Abstract
Purpose/Background:
Muscle soreness can negatively interfere with the activities of daily living as well as sports performance. In the working environment, a common problem is muscle tenderness, soreness and pain, especially for workers frequently exposed to unilateral high repetitive movements tasks. The aim of the study is therefore to investigate the acute effect of massage applied using a simple device Thera‐band roller Massager on laboratory induced hamstring muscle soreness, and the potential cross over effect to the non‐massaged limb.
Methods:
22 healthy untrained men (Mean age 34 +/− 7 years; mean height 181.7 +/− 6.9 cm; mean weight 80.6 +/− 6.4 kg; BMI: 24.5 +/− 1.3) with no prior history of knee, low back or neck injury or other adverse health issues were recruited. Participants visited the researchers on two separate occasions, separated by 48 hours, each time providing a soreness rating (modified visual analog scale 0‐10), and being tested for pressure pain threshold (PPT) and active range of motion (ROM) of the hamstring muscles. During the first visit, delayed onset muscular soreness of the hamstring muscles was induced by 10 x 10 repetitions of the stiff‐legged dead‐lift. On the second visit participants received either 1) 10 minutes of roller massage on one leg, while the contralateral leg served as a cross over control, or 2) Resting for 10 minutes with no massage at all. Measurement of soreness, PPT and ROM were taken immediately before and at 0, 10, 30 and 60 min. after treatment.
Results:
There was a significant group by time interaction for soreness (p < 0.0001) and PPT (p = 0.0007), with the massage group experiencing reduced soreness and increasing PPT compared with the control group. There was no group by time interaction for ROM (p = 0.18). At 10 min. post massage there was a significant reduction in soreness of the non‐massaged limb in the cross over control group compared to controls but this effect was lost 30 minutes post massage.
Conclusion:
Massage with a roller device reduces muscle soreness and is accompanied by a higher PPT of the affected muscle.
Level of Evidence:
2c; outcomes research
Keywords: Cross over effect, delayed onset muscle soreness hyperalgesia, pain
Introduction
Unaccustomed muscle work, especially that which is eccentric in nature, can cause post‐exercise soreness, usually referred to as delayed onset muscle soreness (DOMS).1–4 Muscle soreness can negatively interfere with the activities of daily living as well as sports performance. Research suggests that DOMS is the result of an inflammatory process caused by micro‐tears in the muscle fibers during unaccustomed repetitive activity and/or eccentric contractions but it has also been suggested that muscle soreness can occur without micro‐trauma.4–6
However, it is not just novel strength training exercises or eccentric contractions that can cause muscle soreness. In the working environment, a common problem is muscle tenderness, soreness and pain, especially for workers frequently exposed to unilateral high repetitive movements tasks. Computer workers commonly experience soreness of several different neck/shoulder muscles.7 Andersen and coworkers reported that 33% and 29% of blue‐ and white‐collar workers respectively, within the general working population, suffered from neck/shoulder pain.8 Furthermore, it seems that highly repetitive movement tasks can physically alter the muscle fiber itself. Andersen et al identified grossly hypertrophied Type 1 muscle fibers with poor capillarization – so called megafibers ‐ in the trapezius muscle of women with trapezius myalgia who worked in monotonous jobs, indicating that high intensity or eccentric muscle contractions are not a prerequisite to cause muscle soreness.9 Furthermore, as muscle pain has been associated with decreased performance in the form of decreased rapid force capacity and maximal muscle strength, continuous overload causing muscle soreness and pain may not only have adverse functional implications during working hours but also during activities of daily living during non‐working hours.10,11
Although not universally accepted as a treatment for muscle soreness, massage is often prescribed to relieve the discomfort of soreness following unaccustomed, eccentric training or high repetition monotonous movement tasks.4,12–15 There is some evidence to support the efficacy of massage in such applications but more conclusive studies are warranted examining timing and type of massage.12,16–19 A recent study concluded that both active warm‐up and massage can reduce severe muscle soreness, but the effect may level off within an hour.20
From functional magnetic resonance imaging studies it has been shown that pain and soreness from DOMS has a cortical projection and Melzack has described pain as a multi‐dimensional experience produced by a complex neural network in the brain.21,22 Central adaptation of pain perception also occurs in response to rehabilitation of pain with strength training, leading to altered pain thresholds in parts of the body not directly trained.23 It is therefore interesting to investigate if changes in the perception of pain following massage have a cross over effect to the non‐massaged limb thereby indicating a change in the central cortical output.
