Abstract
Summary
Background:
The human intestine is colonized with trillions of microorganisms important to health and disease. There has been an intensive effort to catalog the species and genetic content of this microbial ecosystem. However, little is known of the ecological interactions between these microbes, a prerequisite to understanding the dynamics and stability of this host-associated microbial community. Here we perform a systematic investigation of public goods-based syntrophic interactions among the abundant human gut bacteria, the Bacteroidales.
Results:
We find evidence for a rich interaction network based on the breakdown and use of polysaccharides. Species that utilize a particular polysaccharide (producers) liberate polysaccharide breakdown products (PBP) that are consumed by other species unable to grow on the polysaccharide alone (recipients). Cross-species gene addition experiments demonstrate that recipients can grow on a polysaccharide if the producer-derived glycoside hydrolase, responsible for PBP generation, is provided. These producer-derived glycoside hydrolases are public goods transported extracellularly in outer membrane vesicles allowing for the creation of PBP and concomitant recipient growth spatially distant from the producer. Recipients can exploit these ecological interactions and conditionally outgrow producers. Finally, we show that these public good-based interactions occur among Bacteroidales species co-resident within a natural human intestinal community.
Conclusions:
This study examines public-goods based syntrophic interactions between bacterial members of the critically important gut microbial ecosystem. This polysaccharide-based network likely represents foundational relationships creating organized ecological units within the intestinal microbiota, knowledge of which can be applied to impact human health.
Introduction
The human intestine is home to a diverse and complex microbial ecosystem composed of trillions of bacteria. This microbial community provides many benefits to the host [1-3], and is also implicated in numerous diseases [1-3]. High throughput compositional and metagenomic analyses have provided a catalog of the members and genes within the human intestine [4-7] however, we know little about the relationships among these bacteria. As the intestinal microbiota is critical to human health, it is essential to understand the ecology of this habitat, i.e. the abiotic and biotic determinants that dictate the composition and dynamics of individuals and groups.
The colon harbors the greatest diversity and number of bacteria in the human body with more than 90% of the members belonging to two phyla: the Bacteroidetes and the Firmicutes [4, 5, 8]. The gut Firmicutes are distantly related to each other and comprise different classes and orders [4, 5, 8]. In contrast, the human gut Bacteroidetes belong to one order of closely related members, the Bacteroidales, with three dominant genera, the Bacteroides, the Parabacteroides, and the Prevotella. Individual strains and species of Bacteroidales are highly abundant and co-exist in the human gut at densities of 109-1010 CFU/gram feces [4, 9]. In addition, the Bacteroidales are significantly more stable over both the lifetime and across generations of humans [4]. Thus the question arises as to what allows both the stability of the Bacteroidales and the co-existence of related species.
Microbes perform social behaviors whereby an individual (the actor) performs a function that affects another individual’s (the recipient’s) fitness, i.e. the ability to reproduce [10]. Cooperative behaviors have a positive effect on the fitness of the recipient and are operational during quorum sensing, biofilm formation and iron scavenging. Central to these cooperative behaviors is the provision of public goods by the actor such as the secretion of autoinducers, polymers and siderophores, which are resources available for the benefit of both producing and non-producing members [10]. Such traits have been shown to be important within clonal populations [10]; however, the impact of these behaviors on closely related microbial species, especially those comprising natural communities [11, 12], is poorly understood. Given the importance of the gut Bacteroidales to human health and the coexistence and stability of numerous closely related species, we sought to determine whether public good-based interactions exist among these species.
The success of the Bacteroidales in the human gut is in large part due to their ability to utilize diet-derived polysaccharides, many of which arrive to the colon undigested by human enzymes. Bacteroidales members have differing abilities to use these various plant polysaccharides [13-16]. As carbohydrates are critical for survival and thus serve as valuable currency, we investigated whether polysaccharide utilization generates public goods that may impact community stability in an ecosystem subject to variable nutrients. In particular, whether carbohydrate breakdown products are liberated by bacteria able to grow on a particular polysaccharide and whether bacteria unable to live on that polysaccharide can utilize these public goods. We demonstrate a rich and diverse polysaccharide utilization network based on the release and use of public goods. This is the first study to address public-goods among the predominant Gram negative bacteria of the human gut, and is one of the first to study these interactions among bacteria from a naturally occurring intestinal community.
Results
Polysaccharide utilization and liberation of polysaccharide breakdown products by Bacteroidales members
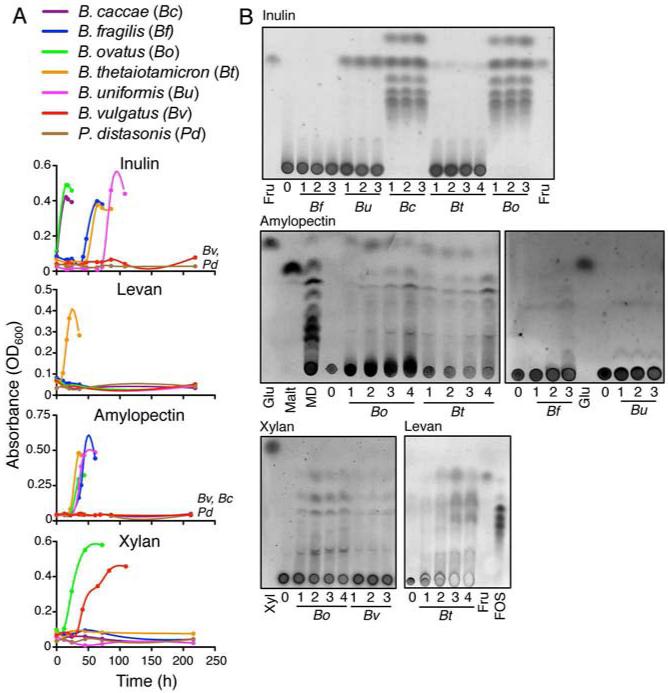
We screened seven type strains of human gut Bacteroidales (Bacteroides caccae, B. fragilis, B. ovatus, B. thetaiotaomicron, B. uniformis, B. vulgatus, and Parabacteroides distasonis) in defined medium with a single polysaccharide as the sole carbohydrate source to identify members that are able (utilizer) or unable (non-utilizer) to grow (Figure 1A). Using four different polysaccharides (the fructose polymers inulin (β1,2) and levan (β2,6), the xylose polymer xylan (β1,4) and the glucose polymer amylopectin), we expanded upon previous studies [13-15] demonstrating that following growth in standard medium, certain Bacteroidales are able to utilize specific polysaccharides for growth, while others cannot (Figure 1A). Five of the strains grew with inulin as the carbon source and two did not (B. vulgatus, P. distasonis). For amylopectin there were near equal numbers of utilizers and non-utilizers; whereas xylan is only utilized by B. ovatus and B. vulgatus, and levan only by B. thetaiotaomicron [15].
