846 | 0.820 | |
| Model 3* | ||||
| Lowest Quartile | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 2.60 (1.10-6.12) | 1.23 (0.46-3.29) | 0.84 (0.30-2.38) | 0.86 (0.40-1.89) |
| Quartile 3 | 1.23 (0.50-3.06) | 0.88 (0.32-2.40) | 1.04 (0.40-2.72) | 0.56 (0.24-1.31) |
| Quartile 4 | 2.95 (1.33-6.52) | 3.10 (1.17-8.24) | 3.03 (1.21-7.62) | 2.40 (1.10-5.22) |
| ROC AUC | 0.788 | 0.859 | 0.836 |
Models are as specified in Table 2.
Finally, we compared the discriminatory power of the addition of oxLDL, AGE-LDL and MDA-LDL concentrations in isolated IC to our model which included standard cardiovascular risk factors (i.e., Model 2 with and without each of our biomarkers). We used areas under the receiver-operating curve (ROC AUC), to compare the discriminatory power of regression models with and without inclusion of each of our biomarkers for the outcome EDIC Year 12 ICA IMT. The ROC AUC for the covariate adjusted model only were 0.831, 0.830 and 0.830, respectively for oxLDL, AGE-LDL and MDA-LDL in isolated IC (i.e., values differed slightly because three individuals were missing different IC values). In comparison, the ROC AUC for models which included oxLDL, AGE-LDL and MDA-LDL in isolated IC were 0.849 (Table 2), 0.839 (Table 3) and 0.846 (Table 4), respectively.
Discussion
Previously, our group reported that high levels of oxLDL and AGE-LDL measured in circulating IC at DCCT baseline, when the patients were free of macrovascular disease, strongly predict progression of carotid artery IMT 8 to14 yrs later after adjusting for conventional risk factors including lipids, albuminuria, HbA1c, blood pressure and smoking(25). Individuals in the highest quartile of oxLDL had a 7-fold increased odds [7.72 (95% CI: 3.27, 18.3)] of having high versus normal ICA IMT relative to those in the lowest quartile of oxLDL, after controlling for treatment group, retinopathy cohort, age, sex, diabetes duration, HbA1c, AER and ultrasonography equipment(25). Adjusting for LDL-cholesterol, HDL-cholesterol, diastolic blood pressure and smoking status attenuated the odds ratios somewhat to 6.11 (95% CI: 2.51, 14.8). Parallel analyses for AGE-LDL resulted in odds ratios of 7.82 (95% CI: 3.17, 19.3) and 6.40 (95% CI: 2.53, 16.2), respectively(25).
In the current study, we extend these findings and report levels of oxLDL-IC, AGE-LDL and MDA-LDL in circulating IC at DCCT closeout (i.e., 5-10 years after DCCT enrollment) are independent predictors for the development and progression of atherosclerosis over a 12 year period. For a biomarker to have clinical utility, it is important to determine the time period over which it predicts disease. At DCCT enrollment participants were young and had a relatively low risk cardiovascular profile (other than having type 1 diabetes). As a result, in our baseline analysis traditional risk factors were not predictive of elevated carotid artery IMT(25). In contrast, in the current study 5-10 years later at DCCT closeout traditional risk factors as well as mLDL in circulating IC independently predict development and progression of atherosclerosis over a 12 year period. Hence, mLDL in circulating IC is predictive very early in the disease process until the time when established risk factors also become predictive.
Prior studies of mLDL have focused on free rather than IC bound mLDL; however, free and IC bound mLDL have distinct properties. Antibodies to mLDL are predominantly of the IgG isotype, subclasses 1 and 3(39-40). Basic science experiments indicate that oxLDL-IC are able to activate the complement system through the classical pathway(41), are more potent activators of human macrophages than the free form oxLDL(42) and have the ability to deliver large concentrations of free and esterified cholesterol to macrophages(43). Additionally, studies indicate that oxLDL-IC is associated with increased macrophage and foam cell formation as well as cell survival (44-45). Hence, oxLDL-IC may be associated with development of atherosclerosis, and may be a mediator, not just a marker, of this pathological process. Results from our analysis of clinical data support this, in that mLDL-IC measured at either the baseline or closeout DCCT examination predict increased carotid IMT, a measure of subclinical atherosclerosis.
