SUMMARY
During each life cycle germ cells preserve and pass on both genetic and epigenetic information. In C. elegans, the ALG-3/4 Argonaute proteins are expressed during male gametogenesis and promote male fertility. Here we show that the CSR-1 Argonaute functions with ALG-3/4 to positively regulate target genes required for spermiogenesis. Our findings suggest that ALG-3/4 functions during spermatogenesis to amplify a small-RNA signal that represents an epigenetic memory of male-specific gene expression. CSR-1, which is abundant in mature sperm, appears to transmit this memory to offspring. Surprisingly, in addition to small RNAs targeting male-specific genes, we show that males also harbor an extensive repertoire of CSR-1 small RNAs targeting oogenesis-specific mRNAs. Together these findings suggest that C. elegans sperm transmit not only the genome but also epigenetic binary signals in the form of Argonaute/small-RNA complexes that constitute a memory of gene expression in preceding generations.
INTRODUCTION
The transmission of information independently of the DNA sequence of the genome is termed epigenetic inheritance. During sexual reproduction both genetic and epigenetic information is passed to the zygote via specialized germ cells known as gametes. Gametogenesis involves dynamic molecular and morphological changes, culminating in the creation of highly specialized sperm and egg cells that package a haploid genome and all of the cellular machinery and epigenetic information necessary to launch zygotic development upon fertilization. Although many of the pathways required for gametogenesis are phylogenetically conserved (Eddy, 2002), especially those that mediate the partitioning of genetic information, very little is known about how gametes package and transmit epigenetic inheritance.
Male gametogenesis is an amazing example of cellular differentiation, in which undifferentiated male germ cells proceed through meiosis and develop into motile spermatozoa. In mammals the process of spermiogenesis, when spermatids differentiate into highly polarized motile spermatozoa, is initiated by a massive wave of gene expression essential for post-meiotic differentiation (Sassone-Corsi, 2002). Shortly thereafter, transcription ceases, and compaction of the haploid male genome ensues. Genome compaction within differentiating spermatids is facilitated by the replacement in chromatin of histones with small basic proteins called protoamines (Wykes and Krawetz, 2003).
The culmination of male gametogenesis is a motile gamete capable of initiating fertilization and delivering a paternal genome complement to an egg. However, genetic material is not the only information packaged in the sperm. Epigenetic information is also transmitted in the form of chromatin (DNA and/or histone) modifications, and RNA. In humans and mice paternal epigenetic factors have been shown to influence metabolism, stress response, and reproduction (Rando, 2012).
In C. elegans epigenetic inheritance involves Argonaute/small RNA pathways. Argonautes are structurally related to ribonuclease H and gain sequence specificity via small guide RNAs. Upon binding, Argonautes can direct endonucleolytic cleavage of target mRNAs, or can recruit cofactors that mediate post-transcriptional or transcriptional silencing (Ghildiyal and Zamore, 2009). In C. elegans mutations that perturb Argonaute pathways often result in infertility (Batista et al., 2008; Buckley et al., 2012; Claycomb et al., 2009; Conine et al., 2010; Gu et al., 2009; Han et al., 2009; Pavelec et al., 2009). For example, the Piwi Argonaute PRG-1, is required for both male and hermaphrodite fertility, and has been linked to transposon and transgene silencing (Batista et al., 2008; Ruby et al., 2006). PRG-1 engages over 30,000 distinct species of genomically-encoded small RNAs, termed Piwi-interacting (pi) RNAs (Batista et al., 2008; Gu et al., 2012). PRG-1/piRNA complexes are thought to utilize imperfect base pairing to scan germline-expressed mRNAs (Bagijn et al., 2012; Lee et al., 2012). When PRG-1/piRNA complexes bind to foreign RNA sequences, such as those produced by a transgene, they initiate the production, via RNA-dependent RNA polymerase (RdRP), of amplified small RNAs called 22G-RNAs (Gu et al., 2009). These 22G-RNAs are in turn loaded onto members of an expanded group of Worm-specific Argonaute (WAGO) proteins (Yigit et al., 2006), which silence gene expression transcriptionally and post-transcriptionally (Buckley et al., 2012; Gu et al., 2009). This form of RNA-induced epigenetic silencing (referred to as RNAe) is then stably transmitted via both the sperm and the egg, apparently indefinitely through subsequent generations (Shirayama et al., 2012).
A major question related to the mechanism by which PRG-1 surveys germline gene expression is how certain mRNAs are recognized as self and protected from silencing. The CSR-1 Argonaute is a candidate factor for mediating self-recognition. CSR-1 is related to WAGOs but engages RdRP-derived small RNAs antisense to most if not all germline-expressed mRNAs. Therefore, it is possible that targeting by CSR-1 prevents PRG-1 recognition of self mRNA. If this model is correct, then a mechanism must exist during gametogenesis to package a cache of CSR-1 22G-RNAs reflecting the state of gene expression during each phase of the germline life cycle.
In this study, we investigate the role of Argonaute small RNA pathways during spermatogenesis in C. elegans. Previous work identified ALG-3 and ALG-4 (ALG-3/4) as redundant AGO-clade Argonautes that promote male fertility (Conine et al., 2010; Han et al., 2009). ALG-3/4 engage a class of Dicer and RdRP-dependent small RNAs termed 26G-RNAs that are antisense to mRNAs expressed during spermatogenesis (Conine et al., 2010; Han et al., 2009; Pavelec et al., 2009). Here we show that sperm transcripts that are targeted by ALG-3/4 26G-RNAs are also targeted by CSR-1 22G-RNAs in the male germline. We show that alg-3/4 and csr-1 mutant males exhibit identical temperature-sensitive sterile phenotypes that result from failed spermiogenesis. Both ALG-3/4 and CSR-1 are required for robust transcription of spermiogenic mRNAs, suggesting that ALG-3/4 and CSR-1 function in the same pathway. Consistent with this, CSR-1 associates with the chromatin of spermiogenic transcripts and localizes to chromatin at the periphery of sperm nuclei in an ALG-3/4–dependent manner.
ALG-3/4 and 26G-RNAs are absent or greatly reduced in mature sperm (Conine et al., 2010). However, we show that CSR-1 and associated 22G-RNAs antisense to ALG-3/4 targets are abundant in mature sperm. Surprisingly, sperm also contain CSR-1 small RNAs antisense to female-specific germline mRNAs. We show that heterozygous offspring of homozygous alg-3/4 or csr-1 males exhibit reduced fertility. Moreover, repeatedly backcrossing heterozygous hermaphrodites to homozygous mutant males results in a progressive loss of fertility (germline-mortal phenotype) that can be rescued by wild-type sperm. Taken together, these findings are consistent with a model in which ALG-3/4 and CSR-1 and their associated small RNAs provide an epigenetic memory of paternal gene expression, thereby ensuring the trans-generational continuity of a robust spermiogenic program.
RESULTS
The previously identified fer genes function in the ALG-3/4 pathway
The infertility phenotypes of alg-3/4 mutant sperm are identical to those of temperature-sensitive fertilization-defective (fer) mutants isolated in genetic screens more than 30 years ago (Argon and Ward, 1980; Hirsh and Vanderslice, 1976). To date, most of the fer genes remain molecularly uncharacterized except for fer-1, which encodes a member of the Ferlin family of membrane proteins required for the fusion of intracellular vesicles during spermiogenesis (Washington and Ward, 2006).