Figure 1. Variation among Bacteroidales members in ability to utilize polysaccharides and to publicly liberate PBP.
(A) Growth curves of bacteria in defined media with indicated polysaccharide as carbohydrate source. (B) Thin layer chromatography (TLC) analyses of polysaccharide breakdown products in the CM of primary utilizers through the growth phases (1-early, 2-mid, 3-late log, −4 stationary) in defined media with indicated polysaccharide as carbohydrate source. The intact polysaccharides do not migrate from the bottom of the TLC plate. PBP are continually generated as they are consumed during bacterial growth due to continued breakdown of polysaccharide. Fru = Fructose, FOS = Fructose oligosaccharides (derived from inulin), Glu = Glucose, Malt = Maltose, MD= Maltodextrin. Data are representative of ≥3 independent experiments, one representative experiment is shown.
We next investigated whether growth of these polysaccharide-utilizing members creates polysaccharide breakdown products (PBP) potentially available to members unable to utilize the polysaccharide alone (potential recipients). Therefore, we determined whether PBP were present in supernatant following growth of a utilizer. This analysis revealed not only that PBP are liberated extracellularly during growth of Bacteroidales, but also unexpected diversity in PBP liberation by different primary utilizers (Figure 1B). For example, B. caccae and B. ovatus grown in inulin liberate the monosaccharide fructose and a variety of oligosaccharides; whereas B. uniformis liberated only fructose, B. thetaiotaomicron only trace amounts of fructose, and B. fragilis liberated no detectable PBP (Figure 1B). Similarly, although all amylopectin utilizers released some form of PBP, growth in amylopectin resulted in qualitative and quantitative differences, with B. ovatus and B. thetaiotaomicron liberating significant amounts of PBP and B. fragilis and B. uniformis very little. Growth of B. ovatus and B. vulgatus on xylan resulted in liberation of oligosaccharides but not xylose, previously described for B. ovatus during growth in xylan [17], whereas B. thetaiotaomicron grown in levan liberated both fructose and oligosaccharides (Figure 1B). Therefore, polysaccharide utilization typically results in the production of freely available PBP, but the profiles vary drastically both qualitatively and quantitatively depending on the Bacteroidales utilizer and the polysaccharide.
Utilization of liberated PBP public goods by non-polysaccharide utilizing Bacteroidales
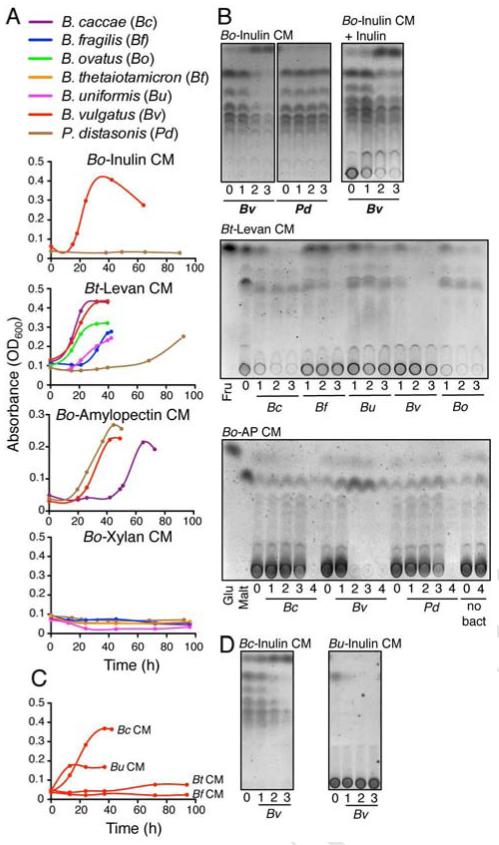
To identify potential recipients that may utilize producer-derived PBP, we screened non-utilizing strains for growth when cultured in filter-sterilized conditioned medium (CM) derived from growth of a PBP producing primary utilizer (for inulin, B. ovatus; for levan, B. thetaiotaomicron; for amylopectin, B. ovatus; for xylan, B. ovatus) (Figure S1). This strategy revealed several non-utilizing bacteria that were able to grow in these CM (Figure 2A). Growth occurred in CM harvested from various phases of growth of the primary utilizer (Figure S2A). Growth of non-utilizers, however, was not universal, and was specific to both polysaccharide and bacteria, as certain non-utilizing species grew readily in various CM (termed “recipients”), while others demonstrated no or delayed growth (termed “non-recipients” and “poor-recipients”, respectively). Of the inulin non-utilizers, B. vulgatus grew in inulin medium conditioned by B. ovatus, but P. distasonis did not. All species that were unable to grow in levan or amylopectin media grew in levan medium conditioned by B. thetaiotaomicron and in amylopectin medium conditioned by B. ovatus. In contrast, no xylan non-utilizers were able to grow in xylan medium conditioned by B. ovatus (Figures 2A and S2). Furthermore, some recipients grew in the CM early (B. caccae, B. vulgatus, B. ovatus in B. thetaiotaomicron levan CM and B. vulgatus and P. distasonis in B. ovatus amylopectin CM), whereas other recipients grew later (P. distasonis in B. thetaiotaomicron levan CM and B. caccae in B. ovatus amylopectin CM) (Figure 2A).