A limitation of our study is that the 467 individuals who had mLDL in circulating IC and IMT measurements available during EDIC were selected for a case-control study and, therefore, were not a random sample of the entire DCCT/EDIC study. To overcome this selection bias we have conducted a weighted analysis(38) and adjusted for DCCT retinopathy cohort, AER, diabetes duration and HbA1c throughout all analysis. However, there may still be some residual confounding which we were unable to account for in our analysis. A second limitation of our study is that our outcome is carotid IMT, a measure of subclinical atherosclerosis. While carotid IMT is an appropriate endpoint for examining development of atherosclerosis and is highly predictive of cardiovascular outcomes(16-17), future studies will examine the association of biomarkers with actual cardiovascular events.
Given the strength of our findings, mLDL-ICs may serve as important biomarkers to predict progression of atherosclerosis. In addition to the results presented, we have completed analyses indicating mLDL measured at the baseline DCCT examination predicted development of coronary artery calcification(46), nephropathy(47) and retinopathy(48). Additionally, we recently report that high levels of MDA-LDL IC are associated with increased risk of myocardial infarction [HR=2.44 (95% CI: 1.03, 5.77)] in the Veteran Affairs Diabetes Trial, a cohort of Veterans with established type 2 diabetes. Knowledge of the role of mLDL in circulating IC on the disease pathway may provide a mechanism through which the development of atherosclerosis or clinical events could be prevented. Future work is required to determine the ability of modified LDL in circulating IC to predict hard cardiovascular events as well as their predictive ability in the general population.
Supplementary Material
Highlights.
Over 90% of modified LDL circulates as part of immune complexes (IC).
Few prior studies have examined modified LDL circulating as part of IC.
oxLDL in circulating IC is a strong predictor of atherosclerosis.
AGE-LDL in circulating IC is a strong predictor of atherosclerosis.
Knowledge of their role in disease may provide mechanisms to target for prevention.
Acknowledgments
This work was supported by a Program Project funded by the National Institutes of Health/NHLBI (PO1 HL 55782), by two R01 Grants funded by NIH/NIDDK (R01 DK081352 and R01 DK088778) and by a Juvenile Diabetes Foundation Grant (2006-49). The work was also supported by the Research Service of the Ralph H. Johnson Department of Veterans Affairs Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translation Science Centers Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases.
Additional support was provided by the National Center for Research Resources through the GCRC program and by Genentech Inc through a Cooperative Research and Development Agreement with the NIDDK.
