Abstract
The neuropeptide oxytocin has been implicated in a wide range of social processes, such as pair bonding, affiliation, and social judgments that may contribute to normal adjustment and psychiatric states. The present experimental study sought to elucidate potential underlying mechanisms by which oxytocin may impact social processes by examining the effects of intranasal oxytocin on basic evaluative processes. Subjects rated slide stimuli from the International Affective Picture System, varying across multiple categories (pleasant, neutral, unpleasant) and social content. Separate ratings for arousal and for the positive and negative components of valence were obtained in the context of a bivariate evaluative space model. Oxytocin did not have an independent significant effect on positivity or negativity ratings, but instead oxytocin treatment altered the interaction between these component processes for social, relative to non-social stimuli regardless of valence conditions. Specifically, oxytocin, relative to vehicle, significantly decreased arousal ratings to threatening human stimuli without altering ratings of threatening animal stimuli. These results indicate that oxytocin may exert its effects through dynamic alterations in the partially separable neural substrates mediating arousal as well as positive and negative evaluations of social stimuli.
Keywords: Affect, evaluation, neuropeptide, oxytocin, social, threat
Introduction
The neuropeptide oxytocin is receiving increased attention in view of its primary role in the regulation of social processes and its potential contribution to psychiatric conditions. Anomalies in oxytocin systems, for example, have been suggested to contribute to a wide range of psychopathologies, ranging from autism (Hollander et al., 2007) and depression (Costa et al., 2009) to schizophrenia (Goldman, 2009) and psychopathy (Lee et al., 2009). Within non-human animals, oxytocin is an important neurochemical substrate underlying social behaviors including social attachment (Carter, 1998), social recognition (Ferguson et al., 2001), aggression (DeVries et al., 1997), and social approach (Young, 2002). In humans, oxytocin attenuates amygdala activation to fearful and threatening stimuli (Kirsch et al., 2005) as well as emotionally laden faces (Domes et al., 2007). Moreover, oxytocin decreases amygdala activation and modulates the expression of evaluative conditioning selectively for socially relevant faces (Petrovic et al., 2008).
In conjunction with its role in neural circuits associated with threat and aversion, oxytocin modulates activity within mesolimbic reward pathways where it is thought to mediate variations in social attachment and approach behaviors (Insel and Young, 2001; Ross et al., 2009). Positive social interactions increase the central release of oxytocin which promotes social bonding and down-regulates reactivity to stressors (Uvnas-Moberg, 1998). Moreover, oxytocin administration in humans enhances the encoding of positive social information (Unkelbach et al., 2008), selectively increases memory for positive social stimuli (Guastella et al., 2008), and slows reaction time to correctly identify fearful facial expressions (Di Simplicio et al., 2009).
Oxytocin modulates positive and negative affect through the differential activation of partially distinct neurobiological substrates. Importantly, previous reports on the effects of oxytocin have utilized bipolar models of affect where positive and negative fall along a single valence dimension ranging from positive to negative, and as a result, precludes consideration of the dynamic interactions between the two systems. The evaluative space model (Cacioppo and Berntson, 1994) postulates that evaluative processes represent the integration of two separable and partially distinct components of the evaluative system. Consistent with the evaluative space model (ESM), evidence suggests that positive and negative affect can be uncorrelated (Larsen et al., 2004), that factors can have functionally different effects on the activation of positive and negative components of valence (i.e. positivity and negativity), and that the same stimulus can evoke differential activation of positive and negative reactions and associated emotional arousal.
Specifically, oxytocin acts upon a widespread network of neural systems to modulate social behavior across diverse contexts. Such broad effects are thought to be the result of dynamic modulation of both approach and avoidance systems allowing social animals to overcome the natural avoidance of proximity and to inhibit defensive behavior, thereby facilitating approach behavior (Carter, 2007; Hammock and Young, 2006; Heinrichs and Gaab, 2007). The present study is the first to evaluate the effects of intranasal oxytocin on basic evaluative processes from the perspective of a bivariate model of affect (Cacioppo and Berntson, 1994; Cacioppo et al., 2010). In order to illuminate potential basic mechanisms of oxytocin actions, we examined the effects of oxytocin on separate components of valence ratings (positivity and negativity ratings) as well as arousal ratings of affective picture stimuli (pleasant, neutral, and unpleasant).
