Abstract
Basophils have emerged in recent years as a small but potent subpopulation of leukocytes capable of bridging innate and adaptive immunity. They can be activated through IgE-dependent and IgE-independent mechanisms to release preformed mediators and to produce Th2 cytokines. In addition to their role in protective immunity to helminths, basophils are major participants in allergic reactions as diverse as anaphylaxis and immediate hypersensitivity reactions, late-phase hypersensitivity reactions, and delayed hypersensitivity reactions. Additionally, basophils have been implicated in the pathophysiology of autoimmune diseases such as lupus nephritis and rheumatoid arthritis, and the modulation of immune responses to bacterial infections, as well as being a feature of myelogenous leukemias. Distinct signals for activation, degranulation, transendothelial migration, and immune regulation are being defined, and demonstrate the important role of basophils in promoting a Th2 microenvironment. These mechanistic insights are driving innovative approaches for diagnostic testing and therapeutic targeting of basophils.
Keywords: Basophil, Allergy, Anaphylaxis, Autoimmunity, Infection, Malignancy
Introduction
Although basophils comprise less than 1 % of peripheral blood leukocytes, emerging insight into basophil biology demonstrates how potent they are for both effector functions and immune regulation. Historically, basophils were primarily associated with immediate hypersensitivity reactions, based on their cell surface expression of the high-affinity IgE receptor (FcƐ RI) and the release of histamine and other atopy mediators upon FcƐ RI crosslinking [1–3]. However, this perception of limited basophil functionality is changing dramatically, with evidence for their expression of numerous cell surface receptors that, when ligated, are capable of transcriptionally activating basophils to produce cytokines that promote and regulate Th2 adaptive immune responses, making them mechanistically important in late-phase hypersensitivity reactions and delayed hypersensitivity reactions, as well as in immediate hypersensitivity reactions [4–28]. There is also evidence that basophils may be important in the pathogenesis of autoimmune diseases [•29–•31], physiologic immune responses to infections [32, 33], and myeloid leukemias [34–38]. Thus, basophils are becoming recognized as a potential target to harness in the therapy of atopy, autoimmunity, and myeloid leukemia [39–42]. Although murine studies have helped elucidate basophil biology, this review will focus on human basophil biology.
Basophil Biology: Origin, Phenotype, and Function
Derived from CD34+ myeloid hematopoietic progenitors in the bone marrow, basophils are phenotypically and functionally distinct from other leukocytes, including mast cells. Although basophils and mast cells do share many characteristics, mast cells reside in tissues whereas basophils reside in the circulation and can be recruited to tissues [11, 17, 43–45]. A key distinction from mast cells is the lack of CD117 (c-kit) expression by basophils and high expression of CD123 (IL-3Rα) by basophils [46]. In vitro derivation of mast cells occurs when CD34+ cells are cultured with stem cell factor (SCF, the ligand for CD117) and IL-6 [47], whereas in vitro derivation of basophils occurs when CD34+ cells are cultured with IL-3 in the absence of SCF [46–•49]. In vivo, basophils enter the circulation with a mature phenotype and survive approximately 5 days [1, •10]. Therefore, the turnover rate is high, with precursor cells constantly being signaled to differentiate into basophils to maintain homeostatic surveillance in the periphery.
Basophils can be morphologically distinguished from other circulating leukocytes by the metachromatic staining of their cytoplasmic granules with Wright Giemsa or Toluidine Blue [7, 50]. Basophils can be phenotypically identified by multi-parameter flow cytometry or immunohistochemistry as FCƐ RI+, CD 123+, and CD303– (to exclude plasmacytoid dendritic cells) [1, 50–52]. Basophils also express a low level of CD203c, which is increased with basophil activation and may correspond to piecemeal degranulation [9]. CD203c is a glycosylated type II transmembrane molecule that belongs to the family of ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP3) enzymes [53]. Basophil activation also induces expression of CD69, while basophil degranulation correlates with expression of CD63 as a consequence of the fusion of the cytoplasmic granule membrane with the cell surface plasma membrane which is termed anaphylactic degranulation [7, 9, 53–56]. CD69 is a member of the C-type animal lectin superfamily that functions as a signal-transmitting receptor, and is expressed on virtually all activated hematopoietic cells [53]. CD63 is a 53-kd tetraspanin family glycoprotein expressed in the membranes of cytoplasmic granules in basophils as well as other granulocytes [16, 55]. Exocytosis leads to fusion of the granule membrane to the cell membrane and hence cell surface CD63 expression where it functions to engage integrins.
Initially, basophils were recognized for their rapid release of histamine and synthesis of leukotriene C4 (LTC4) following crosslinking of IgE bound to their FcƐ RI, and subsequently for their synthesis of IL-4 and IL-13 in response to FcƐ RI crosslinking [13, 55, 56]. Basophils contain approximately 1 pg of histamine/cell, and can synthesize more IL-4 and IL-13/cell than other leukocytes [•10, 13–16]. Thus, basophils have the ability to bridge innate and adaptive immunity, including the capacity to induce and propagate Th2 immune responses [13–28]. The complexity of basophil activation is further evidenced by their responsiveness to IgE-independent mechanisms involving ligation of toll-like receptors (TLR) 2 and 4, IL-3R, IL-5R, IL-18R, IL33R (ST2), C5aR, leukocyte inhibitory receptors (LIR), chemokine receptor (CCR) 2, CCR3, chemoattractant receptor homologous Th2 (CRTH2), granulocyte macrophage colony stimulating factor receptor (GM-CSFR, CD116), CD32 (FcγRII), CD62L, and CD40L [11, 16, 19, 44, 48, 53, 57–65]. Basophil degranulation is largely restricted to signals induced by FcƐ RI crosslinking, or the anaphylatoxin C5a, and to a lesser extent IL-3 [59–62]. Other IgE-independent stimuli promote production of predominantly Th2 cytokines (IL-4, IL-13, and to a lesser extent IL-5), but these stimuli alone do not induce basophil degranulation [9, 32, 48, 59, 66]. A summary of the functional effects of ligands that can stimulate human basophils is shown in Table 1.