The concept of cross‐education (cross over) was first described over a century ago in 1874 by Scripture et al and an abundance of literature has been published on cross education training effects.24 Alternately, a cross over fatigue effect, whereby the fatigue of an ipsilateral limb results in fatigue of a contralateral limb, has been examined by several researchers. These authors investigated force or strength and fatigue effects of the elbow flexors, intrinsic hand muscles and quadriceps.25–30 Paillard et al demonstrated contralateral postural control deficits following fatigue of the opposite quadriceps.31 The only study to identify a cross over effect with the hamstrings reported contralateral strength deficits after anterior cruciate ligament reconstruction of the contralateral knee.32 With few studies examining the immediate cross over effects associated with the lower limbs, only one study examining the hamstrings (for surgical effects) and no studies examining possible cross over pain effects, it is important to provide further information on this phenomenon.
In jobs with a high prevalence of muscle pain and soreness brought on by repetitive tasks, massage could potentially help reduce the negative effects of monotonous movement on the musculoskeletal system. In order for this to work the massage has to be easily administered and simply executed. The aim of the study is therefore to investigate the acute effect of massage applied using a Thera‐band Roller Massager (RM) on laboratory induced hamstring muscle soreness, and the potential cross over effect to the non‐massaged limb.
The authors hypothesize that significant acute reductions would occur in perceived soreness (VAS), pressure pain threshold (PPT) and active range of motion (ROM) in 1) the massaged limb compared with the non‐massaged limb and a non‐massaged control group, and 2) a significant cross over effect to the non‐massaged limb would occur in the massage group when compared with a non‐massaged control group.
Methods
Study design & participants
This study design used a randomized controlled design (with a control group) to investigate the acute effects of 10 minutes of massage, provided using a RM, on post‐exercise induced DOMS of the hamstring muscles.
22 healthy untrained men (Mean age 34 +/− 7 years; mean height 181.7 +/− 6.9 cm; mean weight 80.6 +/− 6.4 kg; BMI: 24.5 +/− 1.3) not participating in sport or fitness activities on a regular basis and with no prior history of knee, low back or neck injury or other adverse health issues were recruited. Exclusion criteria were 1) contraindications to strength training (including back and neck surgery, knee surgery, knee replacement) 2) blood pressure over 160/100 or other potentially harmful cardiovascular conditions.
The participants were given written and verbal information regarding the study and signed an informed consent form. The local ethical committee approved the study (H‐3‐2010‐062) and the trial was registered, prior to subject enrollment, at clinicaltrials.gov (No. NCT01478464). Furthermore, participants were instructed to not use any over‐the‐counter pain relievers, such as aspirin and acetaminophen, which potentially could interfere with their perception of or ability to accurately report soreness. Finally, the participants were instructed to keep their diet, hydration, and sleep pattern as they normally would.
Study design
All the participants visited the researchers on two occasions separated by approximately 48 hours. On the first occasion descriptive measurements on age, height, weight and BMI were obtained as well as baseline measurements on VAS, PPT and ROM. At the conclusion of the first visit the participants also received the DOMS‐inducing training. On the second occasion, the randomization and allocation to massage group (MA), massage group‐control leg (MC) and control (CO) were performed using a computer‐generated random numbers table. Subsequently, treatment was administered.
Measurements
The outcome measures consisted of a numerical soreness rating, PPT and a ROM measurement. The numerical pain rating was assessed using a VAS ranging from 0‐10 with “0” being “No soreness at all” and “10” being “the worst imaginable soreness” of the entire hamstring.33
Hamstring flexibility (ROM) by a one‐legged sit and reach test was tested.17 Briefly, the participants sat in a chair with one leg straight out in front of them, resting the heel on a box. By flexing at the hip and keeping the knee straight and locked, the forward reach (arms straight, index fingers touching and extended) was tested. In the fully hip‐flexed position, the distance from the tip of the index fingers to the heel of the elevated foot was measured. The test was carried out for both legs, one leg at a time.
Finally, in order to obtain a quantifiable measurement of soreness, the long head of the biceps femoris muscle and semitendinosus muscle were palpated on both legs, the muscle belly located and three central points on each muscle belly superior to inferior and equally distributed marked. A pressure algometer was used to measure pressure pain threshold (Somedic Algometer, Somedic Production AB, Sollentuna, Sweden) on the marked spots of both legs going from lateral and proximal‐distal to medial and distal‐proximal.34,35 The reason for measuring PPT in the aforementioned locations on the hamstring muscles was to avoid increases in sensitivity due to the repeated pressure applied, as well as to be able to collectively describe the soreness of the back of the thigh as a whole.
Outcome measures
The primary outcome of interest was VAS with PPT and ROM as secondary outcomes.