Methods and materials
Subjects
Forty-five participants (22 females and 23 males; mean age 24.22, SD 5.3) were included in this study. Participants were scheduled for an initial screening visit to determine eligibility for the study. During this visit, they completed the Health and Health Behaviors questionnaire to determine whether they were currently suffering from any psychiatric or medical condition. The participants disclosed no history of mental or psychiatric illness, and did not suffer from any chronic medical condition. Prior to the study, participants provided written informed consent and were asked to fast 5 h prior to each visit. Participants were then assigned to either the oxytocin or vehicle group in a random, double-blind manner. The study protocol was approved by the Institutional Review Board of The Ohio State University.
Experimental design
Upon arriving to the experimental session, individuals completed the first round of the evaluative task. Following the completion of the baseline evaluative task, individuals were randomly assigned to receive the intranasal preparation containing either oxytocin (20 IU, Pharmacy Specialist, Altamonte Springs, Florida, n =24) or vehicle (n =21). Participants had previously been instructed on and practiced the administration procedure and were observed throughout to ensure appropriate dosing. Forty-five minutes following intranasal application, individuals completed the second round of the evaluative task. The dosage (20 IU) and timing (45 min post-administration) used in this study was chosen based upon previous reports on the effects of oxytocin on psychological variables (Guastella et al., 2008; Kirsch et al., 2005; Kosfeld et al., 2005). All studies were conducted between 12:00 p.m. and 2:00 p.m. After completion of the study, participants were paid US$100 for the participation.
Stimuli set and evaluative task
Stimuli of the evaluative task were pleasant, unpleasant, and neutral pictures from the International Affective Picture System (IAPS; Lang et al., 1999). Pleasant and unpleasant pictures were matched on normative arousal ratings and on evaluative extremity from the neutral point of the normative scale. Stimuli consisted of eight slides per session of pleasant non-social (e.g. cake), pleasant social (e.g. children playing), neutral non-social (e.g. chair), neutral social (e.g. neutral face), unpleasant non-social (e.g. garbage), and unpleasant social (e.g. frightened child). In conjunction with the ratings mentioned above, a separate group of IAPS slides were utilized in order to determine the potential effects of oxytocin on subjective ratings of threatening human (e.g. terrorist) versus animals (e.g. snake) stimuli. All stimuli were carefully selected to sample the range of affective ratings, distinct emotions, as well as social versus non-social and human threat versus animal threat contexts.
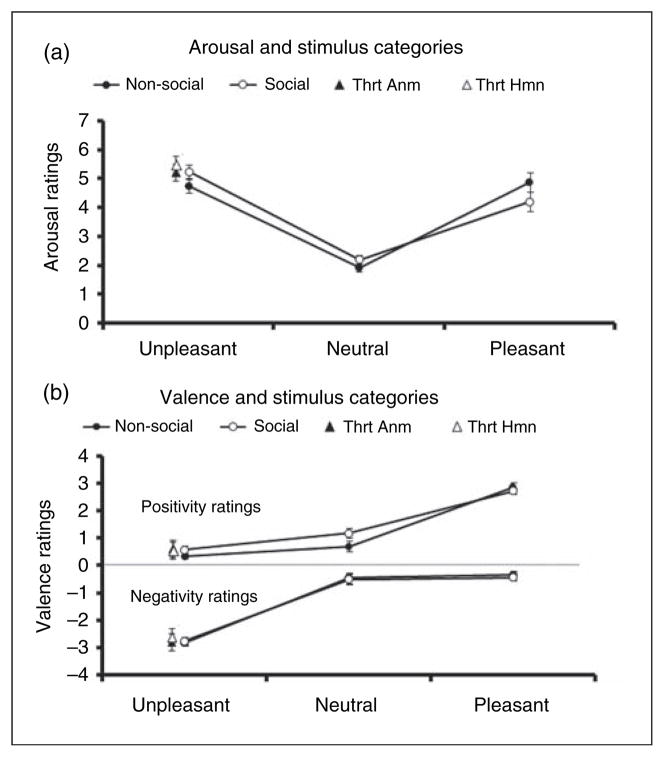
