Abstract
Introduction/Purpose
The incidence of venous thromboembolism (VTE) after bariatric surgery is uncertain.
Materials and Methods
Using the resources of the Rochester Epidemiology Project and the Mayo Bariatric Surgery Registry, we identified all residents of Olmsted County, Minnesota, with incident VTE after undergoing bariatric surgery from 1987 through 2005. Using the dates of bariatric surgery and VTE events, we determined the cumulative incidence of VTE after bariatric surgery using the Kaplan-Meier estimator. Cox proportional hazards modeling was used to assess patient age, sex, weight, and body mass index as potential predictors of VTE after bariatric surgery.
Results
We identified 396 residents who underwent 402 bariatric operations. The most common operation was an open Roux-en-Y gastric bypass (n=228). Eight patients had VTE develop within 6 months (7 within 1 month) after surgery; 5 events occurred after hospital discharge but within 1 month after bariatric surgery. The cumulative incidence of VTE at 7, 30, 90, and 180 days was 0.3%, 1.9%, 2.1%, and 2.1%, respectively (180-day 95% CI, 0.7%-3.6%). Patient age was a predictor of postoperative VTE (hazard ratio, 1.89 per 10-year increase in age; 95% CI, 1.01–3.55; P=.05).
Conclusion
In our population-based study, bariatric surgery had a high risk of VTE, especially for older patients. Because most VTE events occurred after hospital discharge, a randomized controlled trial of extended outpatient thromboprophylaxis is warranted in patients undergoing open Roux-en-Y gastric bypass for medically complicated obesity.
Keywords: bariatric surgery, deep vein thrombosis, epidemiology, obesity, pulmonary embolism, venous thromboembolism
Introduction
An estimated 171,000 bariatric operations were performed in the United States in 2005; this was 10 times the number performed in 1994 (1). Although the potential benefits of bariatric surgery are substantial, so are the risks. In a 2004 meta-analysis, the 30-day postoperative mortality rate was 0.1% for purely restrictive procedures (gastric banding or gastroplasty), 0.5% for gastric bypass, and 1.1% for biliopancreatic diversion or duodenal switch (2).
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and its complication, pulmonary embolism (PE), is a common cause of morbidity and mortality after bariatric surgery. The reported postoperative incidence of VTE, however, varies widely, from 0.2% to 1.3% at 30 days (3,4) to 0.42% at 90 days (5). The postoperative incidence of fatal PE is also uncertain. An autopsy study of 10 patients who died after Roux-en-Y gastric bypass (RYGP) showed that 3 died of PE (6). Reported risk factors for VTE after bariatric surgery include the type of operation performed (with a greater risk for open vs laparoscopic procedures and for RYGP vs adjustable gastric banding) (3,4), patient age greater than 50 years, postoperative anastomotic leak, history of smoking, and prior VTE (7).
Residents of Olmsted County, Minnesota (2010 census population, 144,248), receive medical care from very few providers; most are seen at Mayo Clinic (with its 2 affiliated hospitals) or another group practice, Olmsted Medical Center (with its affiliated hospital). Rochester, the county seat, is geographically isolated from other urban centers and is home to Mayo Clinic, one of the world’s largest private medical centers. Since 1907, every Mayo Clinic patient has been assigned a unique identifier, and all outpatient and inpatient information is contained within a unit medical record. The Rochester Epidemiology Project (REP), a medical records linkage system that also includes all non-Mayo providers of care to county residents (8), facilitates population-based studies of diseases diagnosed and treated in Olmsted County. As of January 1, 2000, 80% of Olmsted County residents were seen at least once by a REP provider within 1 year and 93% within 3 years (9). Because of complete case ascertainment, this resource has the unique ability to determine the natural history of diseases and avoid referral bias (8).
To estimate the incidence of VTE after bariatric surgery, we analyzed a historical cohort of all Olmsted County residents undergoing bariatric surgery from 1987 through 2005. Our secondary goal was to identify risk factors for VTE after bariatric surgery.