Figure 2. Specific non-polysaccharide utilizing Bacteroidales members can utilize liberated PBP public goods.
(A) Growth curves of non-primary utilizing bacteria in media conditioned by PBP-liberating utilizers. (B) TLC analysis of culture supernatants of recipient bacteria grown in the CM of primary utilizers . As P.distasonis did not grow in Bo-Inulin CM, its time points correspond to the growth points of B. vulgatus in the same CM through the growth phases (1-mid, 2-late log, 3-stationary). (C) Growth curves of B. vulgatus in inulin media conditioned by different species of inulin-utilizing Bacteroidales. (D) TLC analysis of culture supernatants of B. vulgatus grown in B. caccae and B. uniformis inulin CM through the growth phases (1-mid, 2-late log, 3-stationary). Data are representative of ≥3 independent experiments, one representative experiment is shown.
Analysis of PBP profiles during recipient growth revealed that the producer-derived PBP are depleted from the CM during recipient growth but not when non-recipients were placed in the CM (Figure 2B), thus demonstrating PBP as public goods. PBP are also utilized by producers as shown by the depletion of PBP when B. ovatus is grown in its own inulin derived CM, and by the depletion of liberated PBP by B. ovatus when inulin is limited (Figure S2B,C). The CM of B. fragilis grown in inulin, which does not contain detectable PBP, does not support the growth of recipients (Figure 2C), further demonstrating that PBP are pubic goods utilized by recipients. Recipient bacteria consumed the different types of PBP liberated by primary utilizers and their growth was limited by the quantity of these PBP (Figures 2C and D). Lack of growth by non-recipients was not due to inhibitory factors or the effects of pH, as all non-recipients grew when glucose was added to the CM (Figure S2D). Together, these data reveal that the liberation of PBP is a trait that varies among polysaccharide utilizing Bacteroidales. In addition, PBP are not universally used by polysaccharide-non-utilizing members, allowing for the designation of recipients and non-recipients.
Addition of producer-derived polysaccharide glycoside hydrolase/polysaccharide lyase genes to recipient bacteria
In Bacteroidales, polysaccharide utilization depends on the presence of clusters of genes termed polysaccharide utilization loci (PUL), each of which encodes products for the utilization of a specific polysaccharide/glycan [16, 18, 19]. PULs typically encode a surface protein that binds the polysaccharide, surface glycoside hydrolases/polysaccharide lyases (GH/PL) that cleave the large polymer to smaller units, an outer membrane protein that imports these cleaved units to the periplasm, periplasmic glycoside hydrolases that degrade the oligosaccharides to monosaccharides, and regulatory proteins [14, 20]. The ability of recipients to grow on producer-derived PBP suggested that they contain all the machinery to utilize a particular polysaccharide except for the surface GH/PL. To explore this possibility, we cloned the genes encoding the B. ovatus inulin PLs (BACOVA_04502 and 04503), the B. thetaiotaomicron levan GH (BT_1760 [15]), and the B. thetaiotaomicron amylopectin GH, SusG (BT_3698 [21]) behind a constitutive promoter and placed them in trans in recipient strains. Addition of these genes enables recipient bacteria to grow on the primary polysaccharide alone (Figure 3A) and to release PBP (Figure 3B). Addition of the BT_3698 gene (susG) allowed B. vulgatus to grow rapidly in amylopectin, but did not support early growth of B. caccae (Figure 3A) despite PBP release and availability (Figure 3B), consistent with its delayed growth in amylopectin CM (Figure 2A). Thus, to grow on a particular polysaccharide, recipient bacteria lack only the GH/PL responsible for initial polysaccharide breakdown and PBP release.
Figure 3. Primary growth and PBP liberation by recipients containing producer- derived GH/PL genes.
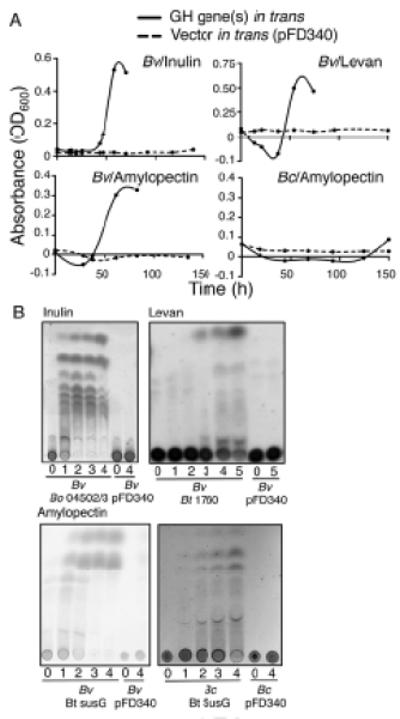
(A). Growth curves of recipient bacteria with genes encoding the B. ovatus inulin PLs (BACOVA_04502 and 04503), the B. thetaiotaomicron levan GH (BT_1760), the B. thetaiotaomicron amylopectin GH (susG, BT_3698), or vector alone (pFD340) in defined polysaccharide media. The initial decrease in OD600 in amylopectin and levan media correspond to rapid degradation of these optically dense polysaccharides. (B) TLC analyses of PBP released from recipient strains containing GH/PL genes in trans, or vector alone (pFD340) through the growth phases (inulin and amylopectin: 1-early, 2-mid, 3-late log, -4 stationary; levan: 1-lag, 2-early, 3-mid, 4-late log, 5-stationary). As recipient with vector alone did not grow in polysaccharide media, its time points correspond to the growth points of recipient strains containing GH/PL genes in trans. Data are representative of ≥3 independent experiments, one representative experiment is shown.
Extracellular secretion of producer-derived GH/PLs
As spatial organization is an important factor in public good-based interactions between microbes [10], we performed experiments on solid media to characterize dimensional properties of public goods release and utilization. Analysis of the growth of amylopectin utilizers on amylopectin plates, which are opaque due to the optically dense polysaccharide, revealed degradation of polysaccharide in extracellular zones surrounding the bacteria (Figure 4A), indicating that amylopectin is degraded at significant distances from the utilizing bacteria. These zones of degradation would not occur simply by the diffusion of PBP, but rather require that the GH/PL is secreted from the bacteria. When PBP recipients were plated adjacent to producers, the early growth recipients (B. vulgatus on B. ovatus amylopectin and inulin plates) but not inefficient/poor recipients (B. caccae on B. ovatus amylopectin plates) showed growth that was inversely proportional to their distance from the producer (Figure 4A).