As described in Berntson et al. (2007), participants rated the picture stimuli on positivity, negativity, and arousal dimensions. Pictures were presented in random order on a computer monitor for 4 s. Participants were instructed to focus on the emotional content of the pictures. After viewing each picture, participants were instructed to rate it on a five-point bivariate scale of positivity and negativity and a univalent scale of how aroused it made them feel (Larsen et al., 2009). Positivity and negativity ratings were registered by moving a mouse pointer and clicking a location in a five-point bivariate grid with the horizontal axis indexing positivity (0 = not at all, 4 = extremely) and the vertical axis indicating negativity (0 = not at all, 4 = extremely). The simple difference between positivity and negativity ratings has been found to correlate very highly (r values >.90) with valence ratings (Larsen et al., 2009). The response grid was presented on the screen immediately after termination of the stimulus picture. After responding, a second screen displayed a single response continuum and the subject was instructed to rate how emotionally aroused they felt to the stimulus on a nine-point scale (1 = not at all, 9 = extremely). Three seconds after completing the ratings, the next slide was presented. Participants displayed the expected changes in evaluative and arousal functions to positive, negative, and threat stimuli during the baseline task (Figure 1).
Figure 1.
Baseline valence and arousal ratings. Mean (SEM) valence (a) and arousal (b) ratings across stimulus categories from Lang et al., (1999). Participants effectively discriminated the stimulus categories and applied valance ratings accordingly. Similarly, all groups also displayed the expected increases in arousal functions to pleasant, unpleasant, and threat stimuli during the baseline task. Thrt Anm: animal threat, Thrt Hmn: human threat.
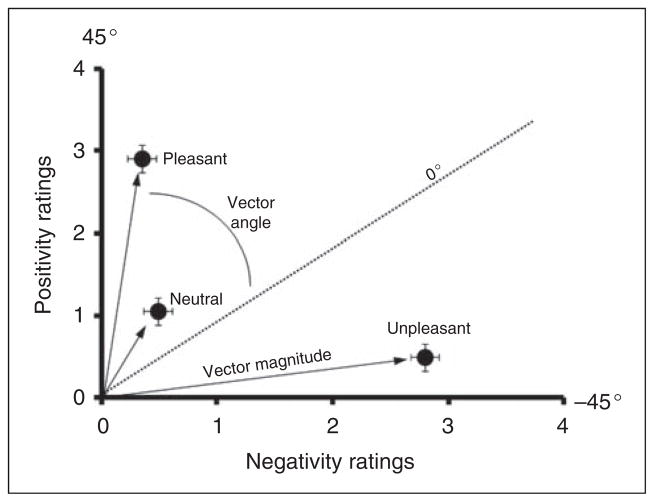
In an attempt to quantify the dynamic interaction subserving evaluative processes, negativity and positivity ratings were transformed into variables constituting a vector within a two-dimensional Cartesian coordinate system with negativity ratings on the abscissa, positivity ratings on the ordinate, and the hypotenuse being the vector magnitude. Individual vector angles were calculated as the interior angles of the junction between the ordinate and the 0° line bisecting the planes (Figure 2). Alterations within the vector angle represent changes in the interaction between positive and negative ratings and changes in vector magnitude represent alterations in the size of the evaluative ratings.
Figure 2.
Bivariate representation of baseline evaluative space ratings in a two-dimensional Cartesian coordinate system, with negativity ratings on the abscissa, positivity ratings on the ordinate, and the hypotenuse being the vector magnitude. The individual vector angles were calculated as the interior angles of the junction between the ordinate and the 0 ° line bisecting the planes. As expected, pleasant pictures had the highest vector angle followed by neutral and unpleasant. Neutral stimuli had the smallest vector magnitude with pleasant and unpleasant stimuli having similar magnitudes.
