Abstract
Study Design:
Experimental design
Background:
Normal breathing mechanics play a key role in posture and spinal stabilization. Breathing Pattern Disorders (BPD) have been shown to contribute to pain and motor control deficits, which can result in dysfunctional movement patterns. The Functional Movement Screen™ (FMS™) has been shown to accurately predict injury in individuals who demonstrate poor movement patterns. The role BPD play on functional movement is not well established. Furthermore, there is currently no single test to clinically diagnose BPD. A variety of methods are used, but correlations between them are poor.
Purpose:
To examine the relationship between BPD and functional movement and identify correlations between different measures of BPD.
Methods:
Breathing was assessed in 34 healthy individuals using a multi‐dimensional approach that included biomechanical, biochemical, breathing related symptoms, and breathing functionality measures. Movement was assessed using the FMS™. Analysis, involving independent t‐tests and Pearson correlation were performed to identify associations between measures.
Results:
Individuals who exhibited biochemical and biomechanical signs of BPD were significantly more likely to score poorly on the FMS™. These studied measures of BPD correlated highly with each other.
Conclusion:
These results demonstrate the importance of diaphragmatic breathing on functional movement. Inefficient breathing could result in muscular imbalance, motor control alterations, and physiological adaptations that are capable of modifying movement. These findings provide evidence for improved breathing evaluations by clinicians.
Level of Evidence:
2B
Keywords: Capnography, diaphragm, FMS™, motor control
Introduction
Functional movement is defined as the ability to produce and maintain an adequate balance of mobility and stability along the kinetic chain while integrating fundamental movement patterns with accuracy and efficiency.1 Postural control deficits, poor balance, altered proprioception, and inefficient motor control have been shown to contribute to pain, disability, and interfere with normal movement.2,3 Identification of risk factors that lead to these problems and contribute to dysfunctional movement patterns could aid injury prevention and performance.
Normal breathing, also known as diaphragmatic breathing, involves synchronized motion of the upper rib cage, lower rib cage, and abdomen.4 Additionally, normal breathing requires adequate use and functionality of the diaphragm muscles.5 Abnormal breathing, known as thoracic breathing, involves breathing from the upper chest, evidenced by greater upper rib cage motion, compared to the lower rib cage.6 Breathing pattern disorders (BPD), defined as inappropriate breathing that is persistent enough to cause symptoms with no apparent organic cause,7 are present in a variety of individuals with musculoskeletal dysfunction.8,9,10,11,12 BPD could be a risk factor for the development of the dysfunction, a result of the dysfunction itself, and an important, clinically measurable attribute to consider in those with musculoskeletal pain.
Individuals with poor posture,13 scapular dyskinesis,14 low back pain,15,11,12 neck pain9,10 and temporomandibular joint pain16 exhibit signs of faulty breathing mechanics. Thoracic breathing is produced by the accessory muscles of respiration (including sternocleidomastoid, upper trapezius, and scalene muscles), dominating over lower rib cage and abdominal motion.6 Over‐activity of these accessory muscles have been linked to neck pain,9 scapular dyskinesis,14 and trigger point formation.17 Vickery7 suggested that decreased abdominal motion, relative to upper thoracic motion, confirms poor diaphragm action. The diaphragm is the key driver of the respiratory pump with attachments onto the lower six ribs, xiphoid process of the sternum, and the lumbar vertebral column (L1‐3).18 Hodges et al.19 stated that since the diaphragm performs both postural and breathing functions, disruption in one function could negatively affect the other.
Roussel et al.11 found a positive correlation between BPD and poor performance of motor control tasks, while O’Sullivan and colleagues20 identified altered motor control strategies and respiratory dysfunction in subjects with sacroiliac joint pain. Hodges et al21 showed diaphragmatic activity can assist with mechanical stabilization of the trunk along with concurrent maintenance of ventilation by increasing intra‐abdominal pressure leading to a stiffening of the lumbar spine.21,22 Poor coordination of the diaphragm may result in compromised stability of the lumbar spine, altered motor control and dysfunctional movement patterns.23
It is proposed that since BPD can affect lumbar, scapula, and cervical motor control, it may have a detrimental effect on functional movement. The Functional Movement Screen™ (FMS™) is a screening tool for assessing the body’s kinetic chain, where the body is evaluated as a linked system of interdependent segments.24,25,26,27,18 The inter‐rater reliability of the FMS™ was recently reported, with weighted κ values ranging from 0.45 to 1.00.18,26 A positive relationship between an individual’s functional movement characteristics, as measured by the FMS™, and injury risk has also been shown.28,29,30 A score of 14 or less (≤14) has been shown to result in an increased risk of injury in an active population.18,28,29,31,32,33
BPD have been characterized as multidimensional, involving biomechanical, biochemical, breathing related symptoms, and breathing function which may or may not co‐exist.34 There is currently no single test utilized to clinically diagnose BPD. Courtney et al.34 investigated a variety of BPD assessment measures and found that correlations between measures were generally not significant. As such, measurements of BPD should include measures that evaluate all dimensions.
Biomechanical diagnosis of a BPD is usually made by clinical observation comparing a patient’s breathing pattern with normal respiratory mechanics.6 The Hi Lo breathing assessment, involving observation of the chest and abdomen motion at rest, in a seated position, is reasonably accurate for determining different types of simulated breathing patterns.35
Thoracic breathing can have an acute effect on respiratory chemistry, specifically a decrease in the level of carbon dioxide () in the bloodstream.36 This causes the pH of the blood to increase, and a state of respiratory alkalosis results.37 Respiratory alkalosis can trigger changes in physiological, psychological, and neuronal states within the body that may negatively affect health, performance, and the musculoskeletal system.14,38 A reliable, time‐sensitive method that is used to assess this biochemical aspect of respiratory function is capnography.37,39 Capnography measures average partial pressure at the end of exhalation, known as end tidal (et) and has good concurrent validity when compared to arterial measures.40,41 Levitsky42 claimed normal ranges were between 35‐40 mmHg, while values of <35mmHg were suggestive of a BPD.