Induction of DOMS
Participants were instructed to perform 10 sets of 10 repetitions of stiff‐legged deadlifts with a kettlebell, separated by 30s of rest. The tempo was set at 1‐2s pace for the concentric and eccentric phases of each repetition. A ramping progression of kettlebell weight was used in the following manner: 1st set: 12 kg, 2nd set: 16 kg, 3rd – 5th set: 24 kg and 6th through 10th set a 32kg kettlebell was lifted. The exercise was demonstrated and supervised by an experienced trainer. Completing 10 sets of 10 repetitions took approximately 10 minutes for all participants.
Experimental treatment
Approximately 48 hours after the receiving the DOMS‐inducing training, the participants were asked to rate their level of soreness of the hamstring muscles of both legs, and the hamstring flexibility and hip flexion ROM and PPT were recorded using the previously described methods, thus establishing a baseline. Each subject was subsequently allocated to either MA or CO by using a block randomization procedure. For the MA, only one leg was randomly assigned for massage treatment the other leg therefore acted as a within subject control leg (MC) in order to test for potential cross over effects of the treatment. The MA was given a 10 minute massage of one leg using a RM while lying prone. A massage roller is very similar to a cooking rolling pin with a dense rubberized surface. The massage was standardized to a constant stroking rhythm going from distal to proximal of the back of the thigh at a speed of 1‐2 sec. per stroke with a moderate pressure. The participants in CO received no massage at all, but rested in a prone position during the 10‐minute period. The outcome measurements were assessed immediately after the cessation of the treatment (referred to as time zero), and then repeated at 10, 30 and 60 minutes post‐massage.
The examiner measuring outcomes was kept blinded to group assignment throughout the experiment and the participants were instructed not to reveal whether massage had been administered or not.
Sample size
Power calculations performed prior to the study showed that 10 participants in each group in an unpaired design (i.e. MA vs. CO group) were necessary for testing the null hypothesis of equality of treatment at an alpha level of 5%, a statistical power of 80%, a standard deviation of 1.5 and a minimally relevant difference in intensity of soreness of 2 on a scale of 0‐10. Finally, an expected cross over effect from the massage to the contralateral leg of 1.5 with a SD of 1.5, given a power of 80% in a paired design.
Statistics
The SAS Mixed procedure, repeated measures ANOVA (SAS statistical software v9.2, Cary, NC USA) was used for statistical analysis of a group by time interaction and performed post hoc tests where appropriate. An alpha level of p < 0.05 is accepted as statistically significant. Results are reported as means (SD) and 95% confidence intervals (95% CI) unless otherwise specified.
Results