Table 1. Functional effects of stimulating human basophils.
| Stimulus | Effects | References | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Degranulation | Leukotriene synthesis | Cytokine sSynthesis | Other | ||
|
|
|
|
|
||
| Fcε RI CL | Histamine | LTC4 | IL-4, IL-13 | PAF | 10, 50 |
| C5a | Histamine | 62, 66, 80 | |||
| IL-3 | Histamine | LTC4 | IL-4, IL-13 | PAF, CD11b, CD18 | 70, 78-80 |
| IL-33 | IL-4, IL-5, IL-6, IL-13 | 64, 71, 75 | |||
| Proteases | IL-4, IL-5, IL-13 | 15, 20, 41 | |||
| Helminths | IL-4, IL-5, IL-13 | 15, 17, 67 | |||
| HIVgp120 | IL-4, IL-13 | 73 | |||
| TLR ligands | IL-4, IL-13 | 63, 65, 98 | |||
| IgD R | IL-1, IL-4 | BAFF, AMP | 28, 74 | ||
| Eotaxins | TEM (CCR3) | 58, 79 | |||
| RANTES | TEM (CCR3) | 79 | |||
| MCP-1 | TEM (CCR2) | 79 | |||
FcεR1 CL Fcε R1 crosslinking; LTC4 leukotriene C4; PAF platelet activation factor; BAFF B cell activating factor;
TLR toll-like receptor; AMP antimicrobial peptide; RANTES: CCL5; CCR chemokine receptor; MCP-1 monocyte chemotactic protein-1; TEM transendothelial migration
In addition to its role in promoting basophil differentiation, IL-3 is a physiologically important enhancer of basophil responsiveness to agonistic factors and of basophil effector functions [11, 67–69]. In particular, basophil degranulation and Th2 cytokine synthesis in response to FcƐ RI crosslinking is enhanced in the presence of IL-3 [70]. The role of IL-3 in basophil biology cannot be overstated. It is essential for many functions throughout the lifecycle of basophils and serves to promote signaling, growth, and mediator release, and often has enhanced effects when combined with other stimuli. For example, IL-3 is synergistic with IL-33 stimulation of basophil synthesis of Th2 cytokines [71]. The only cytokines reported to negatively regulate basophils are the type 1 interferons which limit IL-3-induced cytokine production, but not FcƐ RI crosslinking-induced degranulation [43]. IgG binding to CD32 can also downregulate basophil responsiveness to FcƐ RI signaling, which has led to novel therapeutic approaches using chimeric molecules that can simultaneously crosslink the FcƐ RI and engage CD32 [72]. Additional stimuli reported capable of activating basophil synthesis of Th2 cytokines include: allergens with endogenous protease activity (i.e., Der p 1), HIV gp120, helminthes (Necator americanus), ligands for TLR 2 and 4, and IgD immune complexes (which also induces basophil secretion of antimicrobial peptides and B cell activating factor) [19, 28, 48, 58, 63, 65, 73, 74].
Recent murine studies have suggested the potential for hematopoietic precursors to differentiate into basophils via an IL-3-dependent pathway and an IL-3-independent, thymic stromal lymphopoietin (TSLP)-dependent pathway [11]. The IL-3-derived basophils have conventional responses to FcƐ RI crosslinking, while TSLP-derived basophils are functionally independent of IgE by virtue of not degranulating in response to FcƐ RI crosslinking, but are able to produce Th2 cytokines in response to IL-3 or IL-33. However, such heterogeneity of basophil development and function has not yet been demonstrated for human basophils. Likewise, murine, but not human, basophils have been reported to produce Th2 cytokines in response to IL-18 [64, 75]. Furthermore, there are conflicting reports as to whether human basophils have the potential to serve as antigen-presenting cells, as has been reported for murine basophils [17–20]. Therefore, the murine data, in the absence of confirmatory human data, should be interpreted cautiously, since genomic responses in mouse models are known to poorly mimic human inflammatory diseases. [•76].
The recruitment of basophils in response to injury, assault, or infection is dependent on both activation and chemotactic factors. IL-3 activation increases basophil expression of CD11b and CD18, thereby potentiating adherence to endothelium [77, 78]. Chemotaxis is mediated predominantly by the CCR3 ligands eotaxin (CCL11) and RANTES (CCL5) [58, 79]. In addition to the constitutively expressed CCR3, basophils also express CCR2 and migrate in response to MCP-1 [79]. Further, basophils can produce chemotactic factors in their microenvironment and further modulate the inflammatory response. Basophils can also release platelet activating factor (PAF) in response to stimulation by IL-3 [78, 80], which then stimulates endothelial cells to increase vascular permeability and allows further migration of immune cells. Activation of TLR 2 and 4 can lead to production of B cell activating factor (BAFF) and IL-13 [63, 65], indicating that the microbiota might modulate immunity by interacting with basophils.