Figure 4. GH/PLs serve as public goods through secretion in outer membrane vesicles.
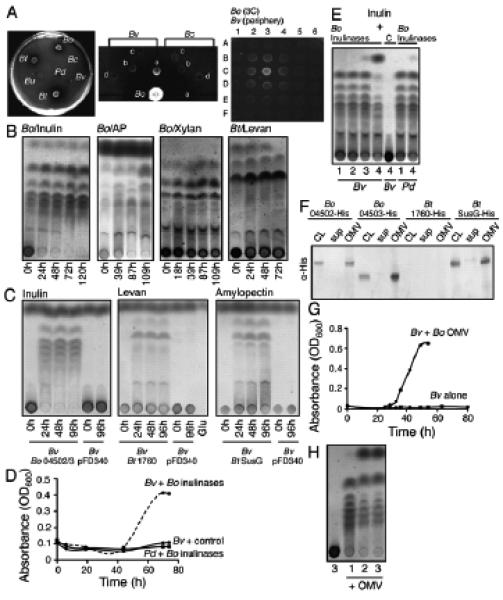
(A) (left panel) Growth of utilizers on defined amylopectin agarose plates demonstrating amylopectin degraded zones surrounding Bf, Bo, and Bt, demonstrating extracellular release of GH. (middle panel) Growth capabilities of recipient (Bv) and late recipient (Bc) plated at various distances (a, b, c, d) from the producer B. ovatus on a defined amylopectin plate (middle). (left) B. vulgatus is only able to grow in the zone of amylopectin degradation, whereas late recipient, B. caccae does not. (Right panel) Growth of recipient B. vulgatus (spotted on all 36 spots except 3C) is dictated by its spatial proximity to the producer B. ovatus (spotted on 3C) on a defined inulin plate (right panel). (B) TLC analyses of GH/PL activity in culture supernatants of PBP-liberating utilizer strains grown in indicated defined medium with extra polysaccharide added and incubated over time. The polysaccharide at the origin of the TLC is degraded with accumulation of PBP. (C) TLC analysis of extracellular GH/PL activity from recipient strains with GH/PL genes in trans or vector alone. Bacteria were cultured in defined glucose medium without polysaccharide as the GH/PL genes are expressed from a constitutive plasmid-borne promoter. Supernatants were harvested, filter sterilized and diluted 1:1 with medium containing the indicated polysaccharide and incubated at 37°C over time prior to TLC analysis. The glucose at the top of the TLC is from the initial growth medium. Glu = Glucose. (D) Growth of B. vulgatus but not P. distasonis in defined inulin medium with purified BACOVA_04502 and BACOVA_04503 added to the medium. B. vulgatus does not grow with material purified from the vector only control. (E) TLC analysis of the resulting media from the samples shown in panel D, through the growth phases (1-lag, 2-lag, 3-late log, 4-stationary), demonstrating PBP consumption by B. vulgatus. C indicates B. vulgatus grown with material prepared from vector-only control. As this B. vulgatus with vector control material and P.distasonis did not grow in Bo-Inulin CM, its time points correspond to the growth points of B. vulgatus with inulinases through the growth phases (F) Western immunoblot analysis of cell lysates (CL), supernatant (sup) or OMV from wild type transconjugants synthesizing His-tagged GH/PLs. BT_1760 was not tracked in this assay. (G) Growth of B. vulgatus in defined inulin medium with added outer membrane vesicles (OMV) isolated from B. ovatus inulin CM. The OMV were harvested from supernatant of B. ovatus grown to log-phase so that the bacteria were actively growing at the time of harvest. (H) TLC analysis of the resulting media from the samples shown in panel G, through the growth phases (1-early, 2-mid, 3-late log) demonstrating PBP consumption by B. vulgatus during growth in OMV + inulin media. The first lane is B. vulgatus cultured inulin medium without OMV at the same timepoint as B. vulgatus with OMV at timepoint 3. Data are representative of ≥2 independent experiments, one representative experiment is shown.
These observations raised the intriguing possibility that in addition to PBP, producer-derived GH/PL themselves are secreted extracellularly and are public goods liberating PBP spatially distant from the producer. We first demonstrated that producer-derived CM contains GH/PL activity as revealed by the depletion of polysaccharide and accumulation of PBP over time in cell-free CM (Figure 4B). There was potent inulinase and amylopectinase activity in B. ovatus-derived CM, and less xylanase and levanase activity in B. ovatus xylan and B. thetaiotaomicron levan CM, respectively. B. fragilis, which does not liberate PBP during growth in inulin (Figure 1B), did not yield any detectable inulinase activity in its CM (Figure S3A). To definitively demonstrate that polysaccharide degradation and PBP liberation was due to producer-derived GH/PL in the CM, we analyzed the CM from recipient bacteria constitutively expressing the GH/PL genes in trans, grown in defined glucose medium. As these CM are devoid of PBP at the start of the assay (Figure 4C), the observed PBP generated upon the addition of polysaccharide is due to the specific extracellular GH/PLs encoded by these genes in trans (Figure 4C).
If these GH/PL are public goods, recipients should grow and utilize polysaccharide when the respective producer-derived GH/PL is added to the growth medium. To test this prediction, we purified recombinant producer derived PLs BACOVA_04502 and BACOVA_04503 (Figure S3B). Addition of these inulinases to inulin medium led to rapid degradation of the polysaccharide with accumulation of PBP (Figure S3B) and supported the growth (Figure 4D) and utilization of PBP (Figure 4E) by the recipient B. vulgatus, but not the non-recipient P. distasonis.