Abbreviations
- AGE-LDL
advanced glycation end products-LDL
- AER
albumin excretion rate
- ROC AUC
area under the receiver- operating curve
- CCA
common carotid arteries
- CT
computed tomography
- CAC
Coronary Artery Calcification
- DCCT
Diabetes Control and Complications Trial
- ETDRS
Early Treatment Diabetic Retinopathy Study
- EDIC
Epidemiology of Diabetes Interventions and Complications
- GEE
generalized estimating equations
- HbA1c
hemoglobin A1c
- IC
immune complexes
- ICA
internal carotid artery
- IMT
intima-media thickness
- MDA -LDL
malondialdehyde-LDL
- mLDL-IC
modified LDL in immune complexes
- oxLDL
oxidized LDL
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Holvoet P, Stassen JM, Van Cleemput J, Collen D, Vanhaecke J. Oxidized low density lipoproteins in patients with transplant-associated coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(1):100–7. doi: 10.1161/01.atv.18.1.100. [DOI] [PubMed] [Google Scholar]
- 3.Lopes-Virella MF, Virella G. The role of immune and inflammatory processes in the development of macrovascular disease in diabetes. Front Biosci. 2003;8:s750–68. doi: 10.2741/1141. [DOI] [PubMed] [Google Scholar]
- 4.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103(11):1547–60. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103(15):1955–60. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 6.Holvoet P, Collen D, Van de Werf F. Malondialdehyde-modified LDL as a marker of acute coronary syndromes. JAMA. 1999;281(18):1718–21. doi: 10.1001/jama.281.18.1718. [DOI] [PubMed] [Google Scholar]
- 7.Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21(5):844–8. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 8.Toshima S, Hasegawa A, Kurabayashi M, Itabe H, Takano T, Sugano J, et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20(10):2243–7. doi: 10.1161/01.atv.20.10.2243. [DOI] [PubMed] [Google Scholar]
- 9.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98(15):1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 10.Virella G, Derrick MB, Pate V, Chassereau C, Thorpe SR, Lopes-Virella MF. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol. 2005;12(1):68–75. doi: 10.1128/CDLI.12.1.68-75.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virella G, Thorpe S, Alderson NL, Derrick MB, Chassereau C, Rhett JM, et al. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Research. 2004;45:1859–67. doi: 10.1194/jlr.M400095-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Lopes-Virella MF, Binzafar N, Rackley S, Takei A, La Via M, Virella G. The uptake of LDL-IC by human macrophages: predominant involvement of the Fc gamma RI receptor. Atherosclerosis. 1997;135(2):161–70. doi: 10.1016/s0021-9150(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 13.Griffith RL, Virella GT, Stevenson HC, Lopes-Virella MF. Low density lipoprotein metabolism by human macrophages activated with low density lipoprotein immune complexes. A possible mechanism of foam cell formation. J Exp Med. 1988;168(3):1041–59. doi: 10.1084/jem.168.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes-Virella MF, Griffith RL, Shunk KA, Virella GT. Enhanced uptake and impaired intracellular metabolism of low density lipoprotein complexed with anti-low density lipoprotein antibodies. Arterioscler Thromb. 1991;11(5):1356–67. doi: 10.1161/01.atv.11.5.1356. [DOI] [PubMed] [Google Scholar]
- 15.Gisinger C, Virella GT, Lopes-Virella MF. Erythrocyte-bound low-density lipoprotein immune complexes lead to cholesteryl ester accumulation in human monocyte-derived macrophages. Clin Immunol Immunopathol. 1991;59(1):37–52. doi: 10.1016/0090-1229(91)90080-t. [DOI] [PubMed] [Google Scholar]
- 16.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–7. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 17.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146(6):483–94. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 18.O'Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22(9):1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 19.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134(3):250–6. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 20.Bots ML, Witteman JC, Grobbee DE. Carotid intima-media wall thickness in elderly women with and without atherosclerosis of the abdominal aorta. Atherosclerosis. 1993;102(1):99–105. doi: 10.1016/0021-9150(93)90088-c. [DOI] [PubMed] [Google Scholar]
- 21.O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson SK, Jr, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23(12):1752–60. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 22.Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke. 1997;28(12):2442–7. doi: 10.1161/01.str.28.12.2442. [DOI] [PubMed] [Google Scholar]