Overall, despite being a minor population of leukocytes, basophils have potent and diverse roles in orchestrating immune responses. Greater elucidation of the mechanisms regulating basophil function should provide insight for developing novel strategies for therapeutic modulation of basophils and hence basophil-mediated disorders (2–8, •10, 39–42, 81).
Basophil Activation Test
Delineation of molecules which can identify basophils and determine their activation state with high sensitivity and specificity has led to use of an in vitro assay, termed the basophil activation test (BAT), with which to investigate the potential role of basophils in disease states [55, 82–•87]. The BAT is a microfluoremetry-based assay that can be performed on peripheral blood. Basophils can be gated as CD123+, FcƐ RI+, and CD303– cells. The basophil activation state is determined by basophil expression of CD69, and the degranulation state is determined by expression of CD203c (piecemeal degranulation) and CD63 (anaphylactic degranulation). Figure 1 experimentally illustrates the microfluoremetry analysis of purified resting basophils before and after in vitro activation by FcƐ RI crosslinking with anti-FcƐ RI mAb. Clinically, the BAT analysis of peripheral blood samples is used largely as a surrogate for basophil involvement in an immune reaction, as was recently reviewed [•84]. However, the clinical value of BAT in patient management will require further investigation.
Figure 1.
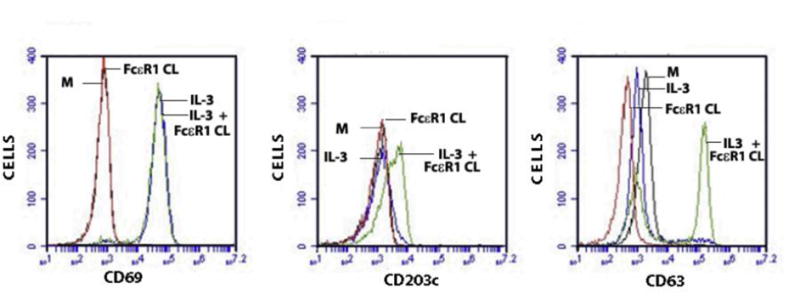
Basophil activation. Human basophils purified from leukopacks from healthy individuals were analyzed by microfluorimetry. The effects of IL-3, alone (blue) or Fcε R1 crosslinking (CL) alone (red), or in combination (green), on the expression of CD69, CD203c, and CD63 are shown as flow cytometry histograms. Unstimulated control basophils in media only (M) are in black
Role of Basophils in Allergic Diseases
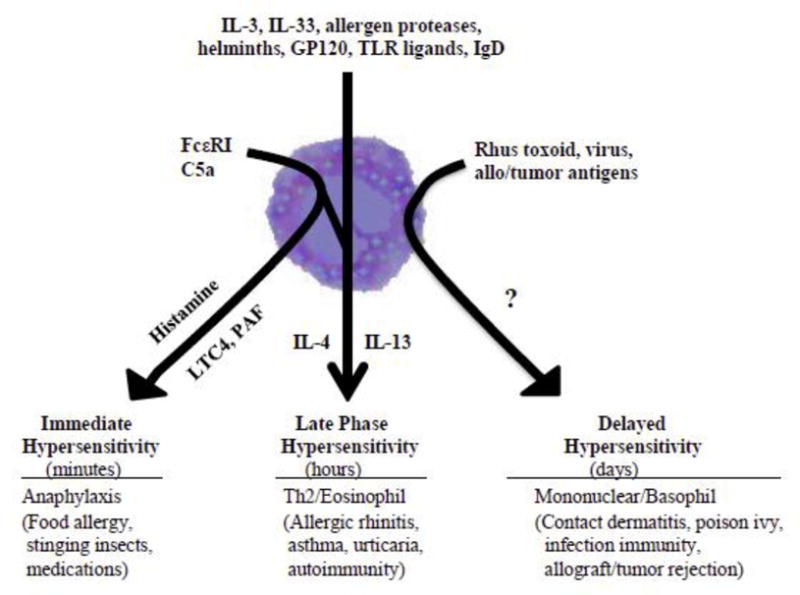
Based on increasing understanding of basophil biology, basophils may prove to be pathophysiologically important in many if not all allergic diseases, including anaphylaxis, allergic rhinitis, asthma, urticaria, and food allergies. The potential for basophils to degranulate for immediate release of histamine, rapidly generate LTC4, and produce Th2 cytokines provides the mechanistic basis whereby basophils can cause immediate hypersensitivity clinical symptoms, as well promote late-phase hypersensitivity reactions, and contribute to delayed hypersensitivity reactions (Fig. 2).
Figure 2. Heterogeneity of human basophil responses in clinical disease.
Immediate Hypersensitivity Reactions
Anaphylaxis is clinically characterized by cardiovascular, cutaneous, respiratory, and gastrointestinal manifestations consequent to basophil and mast cell degranulation, and is usually IgE-dependent [1, 3, 11, 16, 33, •49, 52, 55, 82– •84]. Although specific triggers of anaphylaxis in a given patient may be elusive at the time of presentation, the most common defined causes include allergy to foods, drugs, and insect stings and bites [52, 82–88], as well as non-IgE-mediated inducers of basophil or mast cell degranulation, such as exercise, physical factors, opiates, and the anaphylatoxin C5a that is generated by immune complex activation of the complement cascade [62, 83, 85]. Efforts to determine the relative roles of basophils and mast cells in an anaphylactic episode have included measurements of histamine, tryptase, and prostaglandin D2 (PGD2), as well as BAT [55, 82–•87]. Elevated tryptase and PGD2 levels suggest mast cell involvement and a positive BAT suggests basophil involvement [16, 33]. A recent study evaluating the kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy suggested that acute clinical reactions were basophil-dependent [•87]. Whether autoantibodies to IgE or FcƐ RI are etiologies for systemic anaphylaxsis, in addition to their role in urticaria, is not clear [•89]. Nonetheless, as a circulating pool of leukocytes, basophils are ideally poised to mediate anaphylaxsis in response to blood-borne triggers.