Gram negative bacteria communicate with and deliver cargo to other cells using various secretion mechanisms, one of which is the release of outer membrane vesicles (OMVs) [22, 23]. The producer-encoded GH/PL(BACOVA_04502, BACOVA_04503, BT_1760 and BT_3698) each contain an N-terminal signal peptidase II (SpII) cleavage site, indicating that they are lipoproteins, most of which have been shown to localize to the outer surface of Bacteroidales species [21, 24]. We hypothesized that the secreted GH/PL public goods would be present in OMVs rather than the soluble fraction due to the lipid moiety of these molecules. To test this hypothesis, we His-tagged these four proteins at their C-termini and placed the recombinant plasmids in their background strains for protein localization studies. Western immunoblot analysis revealed that these GH/PLs are present in the OMV fraction of the supernatant (Figure 4F). OMVs isolated from B. ovatus grown to log phase in inulin medium were able to degrade inulin (Figure S3C) and supported the growth of recipient B. vulgatus in inulin (Figure 4G) with concomitant PBP depletion (Figure 4H). These data reveal that GH/PL public goods are carried by OMVs and demonstrate a role for OMVs in ecological interactions among Bacteroidales.
Inducible polysaccharide utilization by some recipient bacteria
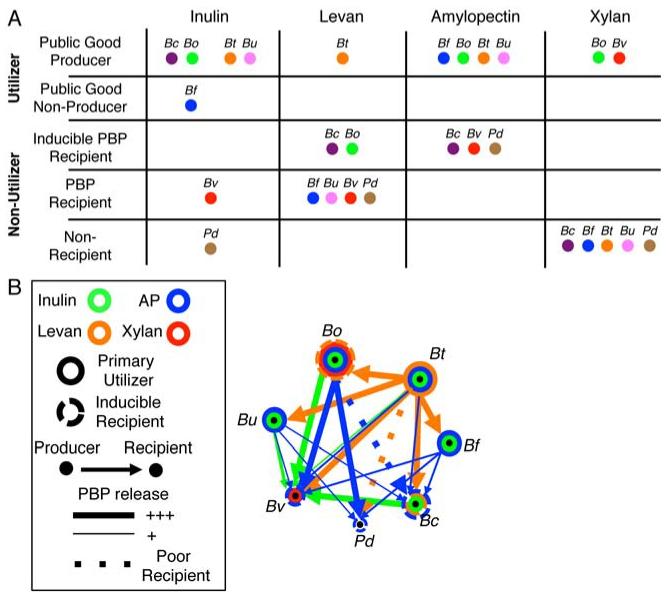
Analysis of PBP consumption by recipients during growth in producer-derived CM revealed that, in some cases, residual polysaccharide was also degraded (Figure 2B). As we demonstrated that cell-free CM derived from producers contained GH/PL activity, we sought to determine the extent to which the producer-derived GH/PLs contributed to this degradation. We boiled CM to inactivate producer-derived GH/PL and repeated the recipient growth experiments. Boiling of B. ovatus inulin CM abolished continued inulin degradation (Figure S4A) but did not affect PBP utilization and growth of recipient B. vulgatus (Figures S4B and C). Therefore, extracellular producer-derived inulinases accounted for the breakdown of inulin during B. vulgatus growth in this CM. In contrast, both B. caccae and B. ovatus grown in boiled B. thetaiotaomicron levan CM, and B. vulgatus grown in boiled B. ovatus amylopectin CM, showed continued robust depletion of polysaccharide not seen in control CM lacking bacteria (Figure S4C). Therefore, these recipients were induced by heat-stable producer-derived factors to catabolize the polysaccharide. This finding is consistent with the observation that under different experimental conditions, B. vulgatus has the ability to grow independently on amylopectin [25]. The ability to utilize polysaccharide was not the result of mutation as CM-induced recipients passaged through standard media were again unable to grow in polysaccharide defined media (Figure S4D). Together, these findings reveal a polysaccharide utilization network with five classes for each polysaccharide: utilizer/pubic goods producer, utilizer/public goods non-producer, inducible polysaccharide-utilizing recipient, non-inducible PBP recipient, and non-recipient (Figures 5A and B).
Figure 5. Ecological classes and network of polysaccharide utilization.
(A) Schematic diagram designating Bacteroidales type strains to one of five ecological classes: utilizer/public good producer, utilizer/public good non-producer, inducible PBP public good recipient, PBP public good recipient (non-inducible), and non-recipient. (B) A network of interactions based on PS utilization for Bacteroidales type strains.
Analysis of fitness benefits during producer-recipient co-culture
We next investigated the effects on fitness to both producers and non-utilizers (recipients and non-recipients) during co-culture. We used a producer auxotrophic for arginine, which allowed analyses of growth dynamics with and without an extrinsic growth limitation to the producer. In inulin, under producer non-limiting conditions, the fitness of B. vulgatus (inulin PBP recipient) increased when co-cultured with B. ovatus, whereas P. distasonis (a non-recipient) did not (Figure 6A). In amylopectin medium, B. vulgatus and P. distasonis, which both utilize liberated PBP and are induced to utilize polysaccharide, thrived in the presence of B. ovatus, whereas B. caccae (a poor recipient) again grew late (Figure 6B). Therefore, recipients, while unable to grow independently, can grow with an appropriate producer, but not outcompete the producer. We did not observe a decrease in fitness to the producer in any circumstance suggesting that in these scenarios, the production of public goods may not itself be costly. Interestingly, however, during amylopectin co-culture with recipients, an appreciable benefit to producer, as demonstrated by augmented growth compared to growth of producer alone, was observed (Figure 6B).
Figure 6. Fitness assays of polysaccharide utilizing and non-utilizing strains in co-culture.