Data analysis
Primary statistical evaluation of evaluative ratings data was by between-within (repeated measures on stimulus categories and drug condition) analysis of variance (ANOVA) with trends, followed up by simple ANOVAs to evaluate pairwise contrasts where significant effects were obtained on the omnibus ANOVA. Due to the multiple comparisons used in the evaluation of valance data, all positivity and negativity ratings were analysed using a Bonferroni correction to limit the potential of type I errors. In order to assure group differences were not the result of extraneous variables, independent t-tests were used to test whether the oxytocin and placebo groups were significantly different with regards to demographic (e.g. age, gender, ethnicity) or baseline psychological (depression, anxiety, loneliness) measures.
Results
Independent samples t-tests revealed that the oxytocin and placebo group were similar (p >0.05) with regards to demographic characteristics (e.g. age, gender, ethnicity) and baseline measures of depression, anxiety, and loneliness (Table 1).
Table 1.
Demographic and psychosocial variables of vehicle and oxytocin groups. Vehicle and oxytocin groups were comparable on demographic and psychosocial variables
| Vehicle | Oxytocin | p-value | |
|---|---|---|---|
| n | 21 | 24 | |
| Gender (% Female) | 52.38 | 45.83 | 0.43 |
| Ethnicity (% Caucasian) | 85.71 | 87.50 | 0.46 |
| Married (%) | 38.10 | 29.17 | 0.22 |
| Age (Years) | 23.91 ± 1.98 | 24.51 ± 1.75 | 0.82 |
| Education (years) | 13.92 ± 1.02 | 13.16 ± 1.39 | 0.66 |
| Annual income (in thousands of $) | 21.23 ± 2.01 | 22.182 ± 1.98 | 0.74 |
| BDI | 3.84 ± 1.27 | 4.95 ± 1.15 | 0.52 |
| BAI | 3.97 ± 1.09 | 5.18 ± 1.01 | 0.42 |
| PSS-10 | 10.77 ± 1.54 | 9.59 ± 1.35 | 0.57 |
| UCLA | 36.86 ± 2.16 | 36.42 ± 2.02 | 0.88 |
BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory, PSS-10: perceived stress scale (10-item), UCLA: UCLA loneliness scale.
Arousal ratings
A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (sociality; social/non-social) × 3 (stimulus categories; neutral, unpleasant, and pleasant) ANOVA on arousal ratings revealed expected differences by category (F2,86 = 194.76, p <0.01) where unpleasant and pleasant stimuli were rated as more arousing than neutral (Figure 1a). Additionally, a significant sociality by stimulus category interaction was detected (F6,258 = 42.89, p <0.01) reflecting the fact that unpleasant social pictures were rated as more arousing compared to unpleasant non-social pictures, whereas pleasant social pictures were rated slightly less arousing than pleasant non-social pictures (Figure 1a).
A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (animal; human/non-human) ANOVA revealed a significant drug by time by animal interaction in the arousal ratings to threat stimuli (F1,43 = 6.87, p = 0.01, f2 = .21). This reflected the fact that oxytocin, relative to vehicle, selectively decreased arousal ratings for threatening human stimuli, but had no effect on arousal ratings to threatening animal stimuli (F1,43 = 0.05, p = 0.82; see Figure 3c).
Figure 3.
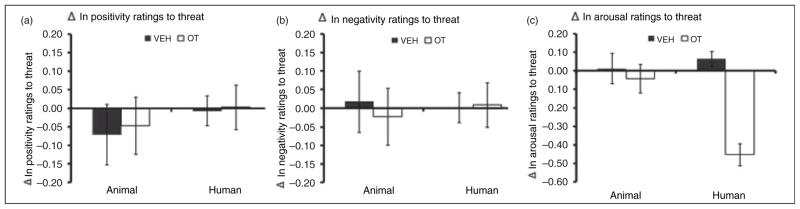
Oxytocin effects on threat ratings. Mean (SEM). (a) Pre/post change scores in positivity ratings to threatening stimuli, (b) pre/post change scores in negativity ratings to threatening stimuli, and (c) pre/post change scores in arousal to threatening stimuli. Oxytocin had no influence on positivity and negativity ratings to threat; it selectively decreased arousal ratings threat. OT: oxytocin (group), VEH: vehicle (group).