Late-Phase Hypersensitivity Reactions
Late-phase hypersensitivity reactions (LPR) occur approximately 6–12 h after an immediate hypersensitivity reaction, and can be observed in allergic rhinitis and severe asthma, and perhaps occur in the context of some urticarias and atopic dermatitis [4–9, •89–91]. The potential contribution of basophils to LPR is based on their presence in target tissues within hours following experimental allergen challenge [6–8, 11, •87, 88, 91]. In cutaneous allergen challenges, the LPR may be comprised of up to 50 % basophils [7, 8, •89]. In nasal allergen challenges and lung segmental allergen challenges, basophils appear within hours, consistent with a LPR [•89, 92, 93]. Basophils detected in bronchoalveolar lavage fluid in individuals with lung inflammation are correlated with increased IL-4 levels, which is consistent with basophil promotion of a Th2 microenvironment [44]. Additionally, in post mortem assessment, lungs of patients with fatal asthma were found to have significantly more basophils than those who died from other causes [93]. The increase in IL-4 in LPR, and the demonstrated capacity for activated basophils to produce large quantities of IL-4, provide correlative evidence for basophils playing an important role in the pathophysiology of LPR with propagation of Th2 immune responses [1, 4, 72, •89–91, •94, -95].
Delayed Hypersensitivity Reactions
Although delayed hypersensitivity reactions are predominantly characterized by mononuclear leukocytes and peak reaction at the site of allergen challenge at 2–3 days, the predominant granulocyte in the reaction is the basophil [6– 8, 33, 93, 96]. Ultrastructural studies of those basophils suggest piecemeal necrosis, rather than anaphylaxis degranulation. Such characteristic basophils have also been found in skin lesions of poison ivy (rhus toxoid)-sensitive patients, as well as other contact dermatoses, skin allograft, and tumor rejection reactions, and gut mucosa of Crohn disease [6–8, 12, 48, 96]. The likely role of basophils in these delayed hypersensitivity reactions is production of IL-4 and IL-13, with enhancement of a Th2 polarizing microenvironment [6, 7, 97].
Role of Basophils in Other Clinical Conditions
Autoimmunity
In addition to the potential role of basophil activation and degranulation by anti-FcƐ RI autoantibodies in the pathogenesis of autoimmune urticaria, basophils have also been implicated in the pathogenesis of lupus nephritis [•29, 30]. This is based largely on correlations between the presence of elevated serum levels of IgE autoantibodies, especially IgE anti-dsDNA, and activated basophils with severity of lupus nephritis [•29]. The proposed model is that IgE anti-dsDNA/dsDNA immune complexes bind to the basophils FcƐ RI, causing basophil activation with homing to lymphoid organs, where the activated basophils produce IL-4 and promote a Th2 adaptive immune response that enhances autoantibody production. Although this scenario is supported in the lyn-/- murine model of lupus nephritis, evidence in humans remains associative. Nonetheless, strategies to include omalizumab as part of other treatment strategies, such as belimumab (a mAb against BAFF), have been proposed as a means of inhibiting basophil participation in the pathogenesis of lupus nephritis [•29, 30].
Basophils are also being implicated in the pathogenesis of rheumatoid arthritis, wherein citrullinated proteins can activate basophils due to IgE anti-citrullinated protein [•31]. This has led to speculation that basophil activation may contribute to the pathogenesis of autoimmune diseases as diverse as anti-glomerular basement membrane disease, membranous nephropathy, or anti-neutrophil cytoplasmic antibody-associated vasculitis [50, •89]. Should evidence solidify around a role for basophils in the pathogenesis of these disorders, it is likely that consideration of indirect therapeutic modulation of basophil activation, such as omalizumab, will be extended to direct therapeutic targeting of basophils to inhibit their activation and promotion of a pathogenic Th2 microenvironment.
Infections
The physiologic role of basophils in protective immunity to helminths is well established [15, 17, 67]. More recently, basophils have also been implicated in the initiation of Th2 immune responses within lymph nodes [17, 20, 27] and enhancing B cell responses to respiratory bacteria, when the basophils are activated by immune complexes consisting of bacterial antigens and IgD bound to the basophil via a putative IgD receptor [28, 74]. These IgD-activated basophils promoted B cell class switching to IgA and IgD and could prevent the replication of H. influenza and Moraxella catarrahalis. Crosslinking of IgD on basophils stimulated the release of immunoactivating, proinflammatory, and antimicrobial mediators [28, 74]. This proinflammatory relationship between IgD and basophils was further implicated in autoinflammatory syndromes with periodic fevers, which are characterized by increased isotype switch to IgD and increased IgD-armed basophils [28]. Also, as discussed previously, activation of TLR 2 and 4 may play a potential role with gut microbiota interactions and could be enhanced upon exogenous bacterial infection [63, 65, 98].