From the first to the second visit there was a highly significant increase in perceived hamstring soreness (VAS) (p < 0.001), a significant decrease in pressure‐applied tenderness (PPT) (p < 0.001), as well as a significant decrease in the sit and reach test (ROM) (p < 0.001) for both legs and all participants. Table 1 displays all relevant descriptive statistics for the two groups.
Table 1.
Descriptive Statistics
| Massage group | Control group | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Number of participants | 11 | 11 |
| Male/female | 11/0 | 11/0 |
| Age (years) | 35 (8) | 33 (7) |
| Weight (kg) | 80.4 (7.8) | 81.4 (4.9) |
| Height (cm) | 181.8 (7.2) | 181.7 (6.6) |
| BMI (kg/m2) | 24.1 (1.6) | 24.5 (0.7) |
| VAS (L) | 1 (0.9) | 1 (0.5) |
| VAS (R) | 1 (0.9) | 1 (0.5) |
| PPT (L) (kPa) | 570 (217) | 459 (178) |
| PPT (R) (kPa) | 524 (180) | 468 (192) |
| ROM (L) (cm) | 24.5 (10.1) | 22.7 (8.6) |
| ROM (R) (cm) | 23.4 (7.9) | 23.6 (9.6) |
BMI = Body mass index; VAS = Perceived soreness; PPT = Pressure‐pain threshold; ROM = Active range of motion
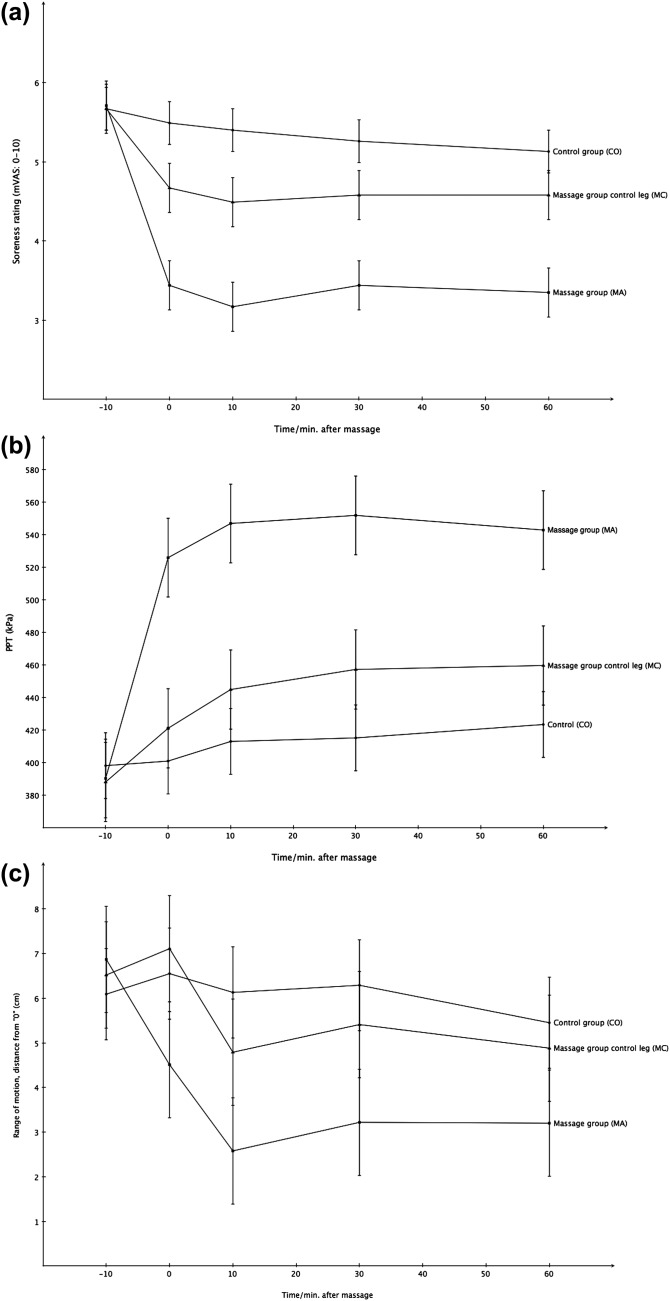
For the second visit there was a significant group by time interaction for soreness (p < 0.0001) and PPT (p = 0.0007), with the massage group experiencing reduced soreness and increasing PPT compared with the control group. There was no group by time interaction for ROM (p = 0.18). Table 2 summarizes the results of the post hoc analysis and Figure 1 shows the mean (+/− SD) reported soreness (1a), PPT (1b) and ROM (1c) over time for the three conditions (MA, MC, CO groups) respectively.
Table 2.
| Outcome measure | Time after administered massage (min.) |
|||||
|---|---|---|---|---|---|---|
| −10 | 0 | 10 | 30 | 60 | ||
| VAS | ||||||
| CO to MA | −0.04 (−0.87 to 0.79) | 2.05 (1.22 to 2.89) * | 2.23 (1.40 to 3.06) * | 1.82 (0.99 to 2.65) * | 1.78 (0.94 to 2.61) * | |
| CO to MC | 0 (−0.83 to 0.83) | 0.82 (−0.01 to 1.65) | 0.91 (0.08 to 1.74) * | 0.68 (−0.15 to 1.51) | 0.55 (−0.29 to 1.38) | |
| MA to MC | 0.04 (−0.56 to 0.65) | −1.23 (−1.83 to −0.63) * | −1.32 (−1.93 to 0.72) * | −1.14 (−1.74 to −0.54) * | −1.23 (−1.83 to −0.63) * | |
| PPT | ||||||
| CO to MA | 7.86 (−56 to 71) | −124.94 (−188 to −61) * | −133.90 (−197 to −70) * | −136.71 (−200 to −73) * | −119.43 (−106 to 22) | |
| CO to MC | 10.06 (−54 to 74) | −20.14 (−84 to 44) | −31.89 (−96 to 32) | −42.07 (−51 to 55) | −36.24 (−100 to 28) | |
| MA to MC | 2.20 (−51 to 55) | 104.80 (52 to 158) * | 102.01 (49 to 155) * | 94.64 (42 to 148) * | 83.20 (30 to 136) * | |
| ROM | ||||||
| CO to MA | −0.78 (−3.99 to 2.42) | 2.04 (−1.17 to 5.25) | 3.55 (0.34 to 6.76) * | 3.07 (−0.14 to 6.27) | 2.25 (−0.96 to 5.46) | |
| CO to MC | −0.43 (−3.62 to 2.76) | −0.56 (−3.75 to 2.63) | 1.34 (−1.85 to 4.53) | 0.88 (−2.31 to 4.07) | 0.56 (−2.63 to 3.76) | |
| MA to MC | 0.35 (−2.04 to 2.74) | −2.60 (−5.00 to −0.21) * | −2.21 (−4.60 to 0.18) | −2.19 (−4.58 to 0.21) | −1.69 (−4.08 to 0.71) | |
Reported as difference between least square means (95% confidence interval) of the three groups.
indicates significance level of p < 0.05
VAS = Perceived soreness; PPT = Pressure‐pain threshold; ROM = active range of motion; CO = Control; MA = Massage group; MC = Massage group control leg
Figure 1.