Positivity and negativity ratings
Positivity ratings of picture stimuli
A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (sociality; social/non-social) × 3 (stimulus categories; neutral, unpleasant, and pleasant) ANOVA revealed expected differences by category (F2,86 = 749.31, p < 0.01). This reflected the higher positivity ratings for pleasant, relative to neutral and unpleasant, stimuli (Figure 1b). In addition, a significant sociality by condition interaction (F1,86 = 37.86, p < 0.01) revealed that social pictures were rated more positively for unpleasant and neutral pictures but not for pleasant pictures (see Figure 1a). No time or drug effects were detected (p >0.05).
A separate analysis was pursued on a different set of slide stimuli depicting threatening content. As illustrated in Figure 1, these stimuli were similar to other unpleasant stimuli in their valence and arousal ratings. A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (animal; human/non-human) ANOVA was utilized to determine potential time, drug, and animal differences on the ratings of these threatening stimuli. No significant main effects or interactions were revealed for any of these factors (p >0.05; Figure 3a).
Negativity ratings of pictures
A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (sociality; social/non-social) × 3 (stimulus categories; neutral, unpleasant, and pleasant) ANOVA revealed expected differences by category (F2,86 = 109.12, p < 0.01) where individuals rated unpleasant pictures more negatively than neutral or pleasant pictures (Figure 1b). No significant effects were detected for drug (p >0.05), time (p >0.05), or sociality (p >0.05).
As expected, negativity ratings of both the human and animal threat slides were significantly higher than neutral slides (t44 = 23.57, p < 0.01; see Figure 1b). Drug effects on the negativity ratings were evaluated by 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (animal; human/non-human) design ANOVA. No significant time, drug, or animal effects were detected for negativity ratings of threatening stimuli.
Vector space derived ratings
Although no significant differences were observed in the separate positive and negative functions alone, it is possible that differences in the patterns of ratings across valences may have been modulated. To further examine this possibility, the positivity and negativity ratings of the picture categories were replotted and analysed in a bivariate evaluative space model, as illustrated in Figure 2. From this bivariate representation, the data can be characterized by two orthogonal vector parameters. The first is a magnitude estimate, capturing the overall evaluative distance from zero, and the second is a vector angle, representing deviation (up to + or − 45 °) from the reciprocal diagonal (Figure 2). A positive value of the vector angle would indicate a predominance of positivity judgments, a negative angle a predominance of negativity judgments, and a 0 angle would represent coactivation, or balanced positivity and negativity.
Vector magnitude
A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (sociality; social/non-social) × 3 (stimulus categories; neutral, unpleasant, and pleasant) ANOVA revealed expected differences by category (F2,86 = 412.89, p <0.01) where the vector magnitude was larger for pleasant and unpleasant stimuli compared to neutral pictures. No significant effects were detected for drug, time (pre/post), or sociality. Analysis of vector magnitude ratings of threatening stimuli according to a 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (animal; human/non-human) ANOVA design failed to reveal any differences between human versus animal threat (Figure 4b). There was no significant drug or pre/post effects detected on vector magnitude (p >0.05).
Figure 4.
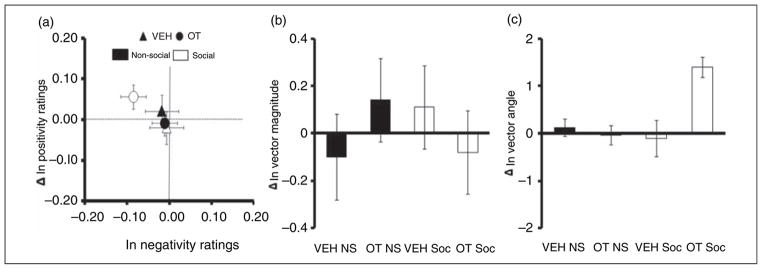
Oxytocin effect on evaluative ratings irrespective of valence category. Mean (SEM). (a) Pre/post scores in positivity and negativity ratings, (b) pre/post scores in vector magnitude scores, and (c) pre/post scores in vector angle scores. Oxytocin had no influence on vector magnitude ratings. However, oxytocin selectively increased vector angle ratings to social stimuli across all categories. NS: non-social, OT: oxytocin, Soc: social, VEH: vehicle.