The Nijmegen Questionnaire (NQ) is currently the most popular symptom questionnaire used to identify BPD.34 This questionnaire includes 4 questions on respiratory symptoms and 12 items on peripheral and central neurovascular or general tension related symptoms.43 It has been used in research studies as a supportive diagnostic tool for identifying anxiety related breathing pattern disorders.44 A score of ≥23 suggests dysfunctional breathing.45
Warburton and Jack46 suggested that breath‐holding ability is an aspect of breathing functionality that is commonly disturbed in individuals with dysfunctional breathing. Times of <20 seconds are proposed to indicate the presence of BPD and to correlate with resting levels.47
To date, the role that BPD play on movement patterns is not well established in the literature. Therefore, the purpose of this experimental study was to investigate the relationship between breathing patterns and functional movement. Due to the lack of consensus on the gold standard measurement for BPD, a range of clinical measures to identify BPD were used and correlations between the different measures were examined. Based on previous literature and current thought2,8 it was hypothesized that individuals with abnormal results in any of the breathing assessments would score poorly on the FMS™ (≤14).
Methods
Experimental Design
Participants took part in an experimental study involving a single 20‐minute testing session, at a Physical Therapy outpatient clinic. Data collection included five different methods of assessment for BPD and the FMS™ as a measure of movement patterns. The aim of the study was to determine the relationship between BPD, as indicated by et measures of <35mmHg,38,42 NQ scores of ≥23,43 respiratory rate (RR) of >16 breaths per minute (bpm) at rest,6 observed faulty breathing patterns via the Hi Lo assessment35 and breath‐hold time (BHT) of <20 seconds,47 and dysfunctional movement, indicated by a low (≤14) FMS™ score.28,31,32 The ordinal scoring criteria, originally described by Cook,2 were used for this study. Additionally, because there are a number of combinations of scores in the FMS™ that can sum to a composite score of ≤14, any participant who scored a 0 in the FMS™, indicating they had pain, or a 1, indicating dysfunction, in any test movement, were classified into a “Fail” group. Individuals who scored only 2 or 3’s were classified into a “Pass” group.
Participants
A convenience sample of 34 healthy men (n = 14; age 32.3 ± 8.0 years; height 180.5 ± 7.3 cm; weight 81.2 ± 11.6 kg) and women (n = 20; age 30.5 ± 5.8 years; height 165.0 ± 5.6 cm; weight 61.2 ± 8.1 kg) volunteered for the study. Participants qualified for the study if they were at least 18 years of age with no self‐reported pain on the day of testing and were not currently under the care of a medical professional for any musculoskeletal complaint. Subjects were included in the study if they participated in physical activity at least 3 times a week and had lived at the testing altitude for at least 3 months (full acclimatization). Exclusion criteria included an injury causing pain or requiring treatment within the past 6 weeks. Previous injury status, health data, and demographic information were assessed via a health questionnaire. The purpose of the study was described to all potential subjects and each signed an informed consent form prior to testing. The rights of each subject were protected. Ethical approval for the study was granted by the Cardiff Metropolitan University Research Ethics Committee.
Procedures
A capnography machine, CapnoTrainer (Better Physiology, Santa Fe, NM), was used to assess et. The CapnoTrainer automatically calibrated to the testing altitude of 2484 meters above sea level. Individuals were fit with a nasal cannula, which attached to a sample line onto the CapnoTrainer. Subjects were seated and directed to breathe nasally and refrain from speaking during the testing duration. Individuals completed the health questionnaire and NQ while baseline measurements were recorded using the CapnoTrainer software (Version M3.1. May 18, 2012). Completion of the questionnaires at this time was to distract the subjects from thinking about their breathing. After three minutes of monitoring, the average resting et measurements and RR were calculated.
Individuals were asked to remove their nasal cannula and were instructed on the breath‐holding protocol34 that involved breathing normally and then, after an exhale, hold their breath. The timer was started and on the first sign of involuntary muscle motion the timer was stopped. A minute between trials was allowed and a total of three trials were performed. The average BHT was recorded for further analysis.
Following the breath‐hold trials, the Hi Lo method of breathing assessment was used to monitor individual breathing patterns in a standing position.6,10 The examiner stood at the front and slightly to the side of the participant. Placing one hand on their sternum and one hand on their upper abdomen the examiner felt for the more dominant pattern of breathing. Testing lasted for 10 breathing cycles. Normal (diaphragmatic) or abnormal (thoracic or paradoxical) breathing patterns were assessed and documented by the examiner. The inter‐observer reliability of the Hi Lo method is moderate, with weighted κ values ranging from 0.42 to 0.47.11
After the breathing assessments were completed, the participants replaced their nasal cannulas and were instructed to perform the FMS™. The test administration procedures, instructions, and scoring process associated with the standardized version of the test were followed to ensure scoring accuracy.2,24 The examiner was Level 1 certified in the FMS™ system. The participant’s et measurements were recorded during the FMS™ to provide data on respiratory chemistry while motor control was being challenged.