Perceived soreness (VAS)
A significant difference between CO and MA was observed in perceived soreness of the hamstrings with lower soreness rating in MA at 0 (p < 0.0001), 10 (p < 0.0001), 30 (p < 0.0001) and 60 (p < 0.0001) minutes post massage. Furthermore, significant differences between MA and MC were also observed at 0 (p = 0.0001), 10 (p < 0.0001), 30 (p = 0.0003) and 60 (p < 0.0001) minutes post massage with lower soreness ratings observed in the MA group. For CO and MC groups a small but significant difference existed only at 10 minutes (p = 0.03) post massage with a lower soreness rating in MC group but this difference was non‐significant at 30 minutes post massage (p = 0.11). The difference approached statistical significance at 0 (p = 0.054), however, was not statistically significant at 30 (p = 0.11) or 60 (p = 0.19) minutes post massage. This trend towards an overall cross over effect in soreness reduction in the MC group as compared with CO group is visible in Figure 1a.
Pressure Pain Threshold (PPT)
PPT was significantly higher for MA group compared with CO group at 0 (p = 0.0002), 10 (p < 0.0001) and 30 (p < 0.0001) minutes post massage. Significant differences were found at all times between MA and MC (p < 0.0001, p = 0.0002, p = 0.0005, p = 0.002 for 0, 10, 30 and 60 minutes post massage, respectively) with higher PPT scores for MA. No statistically significant differences existed between the CO and MC groups.
Range of Motion (ROM)
A significant increase in ROM was found in MA at 10 minutes post massage (p = 0.03) as compared to CO group, no other differences were statistically significantly different at the other time intervals. A significant difference in ROM was also observed between MA and MC groups immediately after the massage (p = 0.03) with MA having the greatest ROM, but this effect was no longer statistically significantly different at 10 minutes post massage (p = 0.07). No differences existed in ROM between CO and MC groups at time point (p>0.05).
Discussion
The results of this single blind randomized controlled trial showed a statistically significant reduction in muscle soreness after massage using a RM. The positive effect of massage on soreness was still significant 60 minutes after the cessation of treatment and was accompanied by significant increases in PPT similar to the pattern seen in VAS over the course of the first 30 minutes, but was no longer statistically significantly different at 60 minutes post treatment. Increases in ROM were not statistically significantly different between groups, except for immediately (0 minutes) and 10 min. after the massage between MA and CO groups, and MA and MC groups, respectively.
Perceived soreness (VAS)
When dealing with soreness in the musculoskeletal system, the use of manually administered massage is becoming increasingly popular in both Europe and the US.36–38 Some authors have reported good results on perceived soreness following massage interventions or combination treatments that included massage whereas others have found little or no change in perceived pain or soreness levels following strenuous or novel exercise.14,16,17,19,39–41 The conflicting results on massage as a muscle soreness reliever may be related to the type of massage administered. The type of massage applied in the current study is of a superficial nature and done at a moderate speed. Previously that type of massage has been shown to activate C‐tactile low‐threshold unmyelinated mechanoreceptors but not myelinated afferents and is commonly experienced as pleasant.42 This description of mechano‐ and nociceptive activation may, at least partially, help explain why some types of massages relieve muscular soreness while others do not.
Pressure Pain Threshold (PPT)
In the current study, the RM was able to reduce mechanical hyperalgesia by increasing pressure thresholds in the affected muscle, which is consistent with findings from several other recent studies using PPT to detect musculoskeletal pain.43–47 For instance, Frey Law et al found that both deep tissue massage and superficial touch reduced mechanical hyperalgesia (increased PPT thresholds) and decreased pain during stretch when combined, but did not cause a change in resting pain levels.46 In animal models massage‐like treatments of stroking and touching have been shown to induce an anti‐nociceptive response by mediating an endogenous release of oxytocin into the plasma and in the central grey matter located around the cerebral aqueduct in the midbrain.48,49 Similarly oxytocin has been shown to induce analgesia in patients with low back pain involving the endogenous opiate peptide system as well as in patients suffering from irritable bowel syndrome and in at least one case of cancer.50–52 Thus, one plausible mechanism to explain the reduction in mechanical hyperalgesia and muscle soreness is the activation of descending inhibitory pathways, using the central gray matter‐opioid system and oxytocin.