Vector angle
A 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (sociality; social/non-social) × 3 (stimulus categories; neutral, unpleasant, and pleasant) ANOVA revealed expected differences by category (F2,86 = 147.19, p <0.01) where the vector angle was largest for pleasant and lowest for unpleasant pictures. Although separate analyses of positivity and negativity ratings failed to reveal differences, a drug × sociality × time interaction was detected for the vector angle (F1,43 = 4.63, p =0.037, f2 = .16; Figure 4c) where oxytocin led to increased vector angles (positivity rating bias) regardless of category, represented by a simultaneous increase in positivity and decrease in negativity ratings (Figure 4a). Analysis of vector angle ratings of threatening stimuli according to a 2 (time; pre/post drug) × 2 (drug; oxytocin/vehicle) × 2 (animal; human/non-human) ANOVA design was utilized to determine drug, pre/post drug, and human versus animal effects on the ratings of threatening stimuli. Vector angle ratings of the human and animal threat slides were not different among groups, nor were there significant drug or pre/post effects were detected for vector angle ratings threatening stimuli (F1,43 = .31, p = 0.58).
Discussion
Intranasal administration of oxytocin significantly decreased arousal ratings to human threat stimuli without altering arousal to animal threat (Figure 3c). Valence ratings were not different with regards to threat stimuli for the oxytocin and vehicle groups. While oxytocin did not significantly alter positivity or negativity ratings independently, it significantly increased the vector angle, a metric that captures the dynamic interplay between positive and negative ratings, to pleasant social stimuli across all categories (Figure 4c). Further demonstrating the selectivity of oxytocin to social stimuli, oxytocin had no effects on non-social stimuli.
The present results may explain and organize other findings in the literature discussed above. Threatening stimuli are rapidly processed through a subcortical pathway that allows for immediate emotional responses that tend to promote defensive behaviors (Ohman, 2005). Intranasal oxytocin diminishes emotion arousal to social threat which may allow for more elaborated processing of social interactions that favor the approach behaviors observed with oxytocin administration. Indeed, recent research suggests that some of the effects of intranasal oxytocin may result from alterations in higher level cognitive processing of social valence (Guastella et al., 2009). Moreover, in conjunction with its effect on social threat, the ability of oxytocin to specifically modulate the interplay between positive and negative social evaluative processes (increase in vector angle) may promote social approach behaviors associated with an increased willingness to trust (Baumgartner et al., 2008).
One potential mechanism for the findings discussed in the present manuscript is the well-described effects of oxytocin on the amygdala. Oxytocin receptors densely populate the central amygdala where they act to inhibit amygdala brainstem functional connectivity (Kirsch et al., 2005). The data presented in this study suggest a far more selective effect of oxytocin on arousal ratings specifically to threatening social stimuli, ostensibly resulting from the activation of distinct subpopulations of oxytocin receptors within the amygdala (Huber et al., 2005). Oxytocin signaling within the mesolimbic reward pathway is especially important in the mediation of motivational and rewarding properties associated with social behavior (Insel and Young, 2001; Ross et al., 2009). Therefore, in conjunction with its known influence on the amygdala, oxytocin may have influenced the dynamic interactivity within valance ratings presented in this study through modulation of a broad neural network associated with both aversion and approach behaviors.
The present study would have benefited from the inclusion of physiological measures of arousal such as skin conductance, so the interpretations of arousal ratings must be limited to the psychological domain. In addition, future studies will be needed to evaluate the potential dose-response relations in the effects of intranasal oxytocin on evaluative processes. Although clear alterations in evaluative ratings were apparent, understanding of the specific neurobiological substrates for this effect will require further neuroimaging studies or other experimental approaches. Although the present results indicate a significant effect of oxytocin on aspects of evaluative processing, because eye tracking was not monitored in this study, we cannot rule out a potential contribution from oxytocin effects on facial visual search and fixation patterns. This remains a possibility, although no close-up facial stimuli were used in the present picture series.