Malignancy
There is a strong association of basophils with some malignancies, particularly acute and chronic myeloid leukemia (AML, CML) [34–36]. Increased numbers of circulating basophils and dysplastic basophils are common features of AML and the accelerated phase of CML [36], and basophil transformation can rarely occur. It is not uncommon in patients with CML to have 70 % blood basophils [37]. In a cohort study of 1,008 patients with myelodysplastic syndromes, basophilia, defined as basophils greater than 250/ul, was associated with reduced survival [38]. Myeloproliferative disorders are also linked to hypersensitivity to IL-3 signaling, with a link of BCR-ABL growth dependence on IL-3 as shown in a murine model [99]. These associations of basophils with myeloid leukemias and poor outcomes have led to the proposal for a clinical trial with an anti-CD123 mAb in patients with CD123+ acute myeloid leukemia in remission with standard chemotherapy, in an effort to delay or prevent recurrence [•40].
Conclusions
Historically, basophils were known for their role in IgE-mediated effector responses manifest as allergic diseases, and their physiologic role in immune responses to helminths. However, it is now evident that basophils are dynamic and can interact with their local environment by responding to various stimuli mediated by both IgE-dependent and IgE- independent mechanisms, and can participate in a broad spectrum of immune-mediated diseases. The multitasking capability of basophils enables them to serve a unique role in bridging innate and adaptive immune responses. A recent provocative study in mice suggested that basophils may differentiate via either an IL-3-dependent or a TSLP-dependent pathway, leading to distinct functional phenotypes, and that TSLP-dependent basophils may be important in the pathogenesis of eosinophilic esophagitis [11, •49, 100–102]. However, verification of such alternative differentiation pathways in humans has not been confirmed. Further understanding of basophil activation mechanisms will help advance development of diagnostics, such as the BAT, for evaluation and management of patients. Understanding the precise mechanisms by which basophils are activated for diverse effector functions will also be important for the development of targeted therapeutics of diseases linked to basophils. Leading therapeutic candidates targeting basophils include omalizumab and anti-CD123 mAb [•29, •40, •84, •87].
Acknowledgments
This work is supported in part by National Institutes of Health grant AI97372.
Footnotes
Conflict of Interest: Jessica L. Cromheecke, Kathleen T. Nguyen, and David P. Huston declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with animal subjects performed by any of the authors. With regard to the authors' research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
References
* Of Importance
- 1.Chirumbolo S. State-of-the-art review about basophil research in immunology and allergy: is the time right to treat these cells with the respect they deserve? Blood Transfus. 2012;10:148–164. doi: 10.2450/2011.0020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130(2):489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siracusa MC, Artis D. Basophil Functions During Type 2 Inflammation: Initiators, Regulators and Effectors. The Open Allergy Journal. 2010;3:46–51. [Google Scholar]
- 4.Wang H, Fang Y, Barrenas F, et al. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation Clinical & Experimental Allergy. Clinical Et Experimental Allergy. 2010;40:1194–1202. doi: 10.1111/j.1365-2222.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 5.Leung DYM, Boguniewicz M, Howell MD, et al. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvorak HF, Hammond ME, Colvin RB, et al. Systemic Expression of Cutaneous Basophil Hypersensitivity. J Immunol. 1977;118:1549–1557. [PubMed] [Google Scholar]
- 7.Dvorak AM, Mihm MC, Dvorak HF. Degranulation of basophilic leukocytes in allergic contact dermatitis reactions in man. J Immunol. 1976;116:687–695. [PubMed] [Google Scholar]
- 8.Dvorak HF, Mihm MC. Basophilic leukocytes in allergic contact dermatitis. J Exp Med. 1972;135:235–254. doi: 10.1084/jem.135.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabato V, Verweij MM, Bridts CH, et al. CD300a is expressed on human basophils and seems to inhibit IgE/FcƐ RI-dependent anaphylactic degranulation Clinical Cytometry. 2012;82B:132–138. doi: 10.1002/cyto.b.21003. [DOI] [PubMed] [Google Scholar]
- 10*.Sokol CL, Medzhitov R. Emerging functions of basophils in protective and allergic immune responses. Mucosal Immunology. 2010;3(2):129–137. doi: 10.1038/mi.2009.137. This review explained the role of basophils in promoting Th2 immune responses. [DOI] [PubMed] [Google Scholar]
- 11.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes IL-3-independent basophils hematopoiesis and type 2 inflammation. Nature. 2012;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends in Immunology. 2010;31(11):407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs BF, Haas H, Falcone FH, et al. Purified human peripheral blood release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. 1996;26:2493–2498. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- 14.MacGlashan DW, Jr, White JM, Huang SK, et al. Secretion of interleukin-4 from basophils; the relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–3016. [PubMed] [Google Scholar]