The similarity of alg-3/4 and fer mutant phenotypes extends to the ultra-structural level as assayed by electron microscopy (Figure 1, see Supplemental Results). For example, alg-3/4 mutants, like the previously characterized fer mutants arrest as round non-polarized spermatids in which sperm-specific organelles fail to undergo a series of stereotypic fusion events and structural reorganizations that drive pseudopod formation. Mutant spermatids also exhibit a missing or abnormal peri-nuclear RNA halo (Figure 1, Ward et al., 1981). Given the striking similarities between the fer and alg-3/4 mutant phenotypes, we asked whether the fer genes might function in the ALG-3/4 pathway. This analysis revealed that fer-2, -3, -4, -6, and -15 exhibit defects in the production of ALG-3/4 pathway 26G-RNAs (Figure S1A). Furthermore, we found that fer-3(hc3) and fer-15(b26) exhibit an Eri phenotype (Figure S1B) and, like other eri mutants, are required for ERGO-1 pathway 26G-RNAs (Figure S1A). Indeed, fer-3 and fer-15 mutations map nearby the ERI-pathway genes eri-3 and rrf-3, respectively (Ward et al., 1981). fer-3(hc3) failed to complement the Fer and Eri phenotypes of eri-3(tm1361) (Figure 1B). Consistent with this finding, and the partial loss of male-specific 26G-RNAs, we identified a nucleotide substitution in exon 3 of eri-3 that is predicted to result in a serine-to-proline missense mutation at amino acid 69 (Figure S1C). fer-15(b26) failed to complement the Fer and Eri phenotypes of rrf-3(pk1426), and we identified a 223 bp deletion that removes exon 6 of rrf-3 and is predicted to shift the reading frame and introduce a premature termination codon (Figure S1C). fer-2, fer-4 and fer-6 mutants were not Eri, and their molecular identities remain to be determined. Taken together, our findings indicate that several previously isolated fer mutants define genes that function in the ALG-3/4-26G-RNA pathway.
Figure 1. Ultrastructural analysis of alg-3/4 spermatozoa.
Transmission electron micrographs of spermatozoa from wild-type (WT, top row) or alg-3/4 males (bottom row) cultured at 20°C (left) or 25°C (center and right). Examples of membranous organelles (black arrowheads) and Fibrous bodies (fb) are indicated. Representative nuclei of WT and alg-3/4 spermatozoa are shown at right. Dotted lines emphasize the RNA halo surrounding the wild-type nucleus. White arrows label large tubule-like structures (visible in cross-section (Ward et al., 1981)) adjacent to alg-3/4 nuclei. See also Figure S1.
Target regulation by ALG-3/4 and 26G-RNAs
Our previous deep-sequencing studies identified 397 genes as high-confidence targets of ALG-3/4–dependent 26G-RNAs using a 10 reads-per-million (rpm) cut-off (Conine et al., 2010). To identify the full repertoire of ALG-3/4 targets, we cloned and deep sequenced small RNAs isolated from alg-3/4 males and WT males cultured at both 20° and 25°C. In total, we identified 1497 genes target ed by 26G-RNAs in wild-type males at a density of at least 5 rpm. We found that 94% (1408) of these genes exhibited a >2-fold reduction in 26G-RNAs in alg-3/4 mutants and were thus designated as ALG-3/4 target genes (Table S1). The remaining 6% of genes targeted by 26G-RNAs that were unaffected in alg-3/4 mutants are likely to be ERGO-1–dependent 26G-RNA targets (Vasale et al., 2010). The expanded list of 1408 ALG-3/4 26G-RNA targets accounts for 63% (617/970) of genes with sperm-specific expression (Reinke et al., 2004).
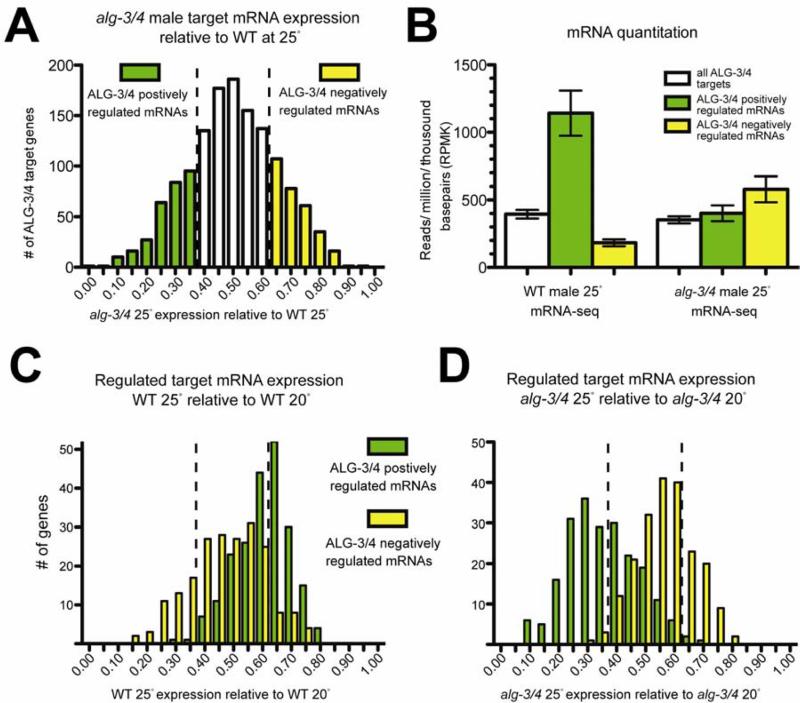
Many ALG-3/4 targets are required for spermiogenesis and are involved in pseudopod formation and sperm motility. For example, these targets include genes that encode MSPs and MSP-related proteins (Burke and Ward, 1983), as well as factors required for sperm motility (Buttery et al., 2003). Because the activities of ALG-3/4 and of many of their target genes are required for spermiogenesis, it therefore seemed unlikely that ALG-3/4 directs the silencing of these targets. To determine how ALG-3/4 and associated 26G-RNAs regulate their targets we performed a combination of mRNA deep sequencing (mRNA-seq) and proteomics on WT and alg-3/4 mutant males grown at 20° and 25°C (Figure 2A and Figure S2A). Finding s from this analysis, which are detailed in Supplemental Information, suggest that ALG-3/4 promote the expression of many targets, and do so by increasing corresponding mRNA levels at elevated temperatures. We found that approximately 214 target mRNAs were positively regulated by 2-fold or more (decreased by 2-fold or more in the mutant), 204 were negatively regulated by 2-fold or more, and 991 target mRNAs did not change (Figure 2A and Table 1). Strikingly, we found that positively-regulated mRNAs were much more abundant in WT males than were non-regulated (4-fold lower) or negatively-regulated (6-fold lower) mRNAs (Figure 2B). This enrichment of positively-regulated mRNAs was completely dependent on ALG-3/4 activity (was abolished in alg-3/4 mutants, Figure 2B). Our proteomic analysis revealed 122 proteins that decreased by at least 1.5 fold in alg-3/4 mutants relative to WT, and 43 that increased (Figure S2A and Table S2). For each protein identified in our proteomic analysis, we compared the change in protein level in alg-3/4 males at 25°C to the change in mRNA (Figure S2B & E). For most ALG-3/4-dependent positively-regulated proteins (81/122, 66%), the decrease in protein level in the alg-3/4 mutant could be explained by a reduction in the corresponding mRNA. By contrast, for 33% (14/43) of ALG-3/4 negatively-regulated proteins, we found that the increase in protein could be attributed to changes in corresponding mRNAs. These data indicate that the ALG-3/4 pathway promotes the expression of many of its targets and does so by increasing their mRNA levels.
Figure 2. ALG-3/4 positively and negatively regulates hundreds of target mRNAs.
(A) Histogram illustrating the enrichment or depletion of ALG-3/4 target mRNAs in alg-3/4 mutants relative to WT at 25°C. Dotted lines indica te a two-fold change, values approaching 1 indicate enrichment (ALG-3/4 negatively-regulated mRNAs), and values approaching 0 indicate depletion (ALG-3/4 positively-regulated mRNAs). In A-D, colored bars indicate ALG-3/4 targets positively-regulated (green) or negatively-regulated (yellow) more than two-fold. Enrichment was calculated as the Reads Per Million (RPM) ratio of alg-3/4 / (alg-3/4 + WT).
(B) Bar graphs showing the average RPM per kilobase (RPMK) for ALG-3/4 regulated targets in WT or alg-3/4 mutant at 25°C.