Statistical Methods
The results were divided into two distinct subgroups that included a continuous variable group (including et, RR, NQ and BHT data) and a breathing pattern group (thoracic or diaphragmatic). Normality of continuous variables was examined and confirmed using the Kolmogorov‐Smirnov test. To identify if associations existed among continuous variables and between continuous variables and FMS™ scores, a Pearson correlation was performed. To examine differences between the means of all continuous variables and FMS™ scores with the breathing pattern group, independent t‐tests were performed. A Kruskal‐Wallis Test was used to examine differences it continuous variables between participants exhibiting pain or dysfunction (Fail group) and participants receiving a score of 2 or 3 in all screens (Pass group). Significance level was set at p ≤ 0.05. All statistical analyses were conducted using SPSS V. 20 (Armonk, NY).
Results
Participants
Descriptive statistics for the entire population are provided in Table 1. The percentage of individuals classified as having a BPD varied depending on the different assessment measure used. Resting et and resting RR were the most sensitive measures of BPD with over 70% of subjects having disordered results (70.59% and 74.29% respectively). Between 50 to 60% of participants had abnormal scores in active et, BHT, and breathing pattern type (58.82%, 55.88% and 52.94% respectively), while only 5.88% scored ≥23 in the NQ. Five of the participants experienced pain with one of the clearing tests during the FMS™, but they were all able to complete the full FMS™ evaluation. Final scores for each of these participants were ≤14. The results from these individuals were included for data analysis, as pain is often a consequence of dysfunctional movement patterns48 and deemed pertinent to the study.
Table 1.
Descriptive statistics
| Variables | Mean ± SD | Min | Max |
|---|---|---|---|
| FMS™ Score | 14.71 ± 1.84 | 12.00 | 19.00 |
| Rest et (mmHg) | 33.70 ± 2.74 | 27.70 | 39.33 |
| Active et (mmHg) | 34.28 ± 2.44 | 29.37 | 40.17 |
| Rest RR (breaths/min) | 18.39 ± 3.41 | 12.25 | 25.2 |
| Active RR (breaths/min) | 24.30 ± 3.06 | 17.65 | 30.64 |
| BHT (sec) | 19.22 ± 5.05 | 10.57 | 34.13 |
| NQ | 9.24 ± 6.43 | 0.00 | 27.00 |
FMS™, Functional Movement Screen™; et, end‐tidal carbon dioxide; RR, respiratory rate; BHT, breath‐hold time; NQ, Nijmegen Questionnaire
Correlation between continuous variables of BPD
Table 2 highlights the correlations between continuous variables of BPD. NQ results were found to be negatively correlated to active et (r = –.351, p < 0.05), and positively correlated to active RR (r = .354, p < 0.05), indicating that subjects who scored higher on the NQ, had a lower et during the FMS™ test and a higher RR. Active RR negatively correlated with active et measurements (r = –.339, p < 0.05), indicating individuals with a high RR during the FMS™ had lower et measurements. Additionally positive correlations were revealed between resting and active et values (r = .690, p < 0.001), and resting and active RR values (r = .567, p < 0.001).
Table 2.
Correlation matrix for continuous variable group of BPD.
| Continuous variables | Rest etCO (mmHg) | Active etCO (mmHg) | Rest RR (breaths/min) | Active RR (breaths/min) | BHT (sec) | NQ |
|---|---|---|---|---|---|---|
| Rest et (mmHg) | 1 | .690** .000 |
−.292 .094 |
−.197 .265 |
.025 .889 |
−.110 .535 |
| Active et (mmHg) | 1 | −.300 .085 |
−.339* .050 |
−.233 .185 |
−.351* .042 |
|
| Rest RR (breaths/min) | 1 | .567** .000 |
−.296 .090 |
.022 .904 |
||
| Active RR (breaths/min) |
1 | −.106 .552 |
.354* .040 |
|||
| BHT (sec) | 1 | .324 .061 |
||||
| NQ | 1 |
et, end‐tidal carbon dioxide; RR, respiratory rate; BHT, breath‐hold time; NQ, Nijmegen Questionnaire.
Correlation is significant at the 0.001 level (2‐tailed)
Correlation is significant at the 0.05 level (2‐tailed)
Differences between continuous variable group and breathing pattern group
Resting et measurements were significantly different between diaphragmatic (mean = 35.47 mmHg) and thoracic breathers (mean = 32.14 mmHg) (t = 4.415, df = 32, p < 0.001). This was also the case for active et measurements between diaphragmatic (mean = 36.14 mmHg) and thoracic breathers (mean = 32.62 mmHg) (t = 6.051, df = 32, p < 0.001). No significant differences were identified between breathing patterns and NQ scores, BHT, or RR.