Materials and Methods
This study was approved by the Mayo Clinic and Olmsted Medical Center institutional review boards. Patients under age 18 or those who refused research authorization were excluded from the study.
We performed a population-based, retrospective, cohort study of all Olmsted County residents undergoing bariatric surgery from January 1, 1987, through December 31, 2005, utilizing the resources of the REP and the Mayo Clinic Bariatric Surgery Database. All bariatric surgery in Olmsted County during this period was performed at Mayo Clinic.
We verified Olmsted County residency for individuals in the Bariatric Surgery Database using the REP. To ensure that we had not missed any Olmsted County residents who had undergone bariatric surgery during this period, we also reviewed the operative reports from residents with the following International Classification of Diseases, Ninth Revision (ICD-9) operative codes: 44.31 (high gastric bypass), 44.38 (laparoscopic gastroenterostomy), 44.39 (other gastroenterostomy), 44.5 (revision of gastric anastomosis), 44.69 (other repair of stomach), 45.91 (small-to-small bowel anastomosis), and 46.93 (revision of small-bowel anastomosis, but only if they also had 54.59, other peritoneal adhesiolysis). REP resources were previously used (10–12) to identify all Olmsted County residents who experienced symptomatic, objectively diagnosed, incident VTE from 1966 through 2005. Incident VTE events were distinguished from recurrent events. Determination of VTE was based on explicit criteria after review of complete, provider-linked medical records by trained nurse abstractors. Records were reviewed from date first seen by a REP provider until death or last REP encounter. Variables abstracted included method of diagnosis, location at symptom onset, and event type (DVT, PE, DVT and PE, or chronic thromboembolic pulmonary hypertension).
As previously described (13), DVT was considered objectively diagnosed when acute symptoms and signs were present and confirmed using venography, compression venous duplex ultrasonography, impedance plethysmography, computed tomographic venography, magnetic resonance imaging, pathologic examination of thrombus removed at operation, or autopsy. PE was considered objectively diagnosed when acute symptoms and signs were present and confirmed using pulmonary angiography, ventilation and perfusion lung scan interpreted as high probability for PE, computed tomographic pulmonary angiography, magnetic resonance imaging, pathologic examination of thrombus removed at operation, or autopsy.
Trained nurse abstractors reviewed the Mayo Clinic medical records of the patient cohort. All operative reports were reviewed by a physician (D.A.F., P.R.D., or K.F.M.). None of the patients had more than one bariatric operation in a given year.
We next determined the time from bariatric surgery to incident VTE. We used the Kaplan-Meier product limit estimator with the Greenwood estimate of standard error to calculate the cumulative incidence of VTE events after bariatric surgery. Patients were censored at the date of their second bariatric surgery, death, last follow-up visit, or December 31, 2005, whichever came first. Cox proportional hazards modeling was used to examine the association of demographic variables (patient age, sex, and calendar year of operation) and risk factors (patient weight, body mass index, and type of bariatric surgery) with incident VTE after bariatric surgery, both univariately and for the risk factors, after adjusting for age and sex. The proportional hazards assumption was examined for each variable by assessing the interaction of the variable and time to event on the log scale. We also used the Kaplan-Meier estimator (with the Greenwood standard error) to calculate the cumulative incidence of death after bariatric surgery. Statistical analyses were performed with SAS software (version 9.2, SAS Institute, Inc). P values of .05 or less were considered statistically significant.
Results
We identified 1,817 bariatric operations in the Bariatric Surgery Database; the majority of these patients resided outside of Olmsted County. We used the REP to verify Olmsted County residency for 434 bariatric operations. After reviewing 425 reports with the ICD-9 codes described above, we identified an additional 4 bariatric operations for our cohort. Twenty-six potential research subjects (who had 28 bariatric operations) refused research authorization, which is their right under Minnesota law (Statute 144.335) (9). Eight patients were excluded because of prior VTE. The final cohort comprised 396 Olmsted County residents who had undergone 402 bariatric operations during the 19-year study period.