(C and D) Histogram illustrating the enrichment of positively- or negatively-regulated ALG-3/4 target mRNAs in WT (C) or alg-3/4 (D) males at 25°C relative to 20°C. Enrichment was calculated as the RPM ratio of 25°C / (25°C + 20°C).
See also Figures S2 and S3, and Tables S1 and S2.
In alg-3/4 mutants, sperm defects are much more severe at high temperature (Conine et al., 2010). We therefore wished to know how ALG-3/4 target mRNA levels changed with temperature in both the wild-type and alg-3/4 mutant strains. We found that, in wild-type males positively-regulated mRNAs increased at 25°C relative to 20°C, whereas negatively-regulated mRNAs tended to decrease at 25°C (Figure 2C). The converse was true for alg-3/4 mutant males: positively-regulated mRNAs decreased dramatically at 25°C compared to 20°C, whereas nega tively-regulated targets increased, albeit less dramatically (Figure 2D). Similar analyses of our proteomic data also corroborate these findings (Figure S2C & S2D). The described changes in transcript levels by mRNA-seq were confirmed using RT-qPCR (Figure S3). These data support the existence of at least two distinct classes of ALG-3/4 target genes: one that is dramatically up-regulated by ALG-3/4 at elevated temperature, and one that is moderately down-regulated by ALG-3/4 at elevated temperature.
ALG-3/4-positively-regulated targets are clearly genes required for spermiogenesis and motility, including many of the MSP and MSP-related genes involved in pseudopod formation, as well as the PP1 phosphatases encoded by gsp-3/4, which are required for multiple aspects of C. elegans sperm development and when mutated exhibit temperature-sensitive (TS) sperm defects similar to alg-3/4 (Wu et al., 2012). ALG-3/4 negatively-regulated targets include genes associated with mitochondrial function (electron transport chain and cellular respiration), but a correlation between reproductive genes and negatively-regulated ALG-3/4 targets was not observed (Table S1 and S2).
To confirm that ALG-3/4 promotes the expression of target genes in the germline, we used immunofluorescence (IF) assays to detect MSP and GSP-3 proteins and fluorescence in situ hybridization (FISH) to detect msp transcripts. Interestingly, IF of MSP and GSP-3, two positively-regulated targets, suggested that these proteins were only slightly reduced in alg-3/4 germlines during spermatogenesis and in haploid spermatids at 20°C compared to WT (Figure 3A). At 2 5°C, however, MSP and GSP-3 were severely reduced throughout the spermatogenic germline of alg-3/4 males compared to WT and failed to accumulate in spermatids (Figure 3A). Although GSP-3 protein was depleted overall, we found that it also accumulated abnormally in the nuclei of alg-3/4 mutants, particularly at 25°C (Figure 3A). In addit ion, we observed more nuclei undergoing chromatin condensation in alg-3/4 males compared to wild-type males (see below). Examining spermatids released from the testes of alg-3/4 mutants raised at 25°C and activated to undergo spermiogene sis in vitro revealed that 12% of alg-3/4 spermatozoa accumulate near WT levels of MSP and GSP-3. Unlike WT spermatozoa, however, these alg-3/4 spermatozoa never formed pseudopods of normal morphology or polarity, with MSP and GSP-3 localized to the leading edge of the pseudopod (Figure 3B). Instead, we observed that: 1) MSP and GSP-3 remained in large punctae that resemble sperm organelles called Fibrous Bodies (FBs) (5%); 2) MSP localized to the cortex and GSP-3 to the cytoplasm of the spermatid but lacked polarity (3%); or 3) GSP-3 remained in the cytoplasm, and spermatids formed a multibranched, spikey pseudopod containing MSP (4%). Consistent with our mRNA-seq and RT-qPCR data, FISH revealed that msp mRNA was indeed reduced in the spermatogenic germline of alg-3/4 males compared to wild-type males (Figure 3C). This reduction was more pronounced at 25°C, with very li ttle signal detected in alg-3/4 male germlines, consistent with the temperature-dependent regulation of expression (Figure 3C).
Figure 3. ALG-3/4 positively-regulate genes required for spermiogenesis.
(A-C) Confocal images of WT and alg-3/4 mutant gonads (A, C) and activated spermatocytes (B). Each column of photographs represent a single specimen imaged with different fluorescence channels (as indicated). A merged image is shown at the bottom. The genotype and temperature at which spermatogenesis occurred are indicated. DNA was visualized by DAPI staining (blue). In (A and B) the sperm proteins MSP (green) and GSP-3 (red) are visualized by Immunofluorescence. In (B) the percentage of WT and alg-3/4 spermatozoa that exhibit the staining pattern shown is indicated. In (C) msp mRNA is visualized by fluorescence in situ hybridization (FISH).
ALG-3/4 promotes the transcription of target genes
Our findings suggest that ALG-3/4 positively regulates targets at the level of the mRNA. We therefore sought to determine whether ALG-3/4 promotes the transcription of positively-regulated targets. Using RNA polymerase II (pol II) chromatin immunoprecipitation (ChIP) followed by qPCR, we found that pol II occupancy of positively-regulated ALG-3/4 targets decreased 1.5- to 3-fold in alg-3/4 males compared to wild-type males cultured at 20°C. Notably, pol I I occupancy was reduced 3- to 7-fold in alg-3/4 males grown at 25°C (Figure 4A). Consistent with t he reduced pol II occupancy in alg-3/4 males, the pre-mRNAs of positively-regulated targets were also decreased in alg-3/4 and fer-15/rrf-3 males by 2- to 4-fold at 20°C and by 4- to 8-fold a t 25°C. These findings suggest that both the Argonaut e (ALG-3/4) and RdRP (FER-15/RRF-3) promote the transcription of positively-regulated targets (Figure 4B). Pol II occupancy of spe-12 and col-122, two control genes expressed in male germline and somatic tissues (respectively) but not targeted by ALG-3/4 26G-RNAs, was not dependent on alg-3/4 or temperature (Figure 4A).
Figure 4. ALG-3/4 promote transcription and CSR-1 nuclear localization in condensing meiotic nuclei.
(A) qPCR analysis of RNA pol II ChIP at ALG-3/4 target genes in wild-type (WT) and alg-3/4 mutants at 20°C and 25°C, normalized to an intergen ic region not occupied by pol II. Data are represented as mean +/− SEM. ALG-3/4 negatively-regulated targets are labeled in grey.
(B) RT-qPCR analysis of ALG-3/4 target pre-mRNAs. Data are normalized to the non-target gpd-2 pre-mRNA and represented as mean +/− SEM.
(C and D) Confocal IF images of dissected WT (left) or alg-3/4 (right) spermatogenic germlines at 25°C stained with antibodies against ( C) elongating RNA pol II (red) and CSR-1 (green), or (D) antibodies against H3K4me2 (green) and Nuclear pore protein (red). White arrows denote spermatocyte nuclei lacking pol II staining. DNA was stained with DAPI (blue).
See also Figure S4.
Curiously, pol II occupancy of the negatively-regulated gene ssp-16 was reduced by 2- and 3-fold in alg-3/4 males at 20° and 25°C, respectively, relative to w ild-type levels (Figure 4A). Pol II occupancy of a second negatively-regulated target f36h12.4 was unchanged at 20°C and reduced nearly 2-fold at 25°C compared to wild-type levels (Figure 4A). The expression of the f36h12.4 pre-mRNA mirrored these findings. Each of the five negatively-regulated targets that we examined showed a similar reduction in pre-mRNA at 25°C (Figure 4B). These results, theref ore, suggest that negatively-regulated ALG-3/4 targets are silenced at a post-transcriptional level.