Associations between measures of BPD and FMS™ score
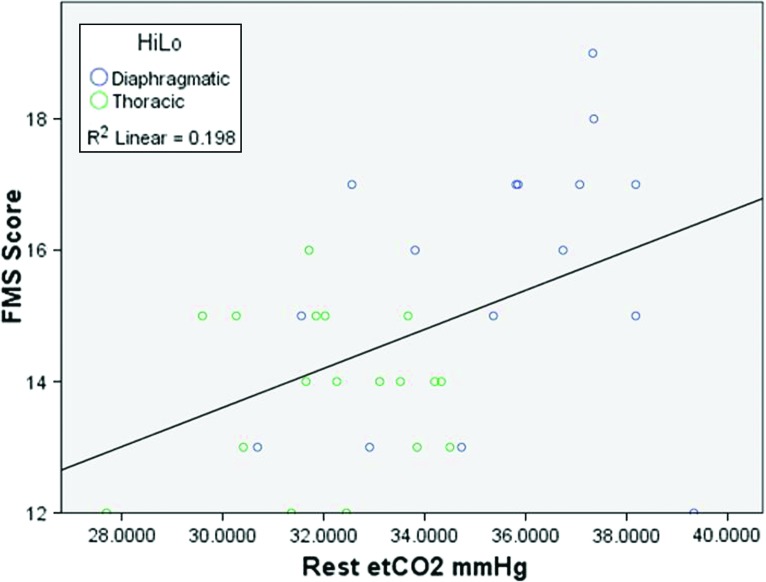
Table 3 shows a breakdown of BPD data for individuals who scored ≤14 on the FMS™ and ≥15. Mean FMS™ scores were significantly higher in diaphragmatic breathers (mean = 15.63) than thoracic breathers (mean = 13.89) (Welch’s t‐test; t = 3.00, df = 23.534, p = 0.006). Therefore, individuals with a less efficient breathing pattern scored worse on the FMS™ compared to those subjects who had normal breathing patterns. Pearson correlation was used to analyze continuous variables and FMS™ scores. Resting et was positively correlated with FMS™ scores (r = 0.445, p = 0.008; Figure 1). There was also a positive correlation between active et and FMS™ scores (r = 0.417, p = 0.014). A negative correlation between active RR and FMS™ score was seen (r = –0.386, p = 0.024), signifying individuals who found the FMS™ test challenging (as indicated by increased RR) scored lower on the FMS™. A significant association was not detected between other measures of BPD and FMS™ scores.
Table 3.
Mean scores ± SD for subsets demonstrating FMS™ scores ≤ 14 and ≥ 15
| Variables | FMS™ Score ≤ 14 n= 16 |
FMS™ Score ≥ 15 n=18 |
|---|---|---|
| Age | 30.6 ± 7.9 | 31.8 ± 5.6 |
| Rest et (mmHg) | 32.9 ± 2.5* | 34.4 ± 2.8* |
| Active et(mmHg) | 33.5 ± 2.5* | 35.0 ± 2.3* |
| Rest RR (breaths/min) | 18.5 ± 2.5 | 18.3 ± 3.6 |
| Active RR (breaths/min) | 25.2 ± 2.7* | 23.5 ± 3.2* |
| BHT (sec) | 19.5 ± 5.8 | 19.0 ± 4.5 |
| NQ | 9.2 ± 7.8 | 9.3 ± 5.2 |
| % Diaphragmatic BP | 25.0% | 66.6% |
| % Thoracic BP | 75.0% | 33.4% |
| FMS™ Score | 13.1 ± 0.8* | 16.1 ± 1.2* |
et, end‐tidal carbon dioxide; RR, respiratory rate; BHT, breath‐hold time; NQ, Nijmegen Questionnaire; BP, Breathing Pattern; FMS™, Functional Movement Screen™
Correlation is significant at the 0.05 level (2‐tailed)
Figure 1.
Associations between measures of BPD and FMS™ Pass and Fail groups
Table 4 shows a breakdown of BPD data for individuals grouped into Pass or Fail on the FMS™. The Kruskal‐Wallis Test revealed a significant difference between the Pass and Fail groups in resting (p = 0.018) and active et values (p = 0.023). There was also a significant difference between active RR (p = 0.006) and FMS™ scores (p = 0.000). No other significant differences were observed between Pass and Fail groups on other measures of BPD.
Table 4.
Mean scores ± SD for subsets demonstrating FMS™ scores classified as Pass or Fail
| Variables | FMS™ Fail (0 or 1’s) n=26 |
FMS™ Pass (2 or 3’s) n=8 |
|---|---|---|
| Age | 31.5 ± 7.2 | 30.4 ± 5.0 |
| Rest et (mmHg) | 33.1 ± 2.6* | 35.7 ± 2.4* |
| Active et(mmHg) | 33.7 ± 2.2* | 36.0 ± 2.3* |
| Rest RR (breaths/min) | 18.9 ± 3.3 | 16.7 ± 3.4 |
| Active RR (breaths/min) | 25.1 ± 2.7* | 21.7 ± 2.9* |
| BHT (sec) | 18.8 ± 5.3 | 20.5 ± 4.1 |
| NQ | 9.8 ± 6.9 | 7.3 ± 4.4 |
| % Diaphragmatic BP | 34.6% | 87.5% |
| % Thoracic BP | 65.4% | 12.5% |
| FMS™ Score | 13.9 ± 1.2** | 17.3 ± 0.9** |
et, end‐tidal carbon dioxide; RR, respiratory rate; BHT, breath‐hold time; NQ, Nijmegen Questionnaire; BP, Breathing Pattern; FMS™, Functional Movement Screen™
Significant at the 0.001 level
Significant at the 0.05 level
Discussion
The purpose of the present study was to investigate whether a relationship exists between breathing pattern and functional movement. It was hypothesized that individuals who would be classified as having a BPD, based on a multi‐dimensional assessment approach, would exhibit dysfunctional movement patterns, corresponding to a low FMS™ score. The results from this study show that a relationship exists between elements of BPD and functional movement. Both biomechanical and biochemical measures of BPD had a significant association with FMS™ scores. A secondary aim of this investigation was to evaluate clinically used measures of BPD in order to identify whether correlations existed between them. It appears there is a correlation between breathing pattern type and et, and between NQ, active RR, and et.
Breathing Pattern Disorders and Functional Movement
The FMS™ was used to assess movement patterns. The mean score for the entire group was 14.7 which is similar to normative values found in active individuals.49,50 In this study, FMS™ scores were significantly higher among diaphragmatic breathers than thoracic breathers (p = 0.006). Seventy‐five percent of individuals who scored ≤14 on the FMS™ were classified as thoracic breathers while 66.6% of individuals who scored ≥15 on the FMS™ were classified as diaphragmatic breathers. Furthermore, 87.5% of individuals who were in the Pass group on the FMS™ were classified as diaphragmatic breathers. These results demonstrate the importance of diaphragmatic breathing on functional movement.