ROM
The present study found increased ROM in the MA group only at 10 minutes post massage as compared to CO group and increased ROM between the MA and MC groups was observed immediately after the massage but at all other times no differences existed. These results are concur with the results of previous studies that have shown little or no effect on ROM and flexibility, or sprint and jump performance following different types of massage.17,53 Huang et al (2010) however showed increased hip flexion following 10 or 30 seconds of deep pressure massage on the musculotendinous junction of the hip flexors using a specific friction massage technique, performed by a manual therapist, using the fingertips.54 It is possible that the application of massage with a RM is superficial in nature and thereby insufficient to increase ROM, as a deeper stimulus targeting the musculotendinous junction is not achieved.
Strengths and Limitations
The examiner‐blind randomized controlled trial design is a significant strength of this study. Furthermore, the type of massage administered with the RM was very easily standardized in time, distance and speed but not in pressure, which is a limitation to the current study but also a limitation in studies where manual therapists are applying the pressure with their hands and fingers. Furthermore, our study design was non‐reliant on the experience level of the administrator, which is a notable strength. Limitations to the current study include not controlling for time of day when administering the massage as well as not providing a sham intervention for the control group. Finally, due to the nature of intervention it was not possible to keep the participants blinded to their group assignment.
Conclusion
The results obtained from this study indicate that massage administered with a RM for 10 minutes has an acute soreness reducing effect accompanied by a higher tolerance to applied pressure in the affected muscle. Furthermore, there is a tendency towards a soreness reducing effect on the contralateral limb indicating a potential cross over effect possibly mediated by central pathways.
References
- 1.Lewis PB, Ruby D, Bush‐Joseph CA. Muscle soreness and delayed‐onset muscle soreness. Clin Sport Med. 2012; 31(2): 255–262 [DOI] [PubMed] [Google Scholar]
- 2.Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001; 537(Pt 2): 333–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J‐G, Malm C, Thornell L‐E. Eccentric contractions leading to DOMS do not cause loss of desmin nor fibre necrosis in human muscle. Histochem Cell Biol. 2002; 118(1): 29–34 [DOI] [PubMed] [Google Scholar]
- 4.Zainuddin Z, Newton M, Sacco P, Nosaka K. Effects of massage on delayed‐onset muscle soreness, swelling, and recovery of muscle function. J Athl Train. 2005; 40(3): 174–180 [PMC free article] [PubMed] [Google Scholar]
- 5.Barbe MF, Barr AE. Inflammation and the pathophysiology of work‐related musculoskeletal disorders. Brain Behav Immun. 2006; 20(5): 423–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr AE, Barbe MF, Clark BD. Systemic Inflammatory Mediators Contribute to Widespread Effects in Work‐Related Musculoskeletal Disorders. Exerc Sport Sci Rev. 2004: 135–142 [DOI] [PubMed] [Google Scholar]
- 7.Andersen LL, Hansen K, Mortensen OS, Zebis MK. Prevalence and anatomical location of muscle tenderness in adults with nonspecific neck/shoulder pain. BMC Musculoskelet Disord. 2011; 12: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen LL, Mortensen OS, Hansen JV, Burr H. A prospective cohort study on severe pain as a risk factor for long‐term sickness absence in blue‐ and white‐collar workers. Occup Environ Med. 2011; 68(8): 590–2 [DOI] [PubMed] [Google Scholar]
- 9.Andersen LL, Suetta C, Andersen JL, Kjaer M, Sjøgaard G. Increased proportion of megafibers in chronically painful muscles. Pain. 2008; 139(3): 588–93 [DOI] [PubMed] [Google Scholar]
- 10.Andersen LL, Holtermann A, Jørgensen MB, Sjøgaard G. Rapid muscle activation and force capacity in conditions of chronic musculoskeletal pain. Clin Biomech (Bristol, Avon). 2008; 23(10): 1237–42 [DOI] [PubMed] [Google Scholar]