Because the WAGO 22G-RNA pathway is required for silencing (Gu et al., 2009), we tested whether the WAGO pathway is required to silence negatively-regulated ALG-3/4 targets at a post-transcriptional level. Indeed, we found that the mRNA levels of several negatively-regulated targets were increased in males lacking rde-3 or deleted for all 12 WAGOs (MAGO-12; Figure S4). These increased levels were similar to those observed in alg-3/4 males (Conine et al., 2010). Strikingly, the pre-mRNA levels of these negatively-regulated targets remained unchanged in rde-3 or MAGO-12 males. Thus as previously hypothesized (Conine et al., 2010), ALG-3/4 negatively regulates a subset of its targets at a post-transcriptional level via the WAGO pathway.
To examine the temporal and spatial regulation of transcription in the male germline by ALG-3/4, we stained male germlines with an antibody that recognizes elongating pol II (Ahn et al., 2004). In wild-type germlines at 25°C, elongating pol II was detected at the periphery of condensing spermatocyte nuclei and in small discrete foci in spermatids (Figure 4C). By contrast, in alg-3/4 germlines at 25°C, elongating pol II disappeared from spermatocyte nuclei at the onset of the condensation process (Figure 4C), but was detected later in spermatogenesis throughout the nucleus in spermatocytes, not just at the nuclear periphery (Figure 4C). We next examined a histone modification associated with actively transcribed chromatin (Histone H3 dimethylated on Lysine 4, H3K4me2, (Kelly et al., 2002). This analysis revealed H3K4me2 localization throughout the nucleus in wild-type spermatocytes at 25°C. However, H3K4me2 was dramatically reduced in alg-3/4 spermatocytes and was present only in small patches of chromatin (Figure 4D).
CSR-1 acts in the ALG-3/4 pathway to promote sperm development
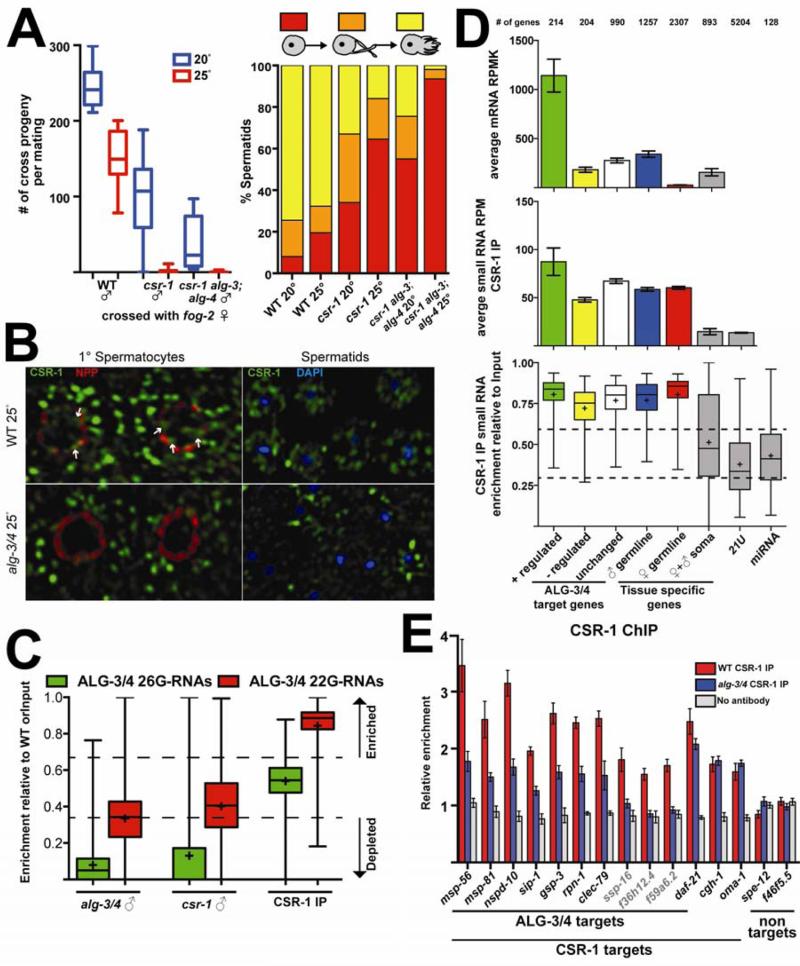
Previous work suggested that the Argonaute CSR-1 and its 22G-RNA cofactors target but do not silence germline-expressed genes (Claycomb et al., 2009), and recent work suggests that CSR-1 promotes germline transgene expression (Seth et al., submitted). Homozygous csr-1(tm892) mutant hermaphrodites are essentially sterile, but produce a few embryos that die due to chromosome segregation defects (Yigit et al., 2006, Claycomb et al., 2009). Although csr-1 males were sterile at 25°C, we were surprised to find that they were ~50% fertile relative to wild-type males at 20°C (Figure 5A). This temperature-dependent sterility was similar to that observed for alg-3/4. Indeed, like alg-3/4 mutant spermatids, csr-1 spermatids failed to complete spermiogenesis at 25°C and arrested as either round spermatids or spermatids with non-motile or spiky pseudopods (Figure 5A).
Figure 5. CSR-1 associates with both male- and female-specific small RNAs in males and positively regulates spermiogenic gene expression.
(A) (Left) Fertility of WT, csr-1, or csr-1 alg-3; alg-4 males at 20°C or 25°C measured in crosses with fog-2 females. Box and whisker plots represent a range of cross progeny from at least 15 independent crosses for each genotype. (Right) In vitro activation of spermatids isolated from csr-1, csr-1 alg-3; alg-4, or WT males grown at 20°C or 25°C. Bar graphs show the percent of spermatids that fail to activate (red), partially activate and form spikey projections (orange), or fully activate and form a pseudopod (yellow) (n>300).
(B) Confocal IF images of primary (1°) spermatocytes (l eft) or spermatids (right) in dissected alg-3/4 or WT germlines stained with antibodies against CSR-1 (green) and a nuclear pore protein (red). DNA was stained with DAPI (blue). White arrows denote chromatin domains where CSR-1 localizes.
(C) Box and whisker plots indicate depletion of 26G-RNAs and 22G-RNAs antisense to ALG-3/4 targets in alg-3/4 and csr-1 males at 25°C. Box and whisker plots to the right indicate enrichment of 22G-RNA and lack of enrichment of 26G-RNAs antisense to ALG-3/4 targets in the CSR-1 IP.
(D) Analysis of mRNA expression and CSR-1 small RNA levels in wild-type males. Categories of gene expression are indicated below the bottom graph. ALG-3/4 targets as defined in Figure 1: positively-regulated (green), negatively-regulated (yellow), and unchanged (White). Male-germline specific genes (blue), includes many ALG-3/4 targets. Female-germline specific genes (red) – not targeted by ALG-3/4. Soma-specific mRNAs and 21U-RNAs are control sequences not enriched by CSR-1 IP. The number of genes in each category is indicated above the graphs. Top graph: mRNA expression as monitored by RNA-Seq in reads per million per kilobase (RPMK). Middle graph: small RNA levels in reads per million (RPM) per gene. Bottom graph: box and whisker plot indicating the enrichment or depletion in CSR-1 IP of small RNAs antisense to genes. Enrichment was calculated as the RPM ratio of FLAG::CSR-1 IP / (FLAG::CSR-1 IP + Input). Dotted lines indicate 2-fold enrichment (upper) or depletion (lower). ‘+’ indicates the average enrichment value for all genes in the category.
(E) qPCR analysis of FLAG::CSR-1 ChIP at genes targeted by ALG-3/4, CSR-1, or neither. ALG-3/4 negatively-regulated targets are indicated in grey text. Data were normalized to y47h10a.3, which is not targeted by ALG-3/4 or CSR-1. Data are represented as mean +/− SEM.
See also Figures S3 and S5, and Table S3.