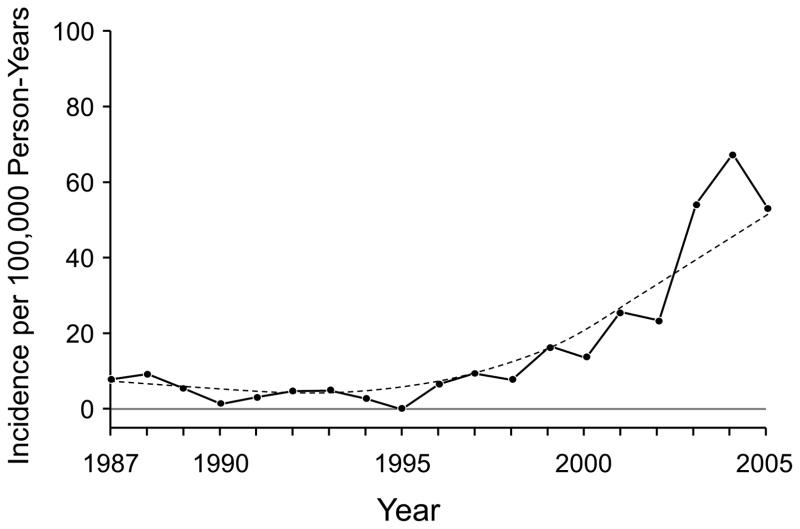
The mean (SD) age at first bariatric surgery was 43.8 (10.2) years (range, 18–76 years); 323 (81.6%) were women. The median weight at first bariatric surgery was 128 kg and the median body mass index was 46.2 kg/m2. Most bariatric operations occurred after 2000 (n=294). The age- and sex-adjusted incidence of bariatric surgery by calendar year is shown in Figure 1.
Figure 1.
Age- and Sex-Adjusted Incidence of Bariatric Surgery per 100,000 Person-Years by Calendar Year. The dashed line shows the smoothed curve.
The most common bariatric operations were open RYGP (n=228 [57%]) and laparoscopic RYGP (n=126 [31%]). Other surgical procedures included laparoscopic biliopancreatic diversion with duodenal switch (n=8), open gastric banding (n=7), revision of bariatric surgery, usually from gastric banding to RYGP (n=30), and other bariatric operations (n=3).
Various strategies of thromboprophylaxis were used postoperatively. External pneumatic compression devices were commonly used (n=320 operations); within this group, subcutaneous unfractionated heparin was administered to 298 patients (93.1%). Pharmacologic thromboprophylaxis included unfractionated heparin (5,000 units, administered subcutaneously twice daily; n=257), unfractionated heparin (5,000 units; administered subcutaneously 3 times daily; n=115); intravenous heparin (n=9); warfarin (n=3), and low-molecular-weight heparin (n=2). Two patients had retrievable inferior vena cava filters placed on the day of bariatric surgery; both were subsequently removed 107 and 120 days after surgery. Thromboprophylaxis with heparin was administered only in the hospital.