- 15.Min B, Prout M, Hu-Li J, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder JT, MacGlashan DW, Jr, Lichtenstein LM. Human basophils: mediator release and cytokine production. Adv Immunol. 2001;77:93–122. doi: 10.1016/s0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183:3033–3039. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 18.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to Th2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 20.Sokol CL, Chu NQ, Yu S, et al. Basophils function as antigen-presenting cells an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khodoun MV, Orekhova T, Potter C, et al. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200:857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Prout M, Ramshaw H, et al. Cutting Edge: Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galli SJ, Franco CB. Basophils are Back! Immunity. 2008;28:495–497. doi: 10.1016/j.immuni.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 25.Min B. Basophils: what they ‘can do’ versus what they ‘actually do’. Nat Immunol. 2008;9:1333–1339. doi: 10.1038/ni.f.217. 2008. [DOI] [PubMed] [Google Scholar]
- 26.Min B. Basophils induce Th2 immunity: is this final answer? Virulence. 2010;1:399–401. doi: 10.4161/viru.1.5.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2007;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Xu W, Wilson M, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell–stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Charles N, Hardwick D, Daugas E, et al. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16(6):701–707. doi: 10.1038/nm.2159. This was the first report potentially linking basophils to the pathogenesis of autoimmune lupus nephritis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warde N. Activated basophils exacerbate lupus nephritis by amplifying production of autoreactive IgE. Nat Rev Rheumatol. 2010;6(8):438. doi: 10.1038/nrrheum.2010.111. [DOI] [PubMed] [Google Scholar]
- 31*.Schuerwegh JM, Ioan-Facsinay A, Dorjee AL, et al. Evidence for a functional role of IgE anticitrullinated protein antibodies in rheumatoid arthritis. PNAS. 2010;107:2586–2591. doi: 10.1073/pnas.0913054107. This article provides the first evidence for the potential role of basophils in rheumatoid arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Anyan WK, Kumagi T, Shimogawara RF, et al. Schistosome eggs have a direct role in the induction of basophils capable of a high level of IL-4 production: Comparative study of single- and bisexual infection of Schistosoma mansoni in vivo. Tropical Medicine and Health. 2010;38(1):13–22. [Google Scholar]
- 33.Pelleau S, Diop S, Dia Badiane M, et al. Enhanced basophil reactivities during severe malaria and their relationship with the plasmodial histamine releasing factor PfTCTP. Infect Immune. 2012;80(8):2963–2970. doi: 10.1128/IAI.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerny-Reiterer S, Ghanim V, Hoermann G, et al. Identification of Basophils as a Major Source of Hepatocyte Growth Factor in Chronic Myeloid Leukemia: A Novel Mechanism of BCR-ABL1– Independent Disease Progression. 2012;14(7):572–584. doi: 10.1593/neo.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuda H, Aritaka N, Ando J, et al. Chronic Myelogenous Leukemia with Mild Basophilia as the Predominant Manifestation at Presentation. Internal Medicine. 2011;50:501–502. doi: 10.2169/internalmedicine.50.4695. [DOI] [PubMed] [Google Scholar]
- 36.Bain BJ, Heller M. Dysplastic basophils in the accelerated phase of chronic myelogenous leukemia. Am J Hematol. 2011;86:949. doi: 10.1002/ajh.22056. [DOI] [PubMed] [Google Scholar]
- 37.Stacchini A, Demurtas A, Godio L. Flow cytometric detection of degranulated basophils in chronic myeloid leukemia in accelerated phase. Clinical Cytometry. 2011;80B:122–124. doi: 10.1002/cyto.b.20566. [DOI] [PubMed] [Google Scholar]
- 38.Wimazal F, Germing U, Kundi M, et al. Evaluation of the prognostic significance of eosinophilia and basophilia in a larger cohort of patients with myelodysplastic syndromes. Cancer. 2010;116:2372–2381. doi: 10.1002/cncr.25036. [DOI] [PubMed] [Google Scholar]
- 39.Rudman SM, Josephs DH, Cambrook H, et al. Harnessing engineered antibodies of the IgE class to combat malignancy : initial assessment of Fc∊RI-mediated basophil activation by a tumour- specific IgE antibody to evaluate the risk of type I hypersensitivity. Clin Exp Allergy. 2011;41:1400–1413. doi: 10.1111/j.1365-2222.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 40*.ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US); 2000. A Study of CSL362 in Patients With CD123+ Acute Myeloid Leukemia Currently in Remission. Internet. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01632852?term=CD123&rank=1 NLM Identifer: NCT01632852 This proposed clinical trial is the first to target CD123, and may establish a future therapy for myeloid leukemia treatment. [Google Scholar]
- 41.Meno KH. Allergen structures and epitopes. Allergy. 2011;66:19–21. doi: 10.1111/j.1398-9995.2011.02625.x. [DOI] [PubMed] [Google Scholar]
- 42.Saini SS, MacGlashan DW., Jr Assessing basophil functional measures during monoclonal anti-IgE therapy. J Immunol Methods. 2012;383:60–64. doi: 10.1016/j.jim.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hida S, Tadachi M, Saito T, et al. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1 / Th2 balance. Blood. 2005;106(6):2011–2017. doi: 10.1182/blood-2005-04-1344. [DOI] [PubMed] [Google Scholar]
- 44.Wakahara K, Baba N, Van VQ, et al. Human basophils interact with memory T cells to augment Th17 responses. Blood. 2012;120:4761–4771. doi: 10.1182/blood-2012-04-424226. [DOI] [PubMed] [Google Scholar]
- 45.Wada T, Ishiwata K, Koseki H, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valent P, Schmidt G, Mayer P, et al. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989;73(7):1763–1769. [PubMed] [Google Scholar]
- 47.Conti P, Kempuraj D, Di Gioaccino M, et al. Interleukin-6 and mast cells. Allergy Asthma Proc. 2002;23(5):331–335. [PubMed] [Google Scholar]