Consistent with the possibility that CSR-1 functions in the same pathway as ALG-3/4, we found that triple csr-1 alg-3; alg-4 mutant males were completely sterile at 25°C with defects in spermiogenesis almost identical to those observed in the csr-1 single, and alg-3/4 double mutants respectively (Figure 5A; Conine et al., 2010). At 20°C csr-1 alg-3; alg-4 males exhibited fertility defects similar to, but slightly more severe, than csr-1 or alg-3/4 males (Figure 5A). Furthermore, csr-1 males grown at 25°C exhibited defects in elongating pol II and H3K4me2 localization in the spermatogenic germline that were indistinguishable from phenotypes observed in alg-3/4 males at 25°C (Figure S5A). Taken together these findings suggest that CSR-1 functions along with ALG-3/4 to promote a chromosomal environment compatible with transcription. Perhaps consistent with this idea, we observed an increase in the number of spermatocytes undergoing nuclear condensation in both alg-3/4 and csr-1 males. The increase was moderate at 20°C (1.2- to 1.4-fold), but enhanced a t 25°C, with both alg-3/4 and csr-1 mutants showing a nearly 2-fold increase in the number of condensing nuclei relative to wild-type (Figure S5B). These observations suggest that spermatocyte nuclei begin to condense prematurely, or fail to complete condensation appropriately, in alg-3/4 and csr-1 mutants.
In the hermaphrodite germline, CSR-1 localizes to perinuclear P-granules and to condensed chromatin in oocytes (Claycomb et al., 2009). In addition CSR-1 was identified in a proteomic study as a protein associated with sperm chromatin (Chu et al., 2006). To examine the expression pattern and localization of CSR-1 during male gametogenesis, we performed immunofluorescence using an antibody that recognizes endogenous CSR-1 (Claycomb et al., 2009). CSR-1 was associated with P-granules throughout the syncytial male germline and into differentiating spermatocytes, where P-granules disperse and disappear. In developing gametes, we found that CSR-1 localized to large cytoplasmic foci and in discrete chromatin domains of spermatocytes undergoing nuclear condensation as well as in haploid spermatids (Figure 5B). In alg-3/4 mutants cultured at 25°C, CSR-1 was present in larg e cytoplasmic foci in spermatocytes undergoing nuclear condensation but was largely absent from chromatin. Notably, in haploid spermatids from alg-3/4 males we observed fewer CSR-1 cytoplasmic and nuclear foci than in haploid spermatids from wild-type males (Figure 5B). Taken together, these findings suggest that CSR-1 functions with ALG-3/4 to promote gene expression in developing spermatocytes.
CSR-1 22G-RNAs target genes also targeted by ALG-3/4 26G-RNAs
The similarities between the cellular and developmental phenotypes of alg-3/4 and csr-1 males are consistent with the possibility that ALG-3/4 and CSR-1 function in the same small RNA pathway. To determine whether CSR-1 is required for the expression of small RNAs antisense to ALG-3/4 targets, we cloned and deep sequenced small RNAs from csr-1 males grown at 25°C. Remarkably, we found that ALG- 3/4-dependent 26G-RNAs – regardless of class, i.e., antisense to positively- or negatively-regulated targets – were strongly depleted in csr-1 males. Indeed, this depletion was to a level resembling that of alg-3/4 mutant males (Figure 5C). On the other hand, 22G-RNAs were only slightly depleted in alg-3/4 and csr-1 males (Figure 5C). A similar minor reduction of 22G-RNAs was observed previously when small RNAs were cloned from csr-1 hermaphrodites (Claycomb et al., 2009). While these findings are consistent with the idea that ALG-3/4 and CSR-1 function in the same pathway, they also suggest that the pathway may not be linear (see Discussion).
To further explore the idea that CSR-1 and ALG-3/4 share targets, we cloned and deep sequenced CSR-1–associated 22G-RNAs. To do this we performed CSR-1-immunoprecipitation (IP) followed by small-RNA deep-sequencing from males expressing a rescuing FLAG::CSR-1 transgene and grown at 25°C. We found that CSR-1 IP enriched small RNAs targeting 5575 genes, including 90% (3775/4190) of the genes previously identified as CSR-1 targets in the hermaphrodite (Claycomb et al., 2009). Surprisingly, we found that small RNAs targeting many female-specific genes were also enriched (Figure 5D; see Supplemental Results). As expected, CSR-1 IP did not enrich miRNAs or 21U-RNAs, nor did it enrich 22G-RNAs previously identified as WAGO-1-associated in hermaphrodites (Claycomb et al., 2009; Gu et al., 2009). We also recovered small RNAs that target ~1800 genes (Table S3) not previously identified as CSR-1 targets, including most annotated male-germline-expressed genes. Notably, using a 2-fold cutoff, FLAG::CSR-1 IP enriched for small RNAs antisense to 82% (1156/1408) of all ALG-3/4 targets defined in this study (Figure 5D), including 88% (188/214) of ALG-3/4 positively-regulated genes, 83% (821/991) of non-regulated genes and 72% (147/204) of ALG-3/4 negatively-regulated genes (Figure 5D). Thus, just as CSR-1 is required for all ALG-3/4 26G-RNAs, these findings indicate that CSR-1 associates with 22G-RNAs targeting most and perhaps all ALG-3/4 targets.
CSR-1 promotes the expression of ALG-3/4 targets
CSR-1 was previously shown to associate with the chromatin of its 22G-RNA target genes in hermaphrodites (Claycomb et al., 2009). To ask whether CSR-1 associates with the chromatin of genes targeted by ALG-3/4 in males, we performed CSR-1 ChIP on WT and alg-3/4 male populations grown at 25°C. We found that in WT males CSR-1 ChIP enriched all of the ALG-3/4 targets assayed, including both positively and negatively regulated targets, by 1.5- to 3-fold relative to a no-antibody control (Figure 5E). This enrichment was not detected in alg-3/4 mutant animals (Figure 5E). CSR-1 ChIP from males also enriched several genes that were previously identified as CSR-1 targets in hermaphrodites, including daf-21, cgh-1 and oma-1 (Claycomb et al., 2009). The latter two, cgh-1 and oma-1, were only weakly expressed in males (Data Not Shown) and were not ALG-3/4 targets. As expected, we found that the association of CSR-1 with these two loci was independent of ALG-3/4 (Figure 5E). CSR-1 ChIP did not enrich spe-12 or f46f5.5, genes that are not targeted by ALG-3/4– or CSR-1–associated small RNAs (Figure 5E). Using RT-qPCR we found that the mRNA and pre-mRNA levels of positively-regulated ALG-3/4 targets were reduced by similar amounts in csr-1 males (see Figure 6C below). By contrast, we found that mRNA levels of ALG-3/4 negatively-regulated targets were unchanged in csr-1 mutant males at 25°C (Figure S3), while pre-mRNA levels decreased (see Figure 6C below). This latter finding – that pre-mRNA levels decrease for ALG-3/4 negatively-regulated targets – was also observed, paradoxically, in alg-3/4 mutant males (Figure 4B). Taken together these findings indicate that targets positively regulated by ALG-3/4 are also positively regulated by CSR-1, and suggest that ALG-3/4 and CSR-1 promote the expression of their targets at a transcriptional level, including a subset of ALG-3/4 targets whose net expression is negatively regulated due to post-transcriptional silencing.
Figure 6. ALG-3/4 and CSR-1 provide a paternal memory of germline gene expression.
(A) Schematic of crosses to assay paternal inheritance of gene expression and sperm function. ‘m’ indicates either csr-1 or alg-3/4 allele.
(B) Repeated mating with alg-3/4 and csr-1 males induces a progressive dominant germline-mortal phenotype. Box and whisker plots indicate the brood sizes of heterozygous hermaphrodite (red/pink) and male (blue/aqua) cross progeny of homozygous mutant fathers (red and blue) or control heterozygous hermaphrodites and males (pink and aqua, respectively) determined in successive generations of mating to homozygous (blue) or heterozygous (aqua) alg-3/4 (left panel) or csr-1 (right panel) mutant males. Fertility of male cross progeny was assayed by mating to fog-2 females.
(C) RT-qPCR analysis of pre-mRNA levels in male cross progeny (as indicated by color). Data are normalized to gpd-2 pre-mRNA and represented as mean +/− SEM. CSR-1 and ALG-3/4 target mRNAs, and a non-target mRNA were assayed (as indicated). An ALG-3/4 negatively-regulated target is indicated in grey text.