Growth of type strains in co-culture or monoculture in (A) defined inulin medium or (B) defined amylopectin medium. The B ovatus strain is an arginine auxotrophic mutant allowing for analyses under both growth limiting (low arginine, 1 μg/ml) and non-limiting (high arginine, 16 μg/ml) conditions. Solid lines represent bacterial counts from monoculture experiments, whereas dotted lines represent bacterial counts from co-culture experiments. Monoculture experiments for recipients were performed under low arginine conditions only, as their growth is not affected by arginine; therefore, this growth curve is used for both producer-limited and not limited experiments. The limit of detection (indicated with * for strains that were below detection) for a given experiment is set at 2 logs below the total density of the culture. Data are representative of ≥3 independent experiments, one representative experiment is shown. Comparison of growth rates: Inulin producer non-limited: Bv in monoculture (-0.08 +/- 1.17) vs. Bv in co-culture (4.62 +/− 0.05), p = 0.027. Bo in monoculture (5.58 +/− 0.24) vs Bo in co-culture with Bv (7.24 +/− 2.4), p = 0.30, not significant (ns). Relative frequency producer : recipient (93.9% +/− 2.4 : 6.1% +/− 2.4. Inulin producer limited: Bv in monoculture (−0.08 +/− 1.17) vs. Bv in co-culture (3.9 +/− 1.2 ), p = 0.005. Relative frequency producer : recipient (5.3% +/− 2.1 : 94.7% +/− 2.1). Bo in monoculture (3.14 +/− 0.37) vs. Bo in co- culture with Bv (3.08 +/− 1.1), p = 0.48, ns. Amylopectin producer non-limited: Bv in monoculture (-2.67 +/− 0.73) vs. Bv in co-culture (4.69 +/− 2.54), p = 0.02. Bo in monoculture (6.06 +/− 1.13) vs. Bo in co-culture with Bv (9.34 +/− 0.89), p = 0.02. Relative frequency producer : recipient (96% +/− 1 : 4% +/− 1). Pd in monoculture (−1.95 +/− 1.07) vs. Pd in co-culture (1.42 +/− 1.56), p = 0.05. Bo in monoculture (6.07 +/− 1.13) vs. Bo in co-culture with Pd (9.88 +/− 0.52), p = 0.045. Relative frequency producer : recipient (92% +/− 2.7 : 8% +/− 2.7). Amylopectin producer limited: Bv in monoculture (− 2.7 +/− 1.15) vs. Bv in co-culture (11.2 +/− 4.1), p = 0.02. Bo in monoculture (6.4 +/− 1.77) vs. Bo in co-culture with Bv (6.22 +/− 1.08), p = 0.42, ns . Relative frequency producer : recipient (4.6% +/− 2.7 : 95.4% +/− 2.7). Pd in monoculture (−1.82 +/− 1.23) vs. Pd in co-culture (5.84 +/− 3.35), p = 0.87, ns. Bo in monoculture (6.37 +/− 1.78) vs Bo in co-culture with Pd (4.9 +/− 0.23), p = 0.21. Relative frequency producer : recipient (15.7% +/− 7.5 : 84.4%+/− 7.5). Limits of detection precluded determination of growth rate of Pd in inulin co-culture and Bc in amylopectin co-culture.
We next tested whether limiting the growth of the producer would allow a recipient to outcompete it. To investigate this possibility, we lowered the arginine concentration in the media so that the producer’s growth was limited (Figure S5A) but sufficient to liberate PBP (Figure S5B). Under these conditions, recipients dependent on PBP (B. vulgutus in co-culture with B. ovatus in inulin) and inducible PBP-recipients (B. vulgutus, P. distasonis, and B. caccae in co-culture with B. ovatus in amylopectin) all outcompeted the producer (Figure 6B) with robust PBP depletion and/or polysaccharide utilization by the recipients (Figure S5B). Together, these data demonstrate that in co-culture, specific Bacteroidales members (recipient but not non-recipient) can benefit from producer-derived public goods, and that extrinsically limiting the growth of the producer allowed recipients to dominate the population.
Bioinformatic analysis of the potential production of GH/PL public goods among gut Bacteroidales.
Having established an ecological network of polysaccharide utilization in Bacteroidales type strains with these four polysaccharides, we next sought to examine the generalizability of these interactions. For the polysaccharides analyzed in this study, B. caccae, B. vulgatus and P. distasonis were typically either recipients or non-recipients, but rarely producers; whereas B. thetaiotaomicron and B. ovatus were more often producers (Figure 5A and B). Having established that SpII containing GH/PLs can be secreted in OMVs as public goods, we used bioinformatics to determine if there are differences in the number of SpII-containing GH/PLs among intestinal Bacteroidales. This analysis revealed that B. caccae, B. vulgatus and P. distasonis type strains encode fewer predicted SpII-containing GH/PLs (28, 28 and 23, respectively) than B. thetaiotaomicron and B. ovatus type strains (54 and 80, respectively) (Tables S1 and S2). To determine if this correlation could be expanded to the species level, we analyzed the genomes of 86 Bacteroides and Parabacteroides strains with draft or completed genomes. This analysis demonstrated a high level of uniformity in the number of SpII-containing GH/PLs among strains of a given species (Table S1), despite differences in the GH/PL gene repertoire within a species. These data suggest that roles as either producers or recipients are a conspecific trait with regard to polysaccharide utilization among the Bacteroidales. Certain species such as B. ovatus and B. cellulosilyticus are more likely to utilize polysaccharide with concomitant production of public goods, i.e. are PBP producers for a larger repertoire of polysaccharides, whereas B. fragilis and P. distasonis are more likely to be recipients in plant polysaccharide utilization webs in the gut ecosystem.
Polysaccharide utilization network among highly abundant Bacteroidales from a natural human-derived ecosystem.
As the Bacteroidales type strains of this study were isolated from different individuals, we sought to determine whether these public good-based interactions occur in co-resident strains. We studied seven Bacteroidales species all co-colonizing a healthy human subject at a minimum concentration of 108 CFU/g feces [9]. Both utilizers and non-utilizers were identified for each of the four polysaccharides (Figure 7A). Among polysaccharide utilizers, individual members demonstrated both PBP producer and non-producer traits (Figure 7B). B. ovatus CL03T12C18 utilized levan, xylan and amylopectin, and liberated significant amounts of PBP during growth in each of these polysaccharides. Conversely, while P. distasonis CL03T12C09 was able to grow in inulin, and B. fragilis CL03T12C07 in amylopectin, these strains did not liberate any detectable PBP. In addition, these data confirm that growth on a particular polysaccharide is not a species-wide property, and support the bioinformatics analysis that certain species such as B. ovatus are producers that utilize many polysaccharides with concomitant PBP release compared to B. fragilis and P. distasonis.