- 48.Schneider E, Thieblemont N, De Moraes ML, Dy M. Basophils: new players in the cytokine network. Eur Cytokine Netw. 2010;21(3):142–153. doi: 10.1684/ecn.2010.0197. [DOI] [PubMed] [Google Scholar]
- 49*.Siracusa MC, Tait Wojno ED, Artis D. Functional heterogeneity in the basophil cell lineage. Adv Immunol. 2012;115:141–159. doi: 10.1016/B978-0-12-394299-9.00005-9. This article provides insight into potential heterogeneity in basophil development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charles N, Dema B, Rivera J. Reply to: Basophils from humans with systemic lupus erythematosus do not express MHC-II. Nat Med. 2012;18:489–490. doi: 10.1038/nm.2663. [DOI] [PubMed] [Google Scholar]
- 52.Ford LS, Bloom KA, Nowak-Wegrzyn AH, et al. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow's milk tolerance. J Allergy Clin Immunol. 2013;131:180–186. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacGlashan D. Jr. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–1377. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crivellato E, Beatrice N, Ribatti D. The history of the controversial relationship between mast cells and basophils. Immunology Letters. 2011;141(1):10–17. doi: 10.1016/j.imlet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Kleine-Tebbe J, Erdmann S, Knol EF, et al. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141:79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- 56.Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301. doi: 10.1016/j.jaci.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Yamada T, Sun Q, Zeibecoglou K, et al. IL-3, IL-5, granulocyte-macrophage colony-stimulating factor receptor alpha-subunit, and common beta-subunit expression by peripheral leukocytes and blood dendritic cells. J Allergy Clin Immunol. 1998;101:677–682. doi: 10.1016/S0091-6749(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 58.Uguccioni M, Mackay C, Ochensberger B, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–1143. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siraganian RP, Hook WA. Mechanism of histamine release by formyl methionine-containing peptides. J Immunol. 1977;119:2078–2083. [PubMed] [Google Scholar]
- 60.Tedeschi A, Salmaso C, Di Donato M, et al. Granulocyte-macrophage colony-stimulating factor and interleukin-3 cause basophil histamine release by a common pathway: downregulation by sodium. Immunology. 1999;96:164–170. doi: 10.1046/j.1365-2567.1999.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bischoff SC, Brunner T, De Weck AL, Dahinden C. Interleukin 5 modifies histamine release and leukotriene generation by human basophils in response to diverse agonists. J Exp Med. 1990;172:1577–82. doi: 10.1084/jem.172.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jürgensen H, Braam U, Kownatzki E, et al. Human C5a induces a substantial histamine release in human basophils but not in tissue mast cells. Int Archs Allergy Appl Immun. 1988;85(4):487–488. doi: 10.1159/000234557. [DOI] [PubMed] [Google Scholar]
- 63.Komiya A, Nagase H, Okugawa S, et al. Expression and Function of Toll-Like Receptors in Human Basophils. Allergy & Immunology. 2006;140(suppl 1):23–27. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 64.Smithgall MD, Comeau MR, Yoon B, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK Cells. Int Immunol. 2008;20(8):1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe T, Yamashita K, Sakurai T, et al. Toll-like receptor activation in basophils contributes to the development of IgG4-related disease. J Gastroenterol. 2012;48(2):247–253. doi: 10.1007/s00535-012-0626-8. [DOI] [PubMed] [Google Scholar]
- 66.Mochizuki A, Mceuen AR, Buckley MG, et al. The release of basogranulin in response to IgE-dependent and IgE-independent stimuli: Validity of basogranulin measurement as an indicator of basophil activation. J Allergy Clin Immunol. 1995;12(1):102–108. doi: 10.1067/mai.2003.1511. [DOI] [PubMed] [Google Scholar]
- 67.Lantz CS, Min B, Tsai M, et al. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Laboratory Investigation. 2008;88:1134–1142. doi: 10.1038/labinvest.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takao K, Tanimoto Y, Fujii M, et al. In vitro expansion of human basophils by interleukin-3 from granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Clin Exp Allergy. 2003;33:1561–1567. doi: 10.1046/j.1365-2222.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- 69.Iikura M, Yamaguchi M, Fujisawa T, et al. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol. 1998;161:1510–1515. [PubMed] [Google Scholar]
- 70.Verweij MM, Sabato V, Nullens S, et al. STAT5 in Human Basophils: IL-3 Is Required for Its FcƐ RI-Mediated Phosphorylation. Clinical Cytometry. 2012;82B:101–106. doi: 10.1002/cyto.b.20629. [DOI] [PubMed] [Google Scholar]
- 71.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, et al. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cady CT, Powell MS, Harbeck RJ, et al. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcγRIIA and FcγRIIB. Immunology Letters. 2010;130:57–65. doi: 10.1016/j.imlet.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patella V, Florio G, Petraroli, Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J Immunol. 2000;164:589–595. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 74.Chen K, Cerutti A. The function and regulation of immunoglobulin D. Current Opinion in Immunology. 2011;23(3):345–352. doi: 10.1016/j.coi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leuko Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76*.Seok J, Shaw Warren H, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. PNAS. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. Evidence was presented that the current mouse model for inflammatory diseases may not be appropriate for translation to understanding human disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzukawa M, Hirai K, Iikura M, et al. IgE- and Fc∊RI-mediated migration of human basophils. Int Immunol. 2005;17:1249–1255. doi: 10.1093/intimm/dxh301. [DOI] [PubMed] [Google Scholar]