CSR-1 and ALG-3/4 provide a paternal memory of past gene expression
The ALG-3/4 proteins are present during spermatogenesis but are eliminated from maturing spermatids (Conine et al., 2010). The CSR-1 protein, however, is abundant in mature sperm (Figure 5B). We therefore wondered if ALG-3/4 and CSR-1 might function together to pass a memory of male-specific gene expression from one generation to the next via CSR-1. To test this idea, we first analyzed the fertility of alg-3/4 and csr-1 heterozygous hermaphrodites (F1) cultured at 25°C. We then mated heterozygous hermaphrodites to homozygous alg-3/4 or csr-1 males, respectively, at the permissive temperature to obtain F2 heterozygous hermaphrodites. In this and subsequent generations, the resulting heterozygous offspring were cultured at 25°C to assay their fertility before being mated to homozygous males at 20°C (Figure 6A). In parallel, as a control, we mated heterozygous hermaphrodites to heterozygous males to obtain heterozygous offspring (Figure 6A). Thus, the maternal genotype was always heterozygous throughout this analysis, whereas the paternal genotype was either homozygous (experimental series) or heterozygous (control series). Remarkably, we found that heterozygous offspring (hermaphrodites or males) derived from homozygous alg-3/4 or csr-1 males became progressively less fertile with each generation at 25°C (Figure 6B). By the sixth generation (F6), the heterozygous offspring of alg-3/4 or csr-1 homozygous males were completely sterile. The sterility of F6 heterozygous hermaphrodites could be rescued by mating to wild-type males, indicating that the sterility was due to a sperm defect. In the control series, on the other hand, the heterozygous offspring of heterozygous males maintained a consistent level of fertility throughout the course of the experiment. Importantly, the impaired fertility of heterozygous alg-3/4 and csr-1 offspring correlated with declining pre-mRNA expression levels of ALG-3/4 targets. By the F5 generation, relative to either control heterozygous males or wild-type males, the heterozygous male offspring of homozygous males exhibited a reduction in pre-mRNA similar to alg-3/4 or csr-1 males at 25°C (Figure 6C). Thus, ALG-3/4 and CSR-1 promot e a paternal epigenetic memory of ALG-3/4 target gene expression.
DISCUSSION
A small RNA feed-forward loop transmits a paternal epigenetic memory of past gene expression
During male gametogenesis germ cells proceed through meiosis and undergo dramatic changes in cellular morphology to produce haploid spermatids containing highly compacted, transcriptionally inert chromatin (Ward et al., 1981). The completion of this process and the subsequent transformation of spermatids into polarized motile spermatozoa capable of fertilization depends on the proper execution of an extensive gene-expression program involving thousands of genes. Here, we have shown that the Argonautes ALG-3/4 and CSR-1 are required during this process to promote robust spermatogenic gene expression. Although ALG-3/4 is absent from mature sperm (Conine et al., 2010), we have shown that CSR-1 is abundant in mature sperm. The propagation of strains lacking CSR-1 or ALG-3/4 activities in the paternal lineage caused a progressive loss of fertility (a germline-mortal phenotype), in which even heterozygous descendants, with a wild-type copy of the respective locus, exhibited complete sperm-specific sterility when assayed at 25°C. The observed infertility involved an arrest as round spermatids with decreased transcription of ALG-3/4 targets, a phenotype identical to that observed in alg-3/4 and csr-1 homozygous mutants. These findings suggest that ALG-3/4 and CSR-1 are not only required to promote spermatogenic gene expression but also act together to transmit an epigenetic memory of paternal gene expression via the sperm.
How might this work? Paternal CSR-1 22G-RNAs delivered via the sperm could enter the zygotic germline (See Model, Figure 7). Later, when spermatogenesis initiates in hermaphrodites and males, CSR-1 targeting could recruit the RdRP-containing ERI complex (Duchaine et al., 2006; Pavelec et al., 2009) to initiate the production of 26G-RNAs that are loaded onto ALG-3/4. In a feed-forward mode, ALG-3/4 could then target cognate transcripts to recruit the EGO-1 RdRP complex to re-amplify CSR-1 22G-RNAs (Claycomb et al., 2009; Conine et al., 2010; Gu et al., 2009). While the initial biogenesis of ALG-3/4 and CSR-1 small RNAs likely requires some template mRNA destruction, the net result of this amplification cycle appears to be increased mRNA levels, perhaps due to feedback on transcription. During spermatogenesis, CSR-1 and its 22G-RNA cofactors might promote gene expression by engaging nascent transcripts on the chromatin of its target genes. For example, CSR-1 could recruit factors that help maintain a transcriptionally active state during spermatogenesis and nuclear condensation, ensuring that spermatids obtain the appropriate level of gene products required for spermiogenesis. Finally, CSR-1 small-RNA complexes could once again become incorporated into mature sperm, thus poised to reinitiate the cycle in the next generation (Figure 7).
Figure 7. Model.
ALG-3/4 and CSR-1 and their small RNA cofactors promote the transcription of spermiogenic genes and provide a memory of past germline gene expression. Black dots in spermatids represent areas of condensed silent chromatin; yellow areas indicate transcriptionally-active chromatin. See Discussion for details.
A protective role for piRNAs across phyla
In many animals, sperm development is inherently temperature sensitive (Rockett et al., 2001). The effects of elevated temperature during sperm development can also be epigenetically transmitted, causing developmental defects in mammalian embryos, including decreased embryonic mass and increased mortality (Jannes et al., 1998; Setchell et al., 1988). However, the molecular mechanism behind the temperature sensitivity of sperm development remains unclear.
Our findings indicate that the gene expression programs required for spermatogenesis in C. elegans are sensitive to temperature. In wild-type males, the expression of many spermiogenesis genes was higher at 25°C than at 20°C. We have shown that ALG-3/4 and CSR-1 are required to initiate and maintain the activation of spermiogenesis genes at elevated temperatures. Failure to maintain spermiogenic gene expression in alg-3/4 and csr-1 mutants correlates with dramatic defects in spermatid activation and infertility in both mutant strains at elevated temperature. Thus, the ALG-3/4 and CSR-1 pathways appear to act as enhancers of gene expression that buffer the effects of temperature on sperm development.
In wild-type animals, the process of spermatogenesis accelerates by greater than 25% at 25°C relative to 20°C, which in turn demands a similar increase in the expression of gene products that support spermiogenesis. Thus, increased gene expression is required in a setting where the rapid onset of meiosis and subsequent chromatin condensation would be expected to shut down transcription. Our findings suggest that the ALG-3/4 and CSR-1 pathways act together to selectively maintain transcriptionally-active chromatin at spermiogenesis genes during meiotic nuclear condensation, while packaging other regions of the genome not essential for spermiogenesis into transcriptionally-inactive chromatin. This would create a burst of transcription of spermiogenesis genes near the end of spermatogenesis, as seen in mammals (Sassone-Corsi, 2002), when chromatin begins to condense in 1° spermatocytes (Shakes et al., 2009).
Interestingly, mice deficient for the PIWI homolog MIWI display spermatogenic arrest at the round spermatid stage (Deng and Lin, 2002), a phenotype similar to that of alg-3/4 and csr-1 mutant sperm. MIWI associates with pachytene piRNAs, ~29-31 nt small RNAs derived from large piRNA genes within non-repetitive genomic regions (Li et al., 2013). Much like ALG-3/4 26G-RNAs, mouse pachytene piRNAs are expressed specifically in developing spermatocytes upon entering the pachytene stage of meiotic prophase I (Girard et al., 2006). Intriguingly, MIWI was shown to associate with the translational machinery as well as with polysomes during early spermiogenesis, leading to speculation that it promotes translation (Lau, 2010). Recent work also suggests that MIWI promotes the stabilization of spermiogenic mRNAs (Nishibu et al., 2012; Vourekas et al., 2012). Interestingly, a protective role for small RNAs and PIWI Argonaute proteins was recently identified in the ciliated protozoan Oxytricha, where piRNAs prevent DNA elimination during genome rearrangement (Fang et al., 2012). These findings raise a thought-provoking possibility that pachytene piRNAs and ALG-3/4/CSR-1 small RNAs provide analogous functions, protecting the genome and buffering gene expression in distant phyla.