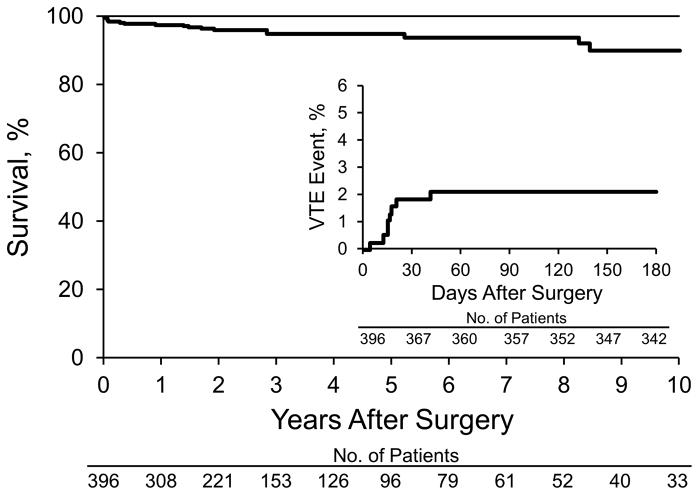
During the study period, a total of 2,416 incident VTE events were documented in Olmsted County residents. Of these, 9 incident VTE events occurred after first bariatric surgery––8 within 6 months after surgery, 7 within 1 month after surgery, and 5 after hospital discharge but within 1 month after bariatric surgery. One fatal pulmonary embolism was diagnosed at autopsy, 20 days after surgery. The median duration of postoperative hospitalization in these 9 patients was 6 days. Characteristics of the 9 patients with VTE events after bariatric surgery are shown in the Table. The cumulative incidence of VTE after bariatric surgery was 0.3% (95% CI, 0%-0.8%) at 7 days; 1.9% (95% CI, 0.5%-3.2%) at 30 days; 2.1% (95% CI, 0.7%-3.5%) at 3 months; and 2.1% (95% CI, 0.7%-3.6%) at 6 months. The cumulative incidence of VTE events after bariatric surgery is shown in Figure 2 (inset).
Figure 2.
Survival Curve After Bariatric Surgery. Inset, Cumulative incidence of venous thromboembolic (VTE) events.
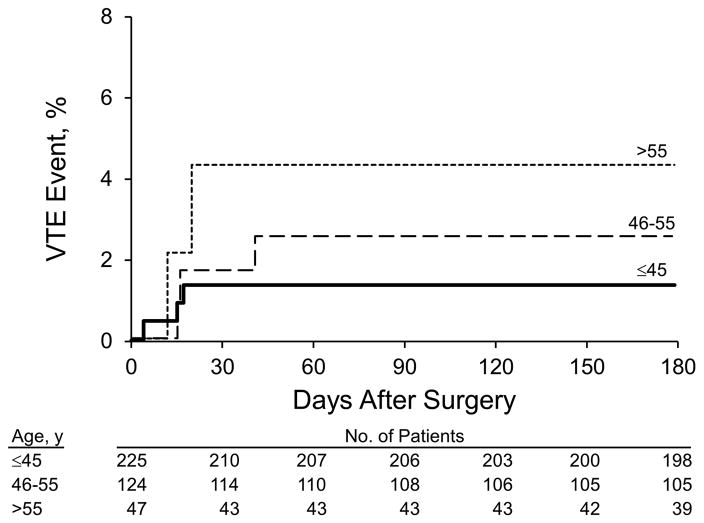
Patient age was associated with incident VTE after first bariatric surgery (hazard ratio, 1.89 per 10-year increase in age; 95% CI, 1.01–3.55; P=.05). Patients were stratified by age (45 years or younger, 46 to 55 years, and 56 years and older), and the Kaplan-Meier estimate for VTE events was calculated (log-rank test, P=.11) (Figure 3). Calendar year and sex were not associated with VTE (P=.32 and P=.33, respectively). After adjusting for age and sex, body mass index at surgery was not associated with VTE risk (P=.54). After adjusting for age and sex, a 10-pound increase in weight had a 7% increase in the hazard ratio of incident VTE events, but this was not statistically significant (P=.14). After adjusting for age and sex, open surgery (RYGP and gastric banding) was not associated with incident VTE (hazard ratio, 1.22; P=.79). Among the subset with either open RYGP or laparoscopic RYGP surgery, open RYGP surgery was not significantly associated with an increased risk of VTE compared with laparoscopic RYGP surgery (hazard ratio, 2.27; 95% CI, 0.26–19.6; P=.44).
Figure 3.
Venous Thromboembolic (VTE) Events After Bariatric Surgery, Stratified by Age.