- 11.Andersen LL, Nielsen PK, Søgaard K, Andersen CH, Skotte J, Sjøgaard G. Torque‐EMG‐velocity relationship in female workers with chronic neck muscle pain. J Biomech. 2008; 41(9): 2029–35 [DOI] [PubMed] [Google Scholar]
- 12.Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sport Med. 2003; 33(2): 145–164 [DOI] [PubMed] [Google Scholar]
- 13.Micklewright D. The effect of soft tissue release on delayed onset muscle soreness: a pilot study. Phys Ther Sport. 2009; 10(1): 19–24 [DOI] [PubMed] [Google Scholar]
- 14.Tiidus P. Massage and Ultrasound as therapeutic modalities in exercise‐induced muscle damage. Can J Appl Physiol. 1999; 24(3): 267–278 [DOI] [PubMed] [Google Scholar]
- 15.Frey Law LA, Evans S, Knudtson J, Nus S, Scholl K, Sluka KA. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008; 9(8): 714–21 [DOI] [PubMed] [Google Scholar]
- 16.Bakowski P, Musielak B, Sip P, Biegaski G. Effects of massage on delayed‐onset muscle soreness. Chir Narzadow Ruchu Ortop Pol. 2003; 73(4): 261–5 [PubMed] [Google Scholar]
- 17.Best TM, Hunter R, Wilcox A, Haq F. Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin J Sport Med. 2008; 18(5): 446–60 [DOI] [PubMed] [Google Scholar]
- 18.Ernst E. Does post‐exercise massage treatment reduce delayed onset muscle soreness? A systematic review. Br J Sport Med. 1998; 32(3): 212–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005; 35(3): 235–56 [DOI] [PubMed] [Google Scholar]
- 20.Andersen LL, Jay K, Andersen CH, et al. Acute effects of massage or active exercise in relieving muscle soreness: Randomized controlled trial. J Strength Cond Res. 2013 [DOI] [PubMed] [Google Scholar]
- 21.Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004 Pain Pract. 2005; 5(2): 85–94 [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann K, Leidl C, Kaschka M, et al. Central Projection of Pain Arising from Delayed Onset Muscle Soreness (DOMS) in Human Subjects. PLoS One. 2012; 7(10): e47230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen LL, Andersen CH, Sundstrup E, Jakobsen MD, Mortensen OS, Zebis MK. Central adaptation of pain perception in response to rehabilitation of musculoskeletal pain: randomized controlled trial. Pain Physician. 15(5): 385–94 [PubMed] [Google Scholar]
- 24.Scripture EW, Smith TL, Brown E. On the education of muscular control and power. Stud Yale Psychol Lab. 1894; 2: 114–19 [Google Scholar]
- 25.Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res. 2003; 150(3): 308–13 [DOI] [PubMed] [Google Scholar]
- 26.Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006; 175(3): 526–35 [DOI] [PubMed] [Google Scholar]
- 27.Zijdewind I, Zwarts MJ, Kernell D. Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett. 1998; 253(1): 41–4 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Maruyama A, Maeda M, et al. Unilateral grip fatigue reduces short interval intracortical inhibition in ipsilateral primary motor cortex. Clin Neurophysiol. 2009; 120(1): 198–203 [DOI] [PubMed] [Google Scholar]
- 29.Martin PG, Rattey J. Central fatigue explains sex differences in muscle fatigue and contralateral cross‐over effects of maximal contractions. Pflugers Arch. 2007; 454(6): 957–69 [DOI] [PubMed] [Google Scholar]
- 30.Rattey J, Martin PG, Kay D, Cannon J, Marino FE. Contralateral muscle fatigue in human quadriceps muscle: evidence for a centrally mediated fatigue response and cross‐over effect. Pflugers Arch. 2006; 452(2): 199–207 [DOI] [PubMed] [Google Scholar]
- 31.Paillard T, Chaubet V, Maitre J, Dumitrescu M, Borel L. Disturbance of contralateral unipedal postural control after stimulated and voluntary contractions of the ipsilateral limb. Neurosci Res. 2010; 68(4): 301–6 [DOI] [PubMed] [Google Scholar]
- 32.Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Contralateral limb strength deficits after anterior cruciate ligament reconstruction using a hamstring tendon graft. Clin Biomech (Bristol, Avon). 2007; 22(5): 543–50 [DOI] [PubMed] [Google Scholar]
- 33.Pincus T, Bergman M, Sokka T, Roth J, Swearingen C, Yazici Y. Visual analog scales in formats other than a 10 centimeter horizontal line to assess pain and other clinical data. J Rheumatol. 2008; 35(8): 1550–8 [PubMed] [Google Scholar]
- 34.Jensen K, Andersen HO, Olesen J, Lindblom U. Pressure‐pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain. 1986; 25(3): 313–23 [DOI] [PubMed] [Google Scholar]