Whole-genome surveillance by Argonaute/small-RNA pathways
While ALG-3/4 and CSR-1 function together to promote the expression of many genes required for spermiogenesis, ALG-3/4 also functions independently of CSR-1 to negatively regulate a subset of its targets via the WAGO gene-silencing pathway. Although CSR-1 22G-RNAs target these same mRNAs, CSR-1 is not required for their silencing (Fig. S3 and Fig. 5). Indeed, perhaps paradoxically, both CSR-1 and ALG-3/4 appear to moderately promote the transcription of these ALG-3/4 negatively-regulated targets. These observations suggest that in males ALG-3/4 and CSR-1 promote the transcription of some targets that are silenced post-transcriptionally via the WAGO/22G-RNA pathway. This complexity might reflect distinct positive and negative regulation of the same target genes by CSR-1 and WAGO at different times during spermatogenesis. However, it is also possible that transcriptional up-regulation of these targets is required to ensure that sufficient template RNA is produced to re-amplify WAGO-22G-RNAs important for post-transcriptional silencing in the next generation. In either case, these findings hint at additional complexity.
Surprisingly, males also contain abundant CSR-1 22G-RNAs targeting female-specific transcripts. We do not know whether these 22G-RNAs are maternal CSR-1/22G-RNA complexes that persist in the male, or alternatively, if they are generated de novo in males, perhaps using transcripts maternally inherited or produced at low levels. Regardless of their origin, this result could indicate that sperm transmit a memory of both paternal and grand-maternal gene expression. CSR-1 is also abundant in oocytes and it seems likely that it could provide similar functions there, delivering epigenetic signals from the mother and possibly the grandfather. Given the relatively much smaller volume of sperm, it is possible that the ALG-3/4 system exists, in part, to amplify the sperm-specific CSR-1 signal, ensuring both the robust expression of sperm genes and the transmission of a memory of sperm-specific gene expression to offspring.
Like CSR-1-dependent small-RNA signals, WAGO-dependent signals are also transmitted via both the egg and the sperm (Ashe et al., 2012; Buckley et al., 2012; Gu et al., 2009; Shirayama et al., 2012). Our analysis of CSR-1 and a previous study of HRDE-1/WAGO-9 (Buckley et al., 2012) suggest that both Argonaute pathways promote germline immortality. Interestingly, when either small RNA pathway is lost, fertility begins to decline gradually over a few generations. These findings suggest that Argonautes act to reinforce and maintain parallel transgenerational epigenetic signals that might, for example, be chromatin mediated. In the respective Argonaute mutants, loss of the small RNA signals may cause a gradual loss, over a period of several generations, of chromatin marks associated with active or silent gene-expression states, resulting in the observed gradual onset of germline mortality.
Recent work on transgenes expressed in the C. elegans germline (Seth et al., 2013, submitted) has identified a CSR-1–dependent transgenerational activating signal (RNAa). This activating signal, which can be transmitted via the sperm, can act within a single generation to reverse a persistent mode of epigenetic silencing referred to as RNA-induced epigenetic gene silencing (RNAe) (Shirayama et al., 2012). Interestingly, while de-silencing was observed immediately after exposure to RNAa, continuous exposure, over many generations, was necessary to render a formerly silent gene capable of durable independent expression (Seth et al., 2013, submitted). The maintenance of RNAe requires members of the WAGO clade of Argonautes, as well as repressive heterochromatin factors and histone modifications (Ashe et al., 2012; Buckley et al., 2012; Shirayama et al., 2012). Perhaps germline mortality occurs gradually, after loss of CSR-1 and ALG-3/4 activity, as silencing marks spread into and gradually silence genes required for spermatogenesis. The findings reported here support the idea that CSR-1 transmits a protective small-RNA-induced trans-activating signal, and provide physiological evidence linking CSR-1 and RNAa more globally to the transmission and maintenance of paternal, and possibly maternal epigenetic memory.
EXPERIMENTAL PROCEDURES
Worm strains and genetics
C. elegans culture and genetics were performed as described (Brenner, 1974). Unless otherwise noted, the “wild-type” (WT) strain in this study is the Bristol N2 strain carrying the fog-2(q71) allele. Alleles listed by chromosome: LGI: fer-1(hc24), fer-1(b232), fer-6(hc23), fer-7(hc34); LGII: neSi1[cb-unc-119(+) 3xflag::csr-1], eri-3(tm1361), fer-3(hc3), rrf-3(pk1426), fer-15(b26); LGIII: alg-4(ok1041), unc-119(ed3), fer-2(hc2); LGIV: alg-3(tm1155), csr-1(tm892), DnT1[unc(n754dm) let](IV;V); LGV: fog-2(q71), fer-4(hc4). The 3xflag::csr-1 transgenic strain was generated by Mos-mediated single-copy insertion (Frokjaer-Jensen et al., 2008) and details are provided in Extended Experimental Procedures. Analysis of Eri phenotypes, male fertility and spermatid activation are described in Extended Experimental Procedures.
Molecular Biology
Males were enriched to >95% homogeneity by filtering through a 35 micron mesh filter (Miller, 2006). Worms were homogenized in a stainless steel dounce in the presence of TRI Reagent (MRC Inc) for RNA isolation or IP buffer (Gu et al., 2009) for IP. RNA was extracted in TRI Reagent (MRC Inc) according to the manufacturer's specifications. The FLAG::CSR-1 IP was performed as described (Gu et al., 2009; Shirayama et al., 2012) using M2 FLAG antibody (Invitrogen). Details of small RNA cloning and data analyses are provided in Extended Experimental Procedures. Perl Scripts are available upon request. Data are available from GEO under the series number GSE49672.
Quantitative PCR and Chromatin IP
Reverse transcription followed by qPCR (RT-qPCR) was performed as described (Batista et al., 2008). To measure pre-mRNA, a qPCR primers was placed within intron sequence. A detailed ChIP method, including antibodies used, is provided in Extended Experimental Procedures. Target enrichment was assayed by qPCR and is relative to a negative control IgG ChIP and a control intergenic region. Primer sequences are available upon request.
Proteomics
Sperm were isolated from fog-2 males fed with 15N-labeled (or heavy) HB101 bacteria for three generations at 20°C and from fog-2 or alg-3/4; fog-2 males fed with unlabeled HB101 bacteria at 20°C or 25°C. Each sperm sample w as homogenized and lysates were centrifuged twice at 10,000 × g for 20 minutes. Proteins were precipitated in trichloroacetic acid and washed in acetone. Proteins were denatured, reduced and alkylated prior to trypsin digestion. The 15N-labeled (or heavy) protein sample served as an internal quantification standard by combining with unlabeled (ie. 14N or light) experimental sperm protein samples. Details of the Multidimensional Protein Identification Technology (MuDPIT) analysis are provided in the Extended Experimental Procedures.
Supplementary Material
Highlights.