Cook2 proposed the musculoskeletal system would migrate toward predictable patterns of movement in response to pain or in the presence of muscle imbalance. The prevalence of altered movement patterns in individuals with disordered breathing patterns have also been explained by Hruska.16 Hruska proposed the existence of a thoracic dominant breathing pattern resulted in hypertonicity of the accessory muscles of breathing, which in turn prevents the diaphragm from returning to an optimal resting position. As the diaphragm plays a key role in pressure generation, the change in the length‐tension relationship of the diaphragm results in altered pressure generation. Lee et al51 suggest that this process challenges deep motor patterns that control trunk stability, resulting in a negative effect on body mechanics and motor control patterns. As normal movement is achieved through a balance of mobility and stability, changes in trunk stability will result in sub‐optimal movement and could lead to dysfunction.11
This is supported by O’Sullivan et al20 who found that individuals who presented with sacroiliac joint pain exhibited altered motor control strategies and alterations of respiratory function while performing a low‐load task. Specifically, a decrease in diaphragmatic excursion was observed. The length and curvature of the diaphragm and the size of the zone of apposition influence its power and efficiency, thus a decrease in diaphragmatic excursion has negative consequences to postural stability and optimal respiration.52 This is in accordance with Roussel et al11, who showed that individuals with low back dysfunction exhibited altered breathing patterns during movements where the core stability muscles were challenged.
Okada et al53 found no significant correlation existed between core stability and FMS™, despite the emphasis fitness professionals have placed on functional movement and core stability. However, a study by Shinkle et al54 established that core strength does have a significant effect on an athlete’s ability to create and transfer forces to the extremities. Neither study evaluated breath control during core testing, which questions whether the individuals used altered breathing patterns in order to establish core stability. Cowen55 showed that a 6‐week yoga program which focused on breathing techniques and breath control could significantly improve FMS™ scores in firefighters. Correct breathing has therefore been proposed as a possible foundation for the correction of dysfunctional movement patterns.13
Capnography measurements to assess biochemical aspects of respiratory function were positively correlated with FMS™ score, both at rest and during the FMS™. A higher et level, indicating efficient respiratory function, was positively correlated with a higher FMS™ score (≥15 on FMS™ and being placed in the Pass group on FMS™. These findings indicate that people who are classified as having a BPD based on capnography measures are more likely to present with dysfunctional movement patterns. Low et leads to respiratory alkalosis, which decreases blood pH.42 Several authors have evaluated the physiological adaptations that occur from a state of respiratory alkalosis5,37,51 and concluded these adaptations are sufficient to cause alterations in motor control and movement. Lee et al51 showed respiratory alkalosis led to smooth muscle constriction, altered electrolyte balance, and decreased tissue oxygenation. Simons et al17 stated that skeletal muscles affected this way became prone to fatigue, dysfunction, and trigger point. The presence of latent trigger points has been shown to alter activation sequences in the entire kinetic chain.56 Chaitow8 proposed that the changes that occur from respiratory alkalosis are capable of modifying normal motor control of skeletal musculature and altering resting muscle tone. These findings would in part explain the results from the current study.
Kepreli et al9 showed that individuals who demonstrate thoracic breathing patterns also have neck pain and a forward head posture.9,57 McLaughlin et al57 demonstrated that individuals who suffered with neck pain presented with poor respiratory chemistry. Interestingly, following an intervention program to address breathing dysfunctions and manual therapy to address thoracic cage mobility, respiratory chemistry improved, pain scores decreased, and functional improvements were observed. A forward head posture has been shown to affect shoulder biomechanics16 and postural balance.58 These factors may also have a negative effect on performance of the FMS™, but further research is needed to draw definite conclusions.
A negative correlation existed between RR during the FMS™ and FMS™ score. This indicates individuals who scored poorly on the FMS™ also had a significantly higher RR during testing. This is further highlighted when considering the significant difference seen between the pass/fail group and RR during the FMS™. This is in accordance with O’Sullivan et al20 who found that individuals who suffered with SIJ pain displayed an increased RR and a decrease in diaphragm excursion during an active straight leg raise task. This task involved lifting the leg 20 cm above the table starting from a supine position and holding for 10 seconds. As the current study investigated individuals who were healthy and pain‐free, comparisons with studies that examined individuals in pain are difficult. However, it is possible that the compensations and dysfunctions seen in this group could eventually lead to pain and injury, since individuals who score poorly on the FMS™ may be at increased risk of injury.28,29
No other measures of BPD were significantly associated with FMS™ outcomes. This could be related to lack of sensitivity of the BPD measures for the chosen population group. The sample group utilized in this study was comprised of healthy individuals. The NQ and BHT have been utilized successfully in studies that examine individuals with suspected BPD and hyperventilation syndrome.43,45,46
Measures of Breathing Pattern Disorders
Breathing pattern and end‐tidal carbon dioxide
Courtney et al34 proposed comprehensive evaluation of breathing dysfunction should include measures of breathing symptoms, breathing patterns, resting et, and functional measures such as BHT. These measures were evaluated in the present study and significant relationships were seen between breathing pattern and et. Individuals who demonstrated lower et values were more likely to demonstrate a thoracic breathing pattern. These results differ from Courtney et al34 who used individuals with concerns about their breathing and found no significant correlation between separate measures of breathing. These differences may be attributed to several factors. Healthy individuals, with no concerns about their breathing were used in the present study and emphasis was placed on FMS™ performance rather than breathing measures. Studies have shown that capnography measures can be improved with breathing re‐education,36,57,59 and it is suggested the individuals with concerns about their breathing may have altered their breathing pattern to one that is more efficient. A study by Perri and Halford10 also examined breathing patterns in healthy individuals and respiration was not mentioned to the group. They found 56.4% of the group exhibited faulty breathing patterns, which is similar to the 53% seen in this study. Individuals who are not concerned or focused on their breathing may not alter their breathing when examined, resulting in higher incidences of observed BPD.