The cumulative mortality rate after first bariatric surgery was 0.3% (95% CI, 0%-0.8%) at 7 days; 1.3% (95% CI, 0.2%-2.5%) at 30 days; 1.3% (95% CI, 0.2%-2.5%) at 3 months; and 1.9% (95% CI, 0.5%-3.2%) at 6 months. Postoperative survival (1–mortality) is shown in Figure 2.
Discussion
In this retrospective, population-based, cohort study of 396 Olmsted County residents undergoing 402 bariatric operations from 1987 through 2005, the most common operations were open RYGP (n=228 [56.7%]) and laparoscopic RYGP (n=126 [31.3%]). Most patients received treatment with both external pneumatic compression devices and subcutaneous unfractionated heparin perioperatively and up until discharge for VTE prophylaxis. The incidence of VTE at 3 months after bariatric surgery was 2.1%, and 5 of 9 (56%) postoperative VTE occurred after hospital discharge and within 1 month postoperatively. Increasing patient age was a significant risk factor for postoperative VTE, with about a 2-fold increase in risk per 10-year increase in age. The cumulative mortality rate at 3 months after surgery was 1.3%.
Perhaps the most interesting finding in our study was that most VTE occurred after hospital discharge. In a study of a large California database of 76 surgical procedures, 56% of all VTE events that occurred within 3 months after elective or emergent surgery occurred after hospital discharge (14). In the bariatric study by Winegar et al (5), 73% of VTE events within 3 months after bariatric surgery occurred after discharge, most within 30 days postoperatively. Although extended prophylaxis after hospital discharge is practiced by some groups, no randomized controlled trials of this practice have been published for patients undergoing bariatric surgery.
Despite combined prophylaxis with unfractionated heparin and external pneumatic compression in most of our patients, 2.1% of our cohort had VTE develop within 3 months after bariatric surgery, suggesting that bariatric surgery is a procedure with a high risk of VTE, similar to total hip or knee arthroplasty. For comparison, a large study from 1998 reported a 3-month cumulative incidence of VTE of 2.8% after total hip arthroplasty and 2.1% after total knee arthroplasty (15). Winegar et al (5) reported a 3-month cumulative incidence of VTE of 0.55% after RYGP; however, the majority of operations (92%) were performed laparoscopically, and the risk of VTE events was 4.5 times greater with an open vs a laparoscopic procedure. In contrast, in the current study, most patients had open bariatric procedures.
Increasing age was a risk factor for postoperative VTE in our population, with a hazard ratio of 1.89 per 10-year increase in age. Similarly, in a study of 660 patients who underwent RYGP, age greater than 50 was associated with an increased risk for postoperative VTE (P=.04) (7). Older age was also associated with an increased risk of postoperative VTE in the study by Winegar et al (5), in which the most common bariatric operation was a RYGP.
The primary strength of our study is its population-based nature, with complete follow-up of patients in the community. We have identified several major weaknesses of this study. First, the small number of VTE events may have limited our ability to identify risk factors for postoperative VTE. Second, most patients in the cohort underwent open RYGP, which is associated with a greater postoperative VTE complication rate than laparoscopic RYGP (3,4). We note that most RYGP is now done laparoscopically at our institution and others. Third, because of the small number of patients who had a duodenal switch with biliopancreatic diversion or gastric banding, we were unable to comment on the postoperative VTE risk in these patients. Fourth, most of our patients received pharmacologic thromboprophylaxis with twice daily unfractionated heparin (5,000 units administered subcutaneously); current guidelines suggest a higher dose of heparin in patients undergoing bariatric surgery (16).
In conclusion, bariatric surgery, particularly open RYGP, has a high risk of VTE for patients with medically complicated obesity. Increasing age is an additional risk factor for postoperative VTE in these patients. Importantly, most VTE events in our patients occurred after hospital discharge. Given the trend of shorter hospital stays for all surgery patients, a randomized controlled trial of extended thromboprophylaxis after hospital discharge is warranted for patients undergoing open RYGP.
Table.