In summary, a single dose of intranasal oxytocin significantly decreases arousal ratings to human threat stimuli. Although oxytocin did not significantly alter positivity and negativity ratings independently, it did modulate the dynamic interaction between positive and negative ratings of social stimuli across all categories. Further highlighting the selective effects of oxytocin, oxytocin had no effect on non-social stimuli in the very same categories. These data provide a more accurate characterization of the influences of oxytocin on affective processes through the usage of a bivariate model of affect. In conjunction with the increasingly rich literature of the selective effects of oxytocin on neural representations of the social world, the present results suggest that oxytocin may influence behavior through dynamic interactions between arousal and approach and avoidance systems. This may have important implications for psychiatric disorders associated with alterations in social behaviors and reactions to social threat, as many psychiatric disorders are characterized by alterations in social approach–avoidance systems (Cacioppo et al., 2007; Goldin et al., 2009; Schaefer et al., 2006). In conjunction with previous studies, the present results demonstrating oxytocin’s ability to decrease subjective arousal to threatening social stimuli while simultaneously altering the dynamic interplay between positive and negative percepts of socially relevant stimuli suggests oxytocin may have therapeutic potential in the understanding and treatment of disorders characterized by social impairments that manifest in alterations in both aversion (e.g. amygdala) and approach (mesolimbic pathway) systems.
Acknowledgments
We thank Thelma Vargo, Betsy Nini, and the entire clinical research center staff for their excellent technical assistance. This study was supported by grants from the Templeton foundation (JTC), the American Heart Association (predoctoral fellowship to KK) and the Clinical Research Center at The Ohio State University, Grant UL1-RR025755 from the National Center of Research Resources of the NIH. All the authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
References
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bechara A, Damasio H, Tranel D, Cacioppo JT. Amygdala contribution to selective dimensions of emotion. Soc Cogn Affect Neurosci. 2007;2:123–129. doi: 10.1093/scan/nsm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Amaral DG, Blanchard JJ, et al. Social neuroscience: progress and implications for mental health. Perspect Psychol Sci. 2007;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychol Bull. 1994;115:401–423. [Google Scholar]
- Cacioppo JT, Berntson GG, Norris CJ, Gollan JK. The evaluative space model: Functional structure and operating characteristics of the affect system. In: Van Lange P, Kruglanski A, Higgins ET, editors. Handbook of Theories of Social Psychology. Thousand Oaks, California: Sage Press; 2010. [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, et al. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34:1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Young WS, III, Nelson RJ. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J Neuroendocrinol. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 2009;23:241–248. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res Rev. 2009;61:210–220. doi: 10.1016/j.brainresrev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Carson DS, Dadds MR, Mitchell PB, Cox RE. Does oxytocin influence the early detection of angry and happy faces? Psychoneuroendocrinology. 2009;34:220–225. doi: 10.1016/j.psyneuen.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Gaab J. Neuroendocrine mechanisms of stress and social interaction: implications for mental disorders. Curr Opin Psychiatry. 2007;20:158–162. doi: 10.1097/YCO.0b013e3280146a13. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings (Technical Report No A-4) Gainesville, Florida: Center for Research in Psychophysiology; 1999. [Google Scholar]
- Larsen JT, McGraw AP, Mellers BA, Cacioppo JT. The agony of victory and thrill of defeat: Mixed emotional reactions to disappointing wins and relieving losses. Psychol Sci. 2004;15:325–330. doi: 10.1111/j.0956-7976.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: a single-item measure of positivity and negativity. Cogn Emot. 2009;23:453–480. [Google Scholar]
- Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology. 2009;34:1567–1573. doi: 10.1016/j.psyneuen.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry. 2006;60:974–986. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Unkelbach C, Guastella AJ, Forgas JP. Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychol Sci. 2008;19:1092–1094. doi: 10.1111/j.1467-9280.2008.02206.x. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]