Figure 7. Polysaccharide-based ecological relationships of naturally co-resident Bacteroidales.
(A) Growth curves of naturally co-resident Bacteroidales strains from human subject CL03 in the four defined polysaccharide media. (B) TLC analysis of PBP release during growth of CL03 strains in defined polysaccharide media. (C) Growth and (D) TLC analysis of PBP consumption by CL03 non-utilizing stains grown in the CM of primary utilizers, through the growth phases (1-early, 2-mid, 3-late log). Producer-derived CM was diluted with fresh polysaccharide containing defined media for recipient growth to assess inducible polysaccharide utilization. Therefore, lane 0 (undiluted producer CM) has less polysaccharide than the subsequent lanes. Data are representative of ≥2 independent experiments, one representative experiment is shown.
To determine if producer-recipient relationships occur among these naturally co- resident strains, growth of non-utilizers was monitored in the CM of two PBP liberating utilizers, B. ovatus CL03T12C18 grown in both levan and xylan, and B. xylanisolvens CL03T12C04 grown in amylopectin. As observed in the type strains, xylan PBP did not support the growth of any non-utilizer (Figure 7C), whereas levan and amylopectin CM supported the growth of some non-utilizers (Figure 7C,D), identifying PBP recipients and non-recipients. TLC analysis showed that B. fragilis CL03T12C07 grew in B. ovatus CL03T12C18 levan CM and P. distasonis CL03T12C09 grew in B. xylanisolvens CL03T12C04 amylopectin CM until the PBP were consumed (Figures 7C and D). These data reveal that abundant, co-resident strains of Bacteroidales derived from a natural human gut ecosystem form a network for polysaccharide utilization based on the production of public goods.
Discussion
This study reveals a complex polysaccharide utilization network among the Bacteroidales and illuminates an important facet of the communal lives of the most abundant Gram negative bacteria of the human intestine. Although metabolic webs are known to exist between members of the intestinal microbiota [26], previously described interactions are based on metabolic by-/waste- products that are not public nor common goods [26] and/or occur between phylogenetically distant members [26-28]. In contrast, public goods production by Bacteroidales, which may have evolved for cooperation among clone-mates, has resulted in a complex polysaccharide utilization network that has the potential for exploitation by recipients. In this network, trait variation exists among polysaccharide utilizers in that certain members liberate public goods, while others do not. This difference is likely due to the synthesis of distinct surface GH/PL among Bacteroidales species for utilization of the same polysaccharide [29]. Therefore, public good producing and non-producing strategies of polysaccharide utilization have simultaneously evolved among highly abundant, co-resident Bacteroidales members. While this study focused on characterizing the complex network of inter-specific interactions, it will be interesting to determine how the production of public goods balances the trade-offs of intra- versus inter-specific competition and/or cooperation among the Bacteroidales
At present, we do not know how the members of the human intestinal microbiota are spatially arranged. It is tempting to speculate that public good-based polysaccharide utilization leads to the formation of spatially organized groups of producers and co-evolved recipients, especially in the lumen where host glycan foraging does not occur. Scaffolding provided by particulate matter has been suggested to be important for public good based dynamics in non-clonal, but closely related socially cohesive groups of bacteria in the ocean [12]. In the human gut, insoluble substrates have been demonstrated to support a specialized microbiota [30], and these insoluble substrates may serve as a scaffold to spatially organize these public goods-based interactions not only between the Bacteroidales, but also Firmicutes, and other less abundant members of this ecosystem.
A more comprehensive picture is emerging to explain how Bacteroidales species stably co-exist at high densities over time. One strategy is the utilization of different polysaccharide substrates, therefore, avoiding direct competition for carbon sources [15, 19, 31]. Another complementary strategy, not mutually exclusive to the first, revealed in this study, is the existence of polysaccharide public good-based interaction networks where certain individuals can persist on carbon sources not supported by their own genes. This polysaccharide utilization network is consistent with the Black Queen hypothesis, which proposes that closely related bacterial species in communities form interdependent interactions marked by the loss of shared diffusible functions [32, 33], which in this network would be the secreted GH/PLs. In addition, reciprocal beneficial relationships or partner feedback mechanisms where factors derived from recipients increase the fitness of the producer [34] have likely evolved within these naturally communities. Indeed, we observe that for certain polysaccharides, co-culture of producers and recipients leads to an increase in producer fitness not seen when producers are cultured alone, suggesting the evolution of mutualistic interactions.
By using a hypothesis based approach, we applied social evolutionary thinking to understand the ecology of abundant members of the human microbiota and revealed a complex network of polysaccharide utilization and characterized the cellular and molecular mechanisms of these interactions. Both hypothesis-based and unbiased approaches to studying social and ecological interactions among the intestinal microbiota will be important in advancing this field. Bioinformatic and experimental approaches, coupled with evaluation of the fitness of different individuals within a group will facilitate our understanding of the ecological dynamics of the intestinal microbiota. Such networks should be considered in both ecological modeling and therapeutically modulating the microbiota for human benefit.
Experimental Procedures
Bacterial strains
Bacteroidales type strains used in this study are Bacteroides caccae ATCC 43185, B. fragilis NCTC 9343, B. ovatus ATCC 8483, B. thetaiotaomicron VPI 5482, B. uniformis ATCC 8492, B. vulgatus ATCC 8482, and Parabacteroides distasonis ATCC 8503. Co-resident Bacteroidales strains isolated from human intestinal ecosystems were previously described [9] and those used in this study include B. caccae CL03T12C61, B. dorei CL03T12C01, B. fragilis CL03T12C07, B. ovatus CL03T12C18, B. xylanisolvens CL03T12C04, Parabacteroides distasonis CL03T12C09 and P. merdae CL03T12C32, all of which coexisted in a human at a density of at least 108/g feces.