Altitude could be another factor as to why correlations were seen in the current study and not seen by Courtney et al.34 The current study was performed at 2484 meters above sea level. Even though the participants were fully acclimated to the altitude, results showed an increased RR at rest (mean 18.34 bpm) compared to normal rates (10‐14 bpm).6 On arrival to altitude, RR increases to compensate for a decrease in the partial pressure of oxygen. This results in a decrease in the partial pressure of in the blood and respiratory alkalosis. At altitudes up to 6000 meters, the kidneys correct the alkalinity of the blood after a few days by removing bicarbonate ions.60 The results of the current study show the mean et level at rest was 33.7 mmHg, which is less than the value of 38.0 mmHg reported by Courtney et al.34 Even after acclimatization to altitude, RR could remain high because an habitual pattern has developed,8 which is reflected in a lower et measure.
Nijmegen questionnaire, respiratory rate and end‐tidal carbon dioxide
A significant correlation between breathing symptoms (NQ), RR and et during the FMS™ were observed in this study. However, it is felt these findings need to be interpreted with caution because values for the NQ are within the normal range. The overall mean score for the entire population was 9.24, with a sum score of 10 reported as normal in European studies.45 Only 5.88% of this sample group scored 23 or more and would be classified as having a BPD from their NQ score. This finding is supported by the work of Thomas and colleagues44 that used the NQ to identify the number of individuals in a community who may suffer from dysfunctional breathing symptoms. They reported 9.5% of adults had symptoms suggestive of dysfunctional breathing according to their NQ score. Considering the high results in other measurements of BPD, the NQ may not be the most accurate method in diagnosing this condition in healthy individuals.
Breath‐hold time
BHT did not correlate with any measures of BPD, even though over 55% of the sample tested demonstrated scores of less than 20 seconds (mean score 19.22 seconds). Stark and Stark47 proposed scores of below 20 seconds indicated the presence of BPD and correlated with resting et levels. This was not the case in the current study. The low BHT may be due to the altitude at which testing was performed. Studies have shown that BHT is inversely related to altitude, which would explain the low BHT scores. Average BHT at sea‐level was found to be approximately 70 seconds.61
Future research
The current study established that a significant relationship existed between BPD and functional movement. However, the outcome of correcting either one of these factors is unknown. Future research should evaluate the role of breathing pattern retraining on movement, injury prevention, and musculoskeletal pain syndrome. If correction of breathing dysfunction can improve these factors, it will provide clinicians valuable evidence for incorporating breathing retraining into their clinical interventions. Ongoing research is also needed to identify the most reliable and valid measure of BPD in athletic and healthy population groups. This study has shown that healthy individuals can exhibit symptoms of BPD.
Limitations
Firstly, the reliability of the Hi Lo breathing assessment was not examined in the current study. Although, fair inter‐observer reliability has been established previously,11 the intra‐observer reliability of this evaluation method remains to be examined. Secondly, all subjects were examined by the same observer, potentially leading to bias amongst data scoring. Future studies should have separate examiners for each breathing assessment method and for the FMS™ in order to reduce experimenter bias. Lastly, the altitude at which testing was performed could also have affected the outcome of the capnography measures.
Conclusion
The present study showed that both biochemical and biomechanical measures of BPD are significantly associated with scores on a screen of functional movement. Individuals who exhibited signs of BPD were likely to demonstrate greater movement dysfunction as represented by lower scores on the FMS™. The reason for these results are likely multifactorial. These findings provide evidence for incorporating breathing evaluations into clinical practice by clinicians and trainers, as they could be contributing to problems with motor control and movement. Furthermore this study found the Hi Lo assessment method and capnography measurements correlated highly, and may be useful in the identification of BPD in healthy individuals. Future research is needed to validate breathing re‐education programs and the role they have in treating pain disorders, preventing injury, and improving movement patterns.