- 78.Bochner BS, McKelvey AA, Sterbinsky SA, et al. IL-3 augments adhesiveness for endothelium and CD11b expression in human basophils but not neutrophils. J Immunol. 1990;145(6):1832–1837. [PubMed] [Google Scholar]
- 79.Iikura M, Ebisawa M, Yamaguchi M, et al. Transendothelial migration of human basophils. J Immunol. 2004;173:5189–5195. doi: 10.4049/jimmunol.173.8.5189. [DOI] [PubMed] [Google Scholar]
- 80.Lie WJ, Homburg E, Kuijers TW, et al. Regulation and kinetics of platelet-activating factor and leukotriene C4 synthesis by activated human basophils. Clin Exp Allergy. 2003;33:1125–1134. doi: 10.1046/j.1365-2222.2003.01726.x. [DOI] [PubMed] [Google Scholar]
- 81.Tsujimura Y, Obata K, Mukai K, et al. Basophils Play a Pivotal Role in Immunoglobulin-G-Mediated but Not Immunoglobulin-E-Mediated Systemic Anaphylaxis. Immunity. 2008;2:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 82.Steiner M, Harrer A, Lang R, et al. Basophil Activation Test for Investigation of IgE-Mediated Mechanisms in Drug Hypersensitivity. Journal of Visualized Experiments. 2011;55:1–6. doi: 10.3791/3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chirumbolo S. Basophil activation test to optimize the diagnosis of adverse effects following immunization to vaccines. Iran J Asthma Immunol. 2013;12:196–202. [PubMed] [Google Scholar]
- 84*.Macglashan DW., Jr Basophil activation testing. J Allergy Immunol. 2013;132(4):777–787. doi: 10.1016/j.jaci.2013.06.038. This review discusses the potential of BAT to assess functional changes in basophils. [DOI] [PubMed] [Google Scholar]
- 85.De Weck AL, Sanz ML, Gamboa PM, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. Int Allergy Immunol. 2008;146:177–189. doi: 10.1159/000115885. [DOI] [PubMed] [Google Scholar]
- 86.Khan FM, Ueno-Yamanouchi A, Serushago B, et al. Basophil activation test compared to skin prick test and fluorescence enzyme immunoassay for aeroallergen-specific Immunoglobulin-E. Allergy Clin Immunol. 2012;8:1–13. doi: 10.1186/1710-1492-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87*.Savage JH, Courneya JP, Sterba PM, et al. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130:1123–1129. doi: 10.1016/j.jaci.2012.05.039. Provided here is the application of omalizumab and its effects in modulating immune response to allergy challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blank S. University Dissertation. Hamburg: 2009. Components and Mechanisms in Diagnosis and Therapy of Hymenoptera Venom Allergy; pp. 1–70. [Google Scholar]
- 89*.Konstantinou GN, Asero R, Ferrer M, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27–36. doi: 10.1111/all.12056. Urticaria as an autoimmune disorder involving basophils is discussed. [DOI] [PubMed] [Google Scholar]
- 90.Gentinetta T, Pecaric-Petkovic T, Wan D, et al. Individual IL-3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol. 2011;128:1227–1234. doi: 10.1016/j.jaci.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 91.Imoto Y, Tokunaga T, Matsumoto Y, et al. Cystatin SN Upregulation in Patients with Seasonal Allergic Rhinitis. PLOS one. 2013;8(8):1–8. doi: 10.1371/journal.pone.0067057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeiger S, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. J Allergy Clin Immunol. 1993;91(3):723–734. doi: 10.1016/0091-6749(93)90191-h. [DOI] [PubMed] [Google Scholar]
- 93.Kepley CL, McFeeley PJ, Oliver JM, Lipscomb MF. Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am J Resp Crit Care Med. 2011;164:1053–1058. doi: 10.1164/ajrccm.164.6.2102025. [DOI] [PubMed] [Google Scholar]
- 94*.Siracusa MC, Kim BS, Spergel JM, Artis D. Basophils and allergic inflammation. J Allergy Clin Immunol. 2013;132:789–801. doi: 10.1016/j.jaci.2013.07.046. This review encapsulates many functions of basophils in allergy but is skewed to mouse biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Botturi K, Langelot M, Lair D, et al. Preventing asthma exacerbations: What are the targets? Pharmacology & Therapeutics. 2011;131(1):114–29. doi: 10.1016/j.pharmthera.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 96.Shelley WB, Resnik SS. Basophil Degranulation Induced By Oral Poison Ivy Antigen. Archives of Dermatology. 1965;92:147–150. doi: 10.1001/archderm.92.2.147. [DOI] [PubMed] [Google Scholar]
- 97.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011;2(3):1–25. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabroe I, Jones EC, Usher LR, et al. Toll-Like Receptor (TLR)2 and TLR4 in Human Peripheral Blood Granulocytes: A Critical Role for Monocytes in Leukocyte Lipopolysaccharide Responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 99.Wong S, McLaughlin J, Cheng D, et al. IL-3 receptor signaling is dispensable for BCR-ABL-induced myeloproliferative disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(20):11630–11635. doi: 10.1073/pnas.2035020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noti M, Tait Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roan F, Bell BD, Stoklasek TA, et al. The multiple facets of thymic thromal lymphopoietin (TSLP) during allergic inflammation and beyond. 2012;91(6):1–10. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bogiatzi SI, Guillot-Delost M, Cappuccio A, et al. Multiple-checkpoint inhibition of thymic stromal lymphopoietin – induced TH2 response by TH17-related cytokines. J Allergy Clin Immunol. 2012;1:233–240. doi: 10.1016/j.jaci.2012.04.038. [DOI] [PubMed] [Google Scholar]