Bacterial culture
For growth in defined or conditioned media, bacteria were inoculated from BHIS plates into BS, cultured overnight to stationary phase, then diluted 1:10 in fresh BS and grown to mid log. At mid log, bacteria were pelleted by centrifugation and washed with sterile phosphate buffered saline (PBS) and then inoculated in either defined or conditioned media. Carbohydrates used to supplement defined media include glucose (G7528, Sigma), levan (L8647, Sigma), amylopectin (10120, Sigma), xylan (X4252, Sigma) and inulin (OraftiHP, Beneo-Orafti group). Conditioned media were prepared by filter sterilizing supernatants from bacteria grown in defined media to late log. For initial experiments, conditioned medium was harvested at all phases of growth and compared for their ability to support recipient growth (Figure S2). For growth of bacteria in conditioned media, harvested conditioned media was diluted 1:1 with fresh defined medium without carbohydrate. In some instances, filter sterilized conditioned media were boiled before being diluted with fresh carbohydrate-free defined medium. All cultures were grown at 37°C under anaerobic conditions. Bacterial growth was quantified by optical density (OD600) using 200 μl of bacterial culture in 96 well flat-bottom microtiter plates using a Powerwave spectrophotometer (Biotek).
Co-culture experiments
For bacterial co-culture experiments, bacteria were grown as indicated for monoculture prior to addition to the defined media. Quantification and differentiation of the two species was performed by plating dilutions on BHIS, followed by replica plating onto defined glucose minimal plates, which do not support the growth of the B. ovatus Δ03533 arginine auxotrophic mutant. Any ambiguous colonies were confirmed using a previously described PCR to differentiate these species [9]. The limit of detection for an outcompeted species was set at two logs below the total density of the culture.
See Supplemental Experimental Procedures for bacterial media, molecular cloning and mutational methods, TLC procedures, and bioinformatics analyses.
Supplementary Material
Highlights.
Gut Bacteroidales form a complex network for polysaccharide (PS) utilization
Non-utilizers grow on PS breakdown products liberated during growth of utilizers
OMVs carry PS degrading enzymes supporting non-utilizer growth distant from producer
These public good-based interactions occur among Bacteroidales co-colonizing humans
Acknowledgements
The authors declare no competing financial interests. We thank Kevin Foster and C. Brandon Ogbunugafor for discussion and critical review of the manuscript. Inulin and FOS were a kind gift from Beneo-Orafti. This work was supported PHS grant R01AI081843 from the NIAID. S. R-N is supported by the PIDS-St Jude Fellowship Program in Basic Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, et al. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, Takami H, Morita H, Sharma VK, Srivastava TP, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zitomersky NL, Coyne MJ, Comstock LE. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infect Immun. 2011;79:2012–2020. doi: 10.1128/IAI.01348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol. 2006;4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 11.Cordero OX, Wildschutte H, Kirkup B, Proehl S, Ngo L, Hussain F, Le Roux F, Mincer T, Polz MF. Ecological populations of bacteria act as socially cohesive units of antibiotic production and resistance. Science. 2012;337:1228–1231. doi: 10.1126/science.1219385. [DOI] [PubMed] [Google Scholar]
- 12.Cordero OX, Ventouras L-A, DeLong EF, Polz MF. Public good dynamics drive evolution of iron acquisition strategies in natural bacterioplankton populations. Proc. Natl. Acad. Sci. USA. 2012;109:20059–20064. doi: 10.1073/pnas.1213344109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salyers AA, Vercellotti JR, West SE, Wilkins TD. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977;33:319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, Mcnulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of Polysaccharide Use in Intestinal Bacteroides Species Determines Diet-Induced Microbiota Alterations. Cell. 2010;141:1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 17.Salyers AA, Gherardini F, O'Brien M. Utilization of xylan by two species of human colonic Bacteroides. Appl Environ Microbiol. 1981;41:1065–1068. doi: 10.1128/aem.41.4.1065-1068.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J. Biol. Chem. 2009;284:24673–24677. doi: 10.1074/jbc.R109.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves AR, Wang GR, Salyers AA. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1997;179:643–649. doi: 10.1128/jb.179.3.643-649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shipman JA, Cho KH, Siegel HA, Salyers AA. Physiological characterization of SusG, an outer membrane protein essential for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1999;181:7206–7211. doi: 10.1128/jb.181.23.7206-7211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 23.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher CM, Coyne MJ, Villa OF, Chatzidaki-Livanis M, Comstock LE. A general O-glycosylation system important to the physiology of a major human intestinal symbiont. Cell. 2009;137:321–331. doi: 10.1016/j.cell.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy RE, Pajeau M, Salyers AA. Role of starch as a substrate for Bacteroides vulgatus growing in the human colon. Appl Environ Microbiol. 1988;54:1911–1916. doi: 10.1128/aem.54.8.1911-1916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischbach MA, Sonnenburg JL. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe. 2011;10:336–347. doi: 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman AL, Mcnulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci. USA. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spence C, Wells WG, Smith CJ. Characterization of the primary starch utilization operon in the obligate anaerobe Bacteroides fragilis: Regulation by carbon source and oxygen. J Bacteriol. 2006;188:4663–4672. doi: 10.1128/JB.00125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker AW, Duncan SH, Harmsen HJM, Holtrop G, Welling GW, Flint HJ. The species composition of the human intestinal microbiota differs between particle-associated and liquid phase communities. Environmental Microbiology. 2008;10:3275–3283. doi: 10.1111/j.1462-2920.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013 doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris JJ, Lenski RE, Zinser ER. The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio. 2012;3 doi: 10.1128/mBio.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachs JL, Hollowell AC. The Origins of Cooperative Bacterial Communities. mBio. 2012;3 doi: 10.1128/mBio.00099-12. e00099–12–e00099–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster KR, Wenseleers T. A general model for the evolution of mutualisms. Journal of Evolutionary Biology. 2006;19:1283–1293. doi: 10.1111/j.1420-9101.2005.01073.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.