Venous Thromboembolism Events After Bariatric Surgery
| Patient | Age, y | Sex | Type of Surgery | Thromboembolic Event | Days after Surgery |
|---|---|---|---|---|---|
| 1 | 56 | Female | Open RYGP | DVT and PE | 20 |
| 2 | 66 | Male | Open RYGP | PE | 2,044 |
| 3 | 52 | Female | Open RYGP | DVT | 16 |
| 4 | 57 | Female | Open RYGP | DVT | 12 |
| 5 | 53 | Male | Open RYGP | PE | 41 |
| 6 | 41 | Male | Open revision | PE | 4 |
| 7 | 41 | Female | Open revision | PE | 15 |
| 8 | 48 | Female | Open RYGP | DVT and PE | 15 |
| 9 | 44 | Female | Laparoscopic RYGP | DVT | 17 |
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; RYGP, Roux-en-Y gastric bypass.
Acknowledgments
Research reported in this publication was supported by grants from the National Heart, Lung and Blood Institute under Award Number HL66216 to Dr. Heit, and the National Institute on Aging under Award Number R01AG034676 of the National Institutes of Health, and by the Mayo Foundation. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Abbreviations
- DVT
deep vein thrombosis
- ICD-9
International Classification of Diseases, Ninth Revision
- PE
pulmonary embolism
- REP
Rochester Epidemiology Project
- RYGP
Roux-en-Y gastric bypass
- VTE
venous thromboembolism
Footnotes
Disclosure of Conflict of Interest
Dr Heit declares that he has served on Advisory Boards for which he has received honoraria.
Dr Sarr declares that he is a consultant for EnteroMedics, Inc; this function is not related to this study.
Drs Froehling, Daniels, Mauck, Collazo-Clavell, Ashrani, and Bailey and Ms Petterson declare no conflicts of interest.
References
- 1.Robinson MK. Surgical treatment of obesity: weighing the facts. N Engl J Med. 2009 Jul 30;361(5):520–1. doi: 10.1056/NEJMe0904837. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004 Oct 13;292(14):1724–37. doi: 10.1001/jama.292.14.1724. Erratum in: JAMA 2005 Apr 13, 293, 14, 1728. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS-NSQIP data. Surg Endosc. 2008 Dec;22(12):2554–63. doi: 10.1007/s00464-008-0074-y. Epub 2008 Sep 20. [DOI] [PubMed] [Google Scholar]
- 4.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009 Jul 30;361(5):445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winegar DA, Sherif B, Pate V, DeMaria EJ. Venous thromboembolism after bariatric surgery performed by Bariatric Surgery Center of Excellence Participants: analysis of the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2011 Mar-Apr;7(2):181–8. doi: 10.1016/j.soard.2010.12.008. Epub 2010 Dec 29. [DOI] [PubMed] [Google Scholar]
- 6.Melinek J, Livingston E, Cortina G, Fishbein MC. Autopsy findings following gastric bypass surgery for morbid obesity. Arch Pathol Lab Med. 2002 Sep;126(9):1091–5. doi: 10.5858/2002-126-1091-AFFGBS. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez R, Haines K, Nelson LG, Gallagher SF, Murr MM. Predictive factors of thromboembolic events in patients undergoing Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006 Jan-Feb;2(1):30–5. doi: 10.1016/j.soard.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012 Dec;41(6):1614–24. doi: 10.1093/ije/dys195. Epub 2012 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998 Mar 23;158(6):585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 11.Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost. 2002 Jun;28( Suppl 2):3–13. doi: 10.1055/s-2002-32312. [DOI] [PubMed] [Google Scholar]
- 12.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):370–2. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ., 3rd Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arterioscler Thromb Vasc Biol. 2009 Sep;29(9):1399–405. doi: 10.1161/ATVBAHA.109.189290. Epub 2009 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003 Sep;90(3):446–55. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]
- 15.White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998 Jul 27;158(14):1525–31. doi: 10.1001/archinte.158.14.1525. [DOI] [PubMed] [Google Scholar]
- 16.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]