Argonautes promote the transcription of spermiogenesis genes
Argonautes promote the robustness of spermatogenesis to high temperature
The Argonaute CSR-1 interacts with chromatin to promote transcription of target genes
Argonautes transmit a transgenerational small-RNA memory of paternal gene expression
ACKNOWLEDMENTS
We thank the Mello laboratory for helpful discussion; E. Youngman and R. Sharma for experimental support; E. Kittler and the UMass Deep Sequencing Core for Illumina sequencing; P. Furcinitti and the UMass Light Microscopy Core for use of the confocal microscope; the Caenorhabditis Genetics Center for providing strains. J.J.M. and J.R.Y. were supported by the National Center for Research Resources (5P41RR011823-17), National Institute of General Medical Sciences (8 P41 GM103533-17), and National Institute on Aging (R01AG027463-04). C.C.M. is a Howard Hughes Medical Institute Investigator and is supported by NIH grant GM058800.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3' end processing. Mol Cell. 2004;13:67–76. doi: 10.1016/s1097-2765(03)00492-1. [DOI] [PubMed] [Google Scholar]
- Argon Y, Ward S. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genetics. 1980;96:413–433. doi: 10.1093/genetics/96.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, Doebley AL, Goldstein LD, Lehrbach NJ, Le Pen J, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagijn MP, Goldstein LD, Sapetschnig A, Weick EM, Bouasker S, Lehrbach NJ, Simard MJ, Miska EA. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science. 2012;337:574–578. doi: 10.1126/science.1220952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, Kennedy S. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature. 2012;489:447–451. doi: 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DJ, Ward S. Identification of a large multigene family encoding the major sperm protein of Caenorhabditis elegans. J Mol Biol. 1983;171:1–29. doi: 10.1016/s0022-2836(83)80312-x. [DOI] [PubMed] [Google Scholar]
- Buttery SM, Ekman GC, Seavy M, Stewart M, Roberts TM. Dissection of the Ascaris sperm motility machinery identifies key proteins involved in major sperm protein-based amoeboid locomotion. Mol Biol Cell. 2003;14:5082–5088. doi: 10.1091/mbc.E03-04-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DS, Liu H, Nix P, Wu TF, Ralston EJ, Yates JR, 3rd, Meyer BJ. Sperm chromatin proteomics identifies evolutionarily conserved fertility factors. Nature. 2006;443:101–105. doi: 10.1038/nature05050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb JM, Batista PJ, Pang KM, Gu W, Vasale JJ, van Wolfswinkel JC, Chaves DA, Shirayama M, Mitani S, Ketting RF, et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139:123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Batista PJ, Gu W, Claycomb JM, Chaves DA, Shirayama M, Mello CC. Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:3588–3593. doi: 10.1073/pnas.0911685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Jr., Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–128. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- Fang W, Wang X, Bracht JR, Nowacki M, Landweber LF. Piwi-interacting RNAs protect DNA against loss during Oxytricha genome rearrangement. Cell. 2012;151:1243–1255. doi: 10.1016/j.cell.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- Gu W, Lee HC, Chaves D, Youngman EM, Pazour GJ, Conte D, Jr., Mello CC. CapSeq and CIP-TAP identify Pol II start sites and reveal capped small RNAs as C. elegans piRNA precursors. Cell. 2012;151:1488–1500. doi: 10.1016/j.cell.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Shirayama M, Conte D, Jr., Vasale J, Batista PJ, Claycomb JM, Moresco JJ, Youngman EM, Keys J, Stoltz MJ, et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol Cell. 2009;36:231–244. doi: 10.1016/j.molcel.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T, Manoharan AP, Harkins TT, Bouffard P, Fitzpatrick C, Chu DS, Thierry-Mieg D, Thierry-Mieg J, Kim JK. 26G endo-siRNAs regulate spermatogenic and zygotic gene expression in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2009;106:18674–18679. doi: 10.1073/pnas.0906378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D, Vanderslice R. Temperature-sensitive developmental mutants of Caenorhabditis elegans. Dev Biol. 1976;49:220–235. doi: 10.1016/0012-1606(76)90268-2. [DOI] [PubMed] [Google Scholar]
- Jannes P, Spiessens C, Van der Auwera I, D'Hooghe T, Verhoeven G, Vanderschueren D. Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum Reprod. 1998;13:372–375. doi: 10.1093/humrep/13.2.372. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Schaner CE, Dernburg AF, Lee MH, Kim SK, Villeneuve AM, Reinke V. X-chromosome silencing in the germline of C. elegans. Development. 2002;129:479–492. doi: 10.1242/dev.129.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC. Small RNAs in the animal gonad: guarding genomes and guiding development. Int J Biochem Cell Biol. 2010;42:1334–1347. doi: 10.1016/j.biocel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Gu W, Shirayama M, Youngman E, Conte D, Jr., Mello CC. C. elegans piRNAs mediate the genome-wide surveillance of germline transcripts. Cell. 2012;150:78–87. doi: 10.1016/j.cell.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, Xu J, Moore MJ, Schimenti JC, Weng Z, et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol Cell. 2013;50:67–81. doi: 10.1016/j.molcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA. Sperm and oocyte isolation methods for biochemical and proteomic analysis. Methods Mol Biol. 2006;351:193–201. doi: 10.1385/1-59745-151-7:193. [DOI] [PubMed] [Google Scholar]
- Nishibu T, Hayashida Y, Tani S, Kurono S, Kojima-Kita K, Ukekawa R, Kurokawa T, Kuramochi-Miyagawa S, Nakano T, Inoue K, et al. Identification of MIWI-associated Poly(A) RNAs by immunoprecipitation with an anti-MIWI monoclonal antibody. Biosci Trends. 2012;6:248–261. doi: 10.5582/bst.2012.v6.5.248. [DOI] [PubMed] [Google Scholar]
- Pavelec DM, Lachowiec J, Duchaine TF, Smith HE, Kennedy S. Requirement for the ERI/DICER complex in endogenous RNA interference and sperm development in Caenorhabditis elegans. Genetics. 2009;183:1283–1295. doi: 10.1534/genetics.109.108134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. Daddy issues: paternal effects on phenotype. Cell. 2012;151:702–708. doi: 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65:229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science. 2002;296:2176–2178. doi: 10.1126/science.1070963. [DOI] [PubMed] [Google Scholar]
- Setchell BP, D'Occhio MJ, Hall MJ, Laurie MS, Tucker MJ, Zupp JL. Is embryonic mortality increased in normal female rats mated to subfertile males? J Reprod Fertil. 1988;82:567–574. doi: 10.1530/jrf.0.0820567. [DOI] [PubMed] [Google Scholar]
- Shakes DC, Wu JC, Sadler PL, Laprade K, Moore LL, Noritake A, Chu DS. Spermatogenesis-specific features of the meiotic program in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000611. doi: 10.1371/journal.pgen.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee HC, Gu W, Ishidate T, Conte D, Jr., Mello CC. piRNAs initiate an epigenetic memory of nonself RNA in the C. elegans germline. Cell. 2012;150:65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasale JJ, Gu W, Thivierge C, Batista PJ, Claycomb JM, Youngman EM, Duchaine TF, Mello CC, Conte D., Jr. Sequential rounds of RNA-dependent RNA transcription drive endogenous small-RNA biogenesis in the ERGO-1/Argonaute pathway. Proc Natl Acad Sci U S A. 2010;107:3582–3587. doi: 10.1073/pnas.0911908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vourekas A, Zheng Q, Alexiou P, Maragkakis M, Kirino Y, Gregory BD, Mourelatos Z. Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nat Struct Mol Biol. 2012;19:773–781. doi: 10.1038/nsmb.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Argon Y, Nelson GA. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J Cell Biol. 1981;91:26–44. doi: 10.1083/jcb.91.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci. 2006;119:2552–2562. doi: 10.1242/jcs.02980. [DOI] [PubMed] [Google Scholar]
- Wu JC, Go AC, Samson M, Cintra T, Mirsoian S, Wu TF, Jow MM, Routman EJ, Chu DS. Sperm development and motility are regulated by PP1 phosphatases in Caenorhabditis elegans. Genetics. 2012;190:143–157. doi: 10.1534/genetics.111.135376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes SM, Krawetz SA. The structural organization of sperm chromatin. J Biol Chem. 2003;278:29471–29477. doi: 10.1074/jbc.M304545200. [DOI] [PubMed] [Google Scholar]
- Yigit E, Batista PJ, Bei Y, Pang KM, Chen CC, Tolia NH, Joshua-Tor L, Mitani S, Simard MJ, Mello CC. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127:747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.