- 35.Persson AL, Brogårdh C, Sjölund BH. Tender or not tender: test‐retest repeatability of pressure pain thresholds in the trapezius and deltoid muscles of healthy women. J Rehabil Med. 2004; 36(1): 17–27 [DOI] [PubMed] [Google Scholar]
- 36.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990‐1997: results of a follow‐up national survey. JAMA. 1998; 280(18): 1569–75 [DOI] [PubMed] [Google Scholar]
- 37.Ernst E, White A. The BBC survey of complementary medicine use in the UK. Complement Ther Med. 2000; 8(1): 32–6 [PubMed] [Google Scholar]
- 38.Mills SY. Regulation in complementary and alternative medicine. BMJ. 2001; 322(7279): 158–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sport Med. 2003; 37(1): 72–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulick DT, Kimura IF, Sitler M, Paolone A, Kelly JD. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996; 31(2): 145–152 [PMC free article] [PubMed] [Google Scholar]
- 41.Jakeman J, Byrne C, Eston R. Efficacy of lower limb compression and combined treatment of manual massage and lower limb compression on symptoms of exercise‐induced muscle damage in women. J Strength …. 2010; 24(11): 3157–3165 [DOI] [PubMed] [Google Scholar]
- 42.Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009; 12(5): 547–8 [DOI] [PubMed] [Google Scholar]
- 43.Anaya‐Terroba L, Arroyo‐Morales M, Fernández‐de‐Las‐Peñas C, Díaz‐Rodríguez L, Cleland JA. Effects of ice massage on pressure pain thresholds and electromyography activity postexercise: a randomized controlled crossover study. J Manipulative Physiol Ther. 2010; 33(3): 212–9 [DOI] [PubMed] [Google Scholar]
- 44.Buttagat V, Eungpinichpong W, Chatchawan U, Arayawichanon P. Therapeutic effects of traditional Thai massage on pain, muscle tension and anxiety in patients with scapulocostal syndrome: a randomized single‐blinded pilot study. J Bodyw Mov Ther. 2012; 16(1): 57–63 [DOI] [PubMed] [Google Scholar]
- 45.Cramer H, Lauche R, Hohmann C, et al. Randomized controlled trial of pulsating cupping (pneumatic pulsation therapy) for chronic neck pain. Forsch Komplementmed. 2011; 18(6): 327–34 [DOI] [PubMed] [Google Scholar]
- 46.Frey Law LA, Evans S, Knudtson J, Nus S, Scholl K, Sluka KA. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008; 9(8): 714–721 [DOI] [PubMed] [Google Scholar]
- 47.Zheng Z, Wang J, Gao Q, et al. Therapeutic evaluation of lumbar tender point deep massage for chronic non‐specific low back pain. J Tradit Chin Med. 2012; 32(4): 534–7 [DOI] [PubMed] [Google Scholar]
- 48.Lund I, Ge Y, Yu L‐C, et al. Repeated massage‐like stimulation induces long‐term effects on nociception: contribution of oxytocinergic mechanisms. Eur J Neurosci. 2002; 16(2): 330–8 [DOI] [PubMed] [Google Scholar]
- 49.Agren G, Lundeberg T, Uvnäs‐Moberg K, Sato A. The oxytocin antagonist 1‐deamino‐2‐D‐Tyr‐(Oet)‐4‐Thr‐8‐Orn‐oxytocin reverses the increase in the withdrawal response latency to thermal, but not mechanical nociceptive stimuli following oxytocin administration or massage‐like stroking in rats. Neurosci Lett. 1995; 187(1): 49–52 [DOI] [PubMed] [Google Scholar]
- 50.Yang J. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine (Phila Pa 1976). 1994; 19(8): 867–71 [DOI] [PubMed] [Google Scholar]
- 51.Louvel D, Delvaux M, Felez A, et al. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut. 1996; 39(5): 741–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madrazo I, Franco‐Bourland RE, León‐Meza VM, Mena I. Intraventricular somatostatin‐14, arginine vasopressin, and oxytocin: analgesic effect in a patient with intractable cancer pain. Appl Neurophysiol. 1987; 50(1–6): 427–31 [DOI] [PubMed] [Google Scholar]
- 53.Delextrat A, Calleja‐González J, Hippocrate A, Clarke ND. Effects of sports massage and intermittent cold‐water immersion on recovery from matches by basketball players. J Sports Sci. 2013; 31(1): 11–9 [DOI] [PubMed] [Google Scholar]
- 54.Huang SY, Di Santo M, Wadden KP, Cappa DF, Alkanani T, Behm DG. Short‐duration massage at the hamstrings musculotendinous junction induces greater range of motion. J Strength Cond Res. 2010; 24(7): 1917–24 [DOI] [PubMed] [Google Scholar]