References
- 1.Mills JD, Taunton JE, Mills WA. The effect of a 10‐week training regimen on lumbo‐pelvic stability and athletic performance in female athletes: a randomized‐controlled trial. Phys Ther Sport. 2005; 6: 60–66 [Google Scholar]
- 2.Cook G. Movement. Functional Movement Systems: Screening, Assessment and Corrective Strategies. Santa Cruz, CA: On Target Publications; 2010 [Google Scholar]
- 3.Devan MR, Pescatello LS, Faghri P, et al. A prospective study of overuse knee injuries among female athletes with muscle imbalances and structural abnormalities. J Athl Train. 2004; 39: 263–267 [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminoff L. What yoga therapists should know about the anatomy of breathing. Int J Yoga Therap. (2006); 16: 67–77 [Google Scholar]
- 5.Pryor JA, Prasad SA. Physiotherapy for Respiratory and Cardiac Problems. Edinburgh, UK: Churchill Livingstone; 2002 [Google Scholar]
- 6.Chaitow L, Bradley D, Gilbert C. Multidisciplinary Approaches to Breathing Pattern Disorders. London, UK: Churchill Livingstone; 2002 [Google Scholar]
- 7.Vickery R. The effect of breathing pattern retraining on performance in competitive cyclists. Available at: http://repositoryaut.lconz.ac.nz/handle/10292/83 Accessed January 7th, 2012 [Google Scholar]
- 8.Chaitow L. Breathing pattern disorders, motor control and low back pain. J Osteop Med. 2004; 7: 33–40 [Google Scholar]
- 9.Kapreli E, Vourazanis E, Billis E, et al. Respiratory dysfunction in chronic neck pain patients. A pilot study. Cephalalgia. 2009; 29(7): 701–710 [DOI] [PubMed] [Google Scholar]
- 10.Perri MA, Halford E. Pain and faulty breathing: a pilot study. J Bodyw Mov Ther. 2004; 8: 297–306 [Google Scholar]
- 11.Roussel NA, Nijs J, Truijen S. Low back pain: Climetric properties of the Tredelenburg test, Active Straight Leg Raise test and Breathing Pattern during Active Straight Leg Raising. J Manipulative Physiol Ther. 2007; 30(4): 270–278 [DOI] [PubMed] [Google Scholar]
- 12.Smith M, Russell A, Hodges P. Disorders of breathing and continence have a stronger association with back pain than obesity and physical activity. Aust J Physiother. 2006; 52: 11–16 [DOI] [PubMed] [Google Scholar]
- 13.Lewitt K. Relationship of faulty respiration to posture, with clinical implications. J Am Osteopath Assoc. 1980; 79(8): 525–528 [PubMed] [Google Scholar]
- 14.Clifton‐Smith T, Rowley J. Breathing pattern disorders and physiotherapy: inspiration for our profession. Phys Ther Rev. 2011; 16: 75–86 [Google Scholar]
- 15.Hestbaek L, Leboeuf‐Yde C, Manniche C. Low back pain: what is the long‐term course? A review of studies of general patient populations. Eur Spine J. 2003; 12(2): 149–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hruska R. Influences of dysfunctional respiratory mechanics on orofacial pain. Dent Clin North Am. 1997; 41(2): 211–227 [PubMed] [Google Scholar]
- 17.Simons DG, Travell JG, Simons IS. Myofascial pain and dysfunction: the trigger point manual: Second Edition. Baltimore, MD: Williams and Wilkins; 1999 [Google Scholar]
- 18.Teyhen DS, Shaffer SW, Lorenson CL, et al. The Functional Movement Screen: a reliability study. J Orthop Sports Phys Ther. 2012; 42(6): 530–40 [DOI] [PubMed] [Google Scholar]
- 19.Hodges P, Sapsford R, Pengel L. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn. 2007; 26: 362–371 [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan PB, Beales DJ, Beetham JA, et al. Altered motor control strategies in subjects with Sacroiliac Joint Pain during the Active Straight‐Leg‐Raise Test. Spine. 2002; 27(1): E1–E8 [DOI] [PubMed] [Google Scholar]
- 21.Hodges PW, Eriksson AE, Shirley D, et al. Intra‐abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005; 38(9): 1873–1880 [DOI] [PubMed] [Google Scholar]
- 22.Kolar P, Sulc J, Kyncl M, et al. Stabilizing function of the diaphragm: dynamic MRI and synchronized spirometric assessment. J Appl Physiol. 2010; 109(4): 1064–1071 [DOI] [PubMed] [Google Scholar]
- 23.Malatova R, Drevikovska P. Testing procedures for abdominal muscles using the muscle dynamometer. Proc Inst Mech Eng H. 2009; 223(8): 1041–1048 [DOI] [PubMed] [Google Scholar]
- 24.Cook G, Burton L, Hoogenboom B. Pre‐participation screening: The use of fundamental movements as an assessment of function ‐Part 1. N Am J Sports Phys Ther. 2006; 1: 62–72 [PMC free article] [PubMed] [Google Scholar]
- 25.Frohm A, Heijne A, Kowalski J, et al. A nine‐test screening battery for athletes: a reliability study. Scand J Med Sci Sports. 2012; 22: 306–15 [DOI] [PubMed] [Google Scholar]
- 26.Minick KI, Kiesel KB, Burton L. Interrater reliability of the Functional Movement Screen. J Strength Cond Res. 2010; 24(2): 479–486 [DOI] [PubMed] [Google Scholar]
- 27.Onate JA, Dewey T, Kollock RO, et al. Real‐time intersession and interrater reliability of the functional movement screen. J Strength Cond Res. 2012; 26(2): 408–15 [DOI] [PubMed] [Google Scholar]
- 28.Chorba RS, Chorba DJ, Bouillon LE, et al. Use of a Functional Movement Screening Tool to determine Injury risk in female collegiate athletes. N Am J Sports Phys Ther. 2010; 5: 47–54 [PMC free article] [PubMed] [Google Scholar]
- 29.Kiesel K, Plisky P, Voight M. Can serious injury in professional football be predicted by a preseason Functional Movement Screen? N Am J Sports Phys Ther. 2007; 2: 147–158 [PMC free article] [PubMed] [Google Scholar]