- 23.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112(10):1018–31. [PubMed] [Google Scholar]
- 24.Li R, Duncan BB, Metcalf PA, Crouse JR, 3rd, Sharrett AR, Tyroler HA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25(12):2377–83. doi: 10.1161/01.str.25.12.2377. [DOI] [PubMed] [Google Scholar]
- 25.Lopes-Virella MF, Hunt KJ, Baker NL, Lachin J, Nathan DM, Virella G. Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes. 2011;60(2):582–9. doi: 10.2337/db10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 27.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348(23):2294–303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Effect of intensive diabetes treatment on carotid artery wall thickness in the Epidemiology of Diabetes Interventions and Complications. Diabetes. 1999;48:383–90. doi: 10.2337/diabetes.48.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes-Virella MF, McHenry MB, Lipsitz S, Yim E, Wilson PF, Lackland DT, et al. Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis. 2007;190(2):359–69. doi: 10.1016/j.atherosclerosis.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Virella G, Carter RE, Saad A, Crosswell EG, Game BA, Lopes-Virella MF. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin Immunol. 2008;127(3):394–400. doi: 10.1016/j.clim.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virella G, Derrick MB, Pate V, Chassereau C, Thorpe SR, Lopes-Virella MF. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol. 2005;12(1):68–75. doi: 10.1128/CDLI.12.1.68-75.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. The DCCT Research Group. Clin Chem. 1987;33(12):2267–71. [PubMed] [Google Scholar]
- 35.Steffes M, Cleary P, Goldstein D, Little R, Wiedmeyer HM, Rohlfing C, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem. 2005;51(4):753–8. doi: 10.1373/clinchem.2004.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48(3-4):171–80. [PubMed] [Google Scholar]
- 37.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98(Suppl):823–33. [PubMed] [Google Scholar]
- 38.Horvitz DG, Thompson DJ. A generalization of sampling without replcemnet from a finite universe. Journal of the American Statistical Association. 1952;47:663–85. [Google Scholar]
- 39.Virella G, Thorpe SR, Alderson NL, Stephan EM, Atchley D, Wagner F, et al. Autoimmune response to advanced glycosylation end-products of human LDL. J Lipid Res. 2003;44(3):487–93. doi: 10.1194/jlr.M200370-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Virella G, Koskinen S, Krings G, Onorato JM, Thorpe SR, Lopes-Virella M. Immunochemical characterization of purified human oxidized low-density lipoprotein antibodies. Clin Immunol. 2000;95(2):135–44. doi: 10.1006/clim.2000.4857. [DOI] [PubMed] [Google Scholar]
- 41.Virella G, Atchley D, Koskinen S, Zheng D, Lopes-Virella MF. Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin Immunol. 2002;105(1):81–92. doi: 10.1006/clim.2002.5269. [DOI] [PubMed] [Google Scholar]
- 42.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res. 2006;47(9):1975–83. doi: 10.1194/jlr.M600064-JLR200. Epub 2006 Jun 27. [DOI] [PubMed] [Google Scholar]
- 43.Virella G, Atchley DH, Koskinen S, Zheng D, Lopes-Virella M. Pro-atherogenic and pro-inflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clinical Immunology. 2002;105:81–92. doi: 10.1006/clim.2002.5269. [DOI] [PubMed] [Google Scholar]
- 44.Al Gadban MM, Smith KJ, Soodavar F, Piansay C, Chassereau C, Twal WO, et al. Differential trafficking of oxidized LDL and oxidized LDL immune complexes in macrophages: impact on oxidative stress. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KJ, Twal WO, Soodavar F, Virella G, Lopes-Virella MF, Hammad SM. Heat shock protein 70B' (HSP70B') expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. J Biol Chem. 2010;285(21):15985–93. doi: 10.1074/jbc.M110.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lopes-Virella MF, Baker NL, Hunt KJ, Lachin J, Nathan D, Virella G. Oxidized LDL immune complexes and coronary artery calcification in type 1 diabetes. Atherosclerosis. 2011;214(2):462–7. doi: 10.1016/j.atherosclerosis.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes-Virella MF, Carter RE, Baker NL, Lachin J, Virella G. High levels of oxidized LDL in circulating immune complexes are associated with increased odds of developing abnormal albuminuria in Type 1 diabetes. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfr454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopes-Virella MF, Baker NL, Hunt KJ, Lyons TJ, Jenkins AJ, Virella G. High concentrations of AGE-LDL and oxidized LDL in circulating immune complexes are associated with progression of retinopathy in type 1 diabetes. Diabetes Care. 2012;35(6):1333–40. doi: 10.2337/dc11-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.