- 30.Peate WF, Bates G, Lunda K, et al. Core strength: a new model for injury prediction and prevention. J Occup Med Toxicol. 2007; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler RJ, Contreras M, Burton L et al. Modifiable risk factors predict injuries in firefighters during training academies. Work. 2013; 46(1): 11–17 [DOI] [PubMed] [Google Scholar]
- 32.Lisman P, O’Connor FG, Deuster PA, et al. Functional Movement Screen and aerobic fitness predict injury in military training. Med Sci Sports Exerc. 2013; 45(4): 636–643 [DOI] [PubMed] [Google Scholar]
- 33.O’Connor FG, Deuster PA, Davis J, et al. Functional Movement Screening: Predicting Injuries in Officer Candidates. Med Sci Sports Exerc. 2011; 43(12): 2224–2230 [DOI] [PubMed] [Google Scholar]
- 34.Courtney R, Greenwood KM, Cohen M. Relationships between measures of dysfunctional breathing in a population with concerns about their breathing. J Bodyw Mov Ther. 2011; 15: 24–34 [DOI] [PubMed] [Google Scholar]
- 35.Courtney R, Reece J. Comparison of the Manual Assessment of Respiratory Motion and the Hi Lo Breathing Assessment in determining a simulated breathing pattern. Int J Osteopath Med. 2009; 12: 86–91 [Google Scholar]
- 36.Garssen B, De Reuiter C, Van Dyke R. Breathing retraining: a rational placebo? Clin Psychol Rev. 1992; 12: 141–53 [Google Scholar]
- 37.Gardner WN. The pathophysiology of hyperventilation disorders. Chest. 1996; 109: 516–534 [DOI] [PubMed] [Google Scholar]
- 38.Laffey JG, Kavanagh BP. Hypocapnia. N Engl J Med. 2002; 347(1): 43–53 [DOI] [PubMed] [Google Scholar]
- 39.Miner JR, Heegaard W, Plummer D. End‐tidal carbon dioxide monitoring during procedural sedation. Acad Emerg Med. 2002; 9(4): 275–280 [DOI] [PubMed] [Google Scholar]
- 40.Litchfield PM. CapnoLearning: Respiratory Fitness and Acid‐Base Regulation. Psychophysiology Today. 2010; 7(1): 6–12 [Google Scholar]
- 41.McLaughlin L. Breathing evaluation and retraining in manual therapy. J Bodyw Mov Ther. 2009; 13: 276–282 [DOI] [PubMed] [Google Scholar]
- 42.Levitsky MG. Pulmonary Physiology. New York, NY: McGraw Hill; 1995 [Google Scholar]
- 43.Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985; 29: 199–206 [DOI] [PubMed] [Google Scholar]
- 44.Thomas M, McKinley RK, Freeman E. Breathing retraining for dysfunctional breathing in asthma: a randomized controlled trail. Thorax. 2003; 58: 110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han J, Stegan K, De Valack C, et al. Influence of breathing therapy on complaints, anxiety and breathing pattern in patients with hyperventilation syndrome and anxiety disorders. J Psychosom Res. 1996; 41(5): 481–493 [DOI] [PubMed] [Google Scholar]
- 46.Warburton C, Jack S. Can you diagnose hyperventilation? Chron Respir Dis. 2006; 3: 113–115 [DOI] [PubMed] [Google Scholar]
- 47.Stark J, Stark R. The carbon dioxide syndrome. Coorparoo: Buteyko On Line Ltd; 2002 [Google Scholar]
- 48.Commerford M, Mottram S. Movement and stability dysfunction – contemporary developments. Man Ther. 2001: 6: 15–26 [DOI] [PubMed] [Google Scholar]
- 49.Bhk FP, Koehle MS. Normative Data for the Functional Movement Screen™ in middle‐aged adults. J Strength Cond Res. 2012. http://doi: 10.1519/JSC.0b013e3182576fa6 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Schneiders AG, Davidsson A, Horman E, et al. Functional Movement Screen: Normative values in a young population. Int J Sports PhysTher. 2011; 6(2): 75–82 [PMC free article] [PubMed] [Google Scholar]
- 51.Lee EK, Seyal M, Mull B, et al. Increased excitability of the human corticospinal system hyperventilation. Clin Neurophysiol. 1998; 109: 263–267 [DOI] [PubMed] [Google Scholar]
- 52.Boyle K, Olinick J, Lewis C. The value of blowing up a balloon. N Am J Sports Phys Ther. 2010; 5: 179–188 [PMC free article] [PubMed] [Google Scholar]
- 53.Okada T, Huxel KC, Nesser TW. Relationship between core stability, functional movement and performance. J Strength Cond Res. 2011; 25(1): 252–261 [DOI] [PubMed] [Google Scholar]
- 54.Shinkle J, Nesser TW, Demchak TJ, et al. Effect of core strength on the measure of power in the extremities. J Strength Cond Res. 2012; 26(2): 373–380 [DOI] [PubMed] [Google Scholar]
- 55.Cowen VS. Functional fitness improvements after a work‐site yoga initiative. J Bodyw Mov Ther. 2010; 14: 50–54 [DOI] [PubMed] [Google Scholar]
- 56.Lucas K, Polus B, Rich P. Latent myofascial trigger points: their effect on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004; 8: 160–166 [Google Scholar]
- 57.McLaughlin L, Goldsmith CH, Coleman K. Breathing evaluation and retraining as an adjunct to manual therapy. Man Ther. 2011; 16: 51–52 [DOI] [PubMed] [Google Scholar]
- 58.Kang JH, Park RY, Lee SU, et al. The effect of the forward head posture on postural balance in long time computer based worker. Ann Rehabil Med. 2012; 36(1): 98–104 http://dx.doi.org/10.5535/arm.2012.36.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehling WE, Hamel KA, Acree M. Randomized, controlled trial of breath therapy for patients with chronic low back pain. Altern Ther Health Med. 2005; 11(4): 44–52 [PubMed] [Google Scholar]
- 60.Green HJ, Roy B. Efficiency after altitude acclimatization. J Appl Physiol. 2001; 91: 1014–1015 [DOI] [PubMed] [Google Scholar]
- 61.Chang L, Landgren CEG. Maximal breath‐holding time and immediate tissue CO2 storage capacity during head‐out immersion in humans. Eur J Appl Physiol. 1996; 73: 210–218 [DOI] [PubMed] [Google Scholar]