Abstract
Study Design:
Case Report.
Background and Purpose:
Dry needling (DN) is an increasingly popular intervention used by clinicians as a treatment of regional neuromusculoskeletal pain. DN is an invasive procedure that involves insertion of a thin monofilament needle directly into a muscle trigger point (MTP) with the intent of stimulating a local twitch response. Current evidence is somewhat limited, but recent literature supports the use of this intervention in specific neuromusculoskeletal conditions. The purpose of this case report is to present the outcomes of DN as a primary treatment intervention in an adolescent subject with subacute posterior knee pain.
Case Description:
The subject was a 16‐year‐old female competitive ballet dancer referred to physical therapy with a two month history of right posterior knee pain. Palpation identified MTPs which reproduced the patient’s primary symptoms. In addition to an exercise program promoting lower extremity flexibility and hip stability, the subject was treated with DN to the right gastrocnemius, soleus, and popliteus muscles.
Outcomes:
The subject reported being pain free on the Numerical Pain Scale and a +7 improvement in perceived change in recovery on the Global Rating of Change at final follow‐up. Physical examination demonstrated no observed impairments or functional limitations, including normal mobility, full strength, and unrestricted execution of dance maneuvers.
Discussion:
The patient was able to return to high level dance training and competition without physical limitations and resumed pre‐injury dynamic movement activities including dancing, running, jumping, and pivoting without pain. DN can be an effective and efficient intervention to assist patients in decreasing pain and returning to high intensity physical activity. Additional research is needed to determine if DN is effective for other body regions and has long‐term positive outcomes.
Level of Evidence:
Level 4
Keywords: Dry needling, knee pain, trigger point, dancing
Background and Purpose
Dancers present unique diagnostic and treatment challenges to the sports physical therapist (PT), with injury rates reported to be as high as 95% annually. The greatest proportion of ballet injuries occur in adolescents between 12 and 18 years of age.1 Since they typically begin dance training at a very early age, with grueling hours of repetitive practice and high performance demands, it is not surprising that the majority of injuries in ballet dancers are due to overuse, with numerous reports indicating lower extremity involvement to be the primary location of injury in this population.1‐8 Even minor injuries can affect training regimens, and up to 59% of young ballet dancers require some form of therapy and training modifications lasting over three weeks duration.1
In a recent study of adolescent dancers, the most common locations for pain were calves (43.7%) followed by the knees (32.7%), and ankles (27.2%).8 The constant “pointe” positions of repetitive plantar‐flexed and toe (or forefoot) weight‐bearing postures are suggested to be possible contributors to the high rate of lower extremity injury in ballet.5,7 The grand plié, a squat‐like movement with full and extreme hip external rotation, is another fundamental movement required of ballet dancers that may lead to lower extremity injury. Any lower extremity deficit may affect muscle activation patterns of the lower extremities due to coping strategies that are utilized as the dancer attempts to continue performing.9
A broad range of conditions can contribute to posterior knee pain, including pathologies to the muscles, bones, ligaments, and/or neurovascular structures.10 Lumbar disc or spinal pathology may refer pain to the posterior aspect of the lower extremity.11 The presence of trigger points (MTP), may also contribute to local and referred pain.12 MTPs are described as localized hyperirritable areas associated with hypersensitive palpable taut bands located in muscle tissue, and are suggested to contribute to joint range of motion restrictions and adversely affect muscle activation.12‐16 MTPs are further described in the literature as either active or latent.17 Active MTPs can be responsible for local pain as well as referred pain or paraesthesia18 and may contribute to spontaneous pain at rest.17 Latent MTPs are focal areas of tenderness and tightness in muscle that may not be directly responsible for referred or local pain unless stimulated; however, latent MTPs are believed to alter muscle activation patterns which may consequently result in limited range of motion or weakness of the muscle involved (Table 1).17‐19
Table 1.
The characteristic features of a myofascial trigger point
| 1. Focal point of tenderness to palpation of the muscle involved |
| 2. Reproduction of pain complaint by trigger‐point palpation (about 3 kg pressure) |
| 3. Palpation reveals an induration of the adjacent muscle (the “taut band”) |
| 4. Restricted range of movement in the muscle involved |
| 5. Often pseudo‐weakness of the muscle involved (no atrophy) |
| 6. Often referred pain on continued (~5 sec) pressure over trigger point |
Adapted from Bennett et al 200717
Key: kg = kilogram, sec = seconds
A variety of treatment techniques can be used to assist with the management of muscle injury, including stretching, soft tissue mobilization, Dry Needling (DN), and injection therapy.14‐16 DN may evoke a neurophysiological “reset”, favorably affecting pain, stability, and motor control of the affected regions.20 There is some evidence indicating that DN used in management of musculoskeletal pain21‐24 and injury to improve function, may alleviate muscle tension.13 It has also been suggested that DN may be a helpful clinical tool to assist in differentiating symptoms originating from muscle tissue and those that may originate from other structures.16
The purpose of this case report is to describe the evaluation and rehabilitation, incorporating the use of DN as an adjunct intervention, for the treatment of posterior knee pain in an adolescent ballet dancer.
Case Description
The subject was a 16‐year‐old female competitive ballet dancer with posterior knee pain. She was first evaluated by her primary care physician approximately three weeks following a “minor twisting injury” to her right knee. She was prescribed rest, ice, and anti‐inflammatory medications for one month. Since there was no noted benefit following her initial care plan, she was referred to an orthopedic surgeon for further evaluation. Soft tissue and structural integrity were confirmed by the surgeon through physical examination, plain radiographs, and magnetic resonance imaging (MRI). The subject was then referred to a PT for further evaluation and treatment.
At the time of the initial PT evaluation, two months following her initial injury, she reported her symptoms had been progressively worsening since the initial onset. She was painfree (0/10) at rest, but stated her pain was 6/10 on a numeric rating scale (NRS) with dancing. She reported that prior to injury, she participated in dance practice approximately 20 hours per week (4 to 5 hours per session). Following the initial onset of pain, she reported being limited to less than 1 to 2 hours per session before experiencing increased posterior knee pain. Despite increasing pain, she reported that she continued to dance 5 to 7 days per week. Her aggravating symptoms were single leg dance positions, jumping, pivoting, and impact activities such as running and fast walking. All symptoms reportedly eased with rest and non‐weightbearing. She reported her general health was good, and she denied all red flags, as well as history of any neurological symptoms. Her goal for physical therapy was a full return to dancing without pain.
Initial Clinical Impression
Given the subjective information, the differential diagnoses included muscle strain or related dysfunction of the gastrocnemius, popliteus, and/or distal hamstrings. Due to the absence of sensory changes, no persistent effusion, denial of instability, and the unremarkable MRI, rare causes of knee pain (cysts, bone tumor, nerve injury, etc.) were considered unlikely.10 A list of differential diagnoses related to knee pain is listed in Table 2.
Table 2.
Potential Causes of Posterior Knee Pain
Tendinopathy, muscle strain, or trigger point (TrP)
|
Nerve injury (palsy)
|
Ligamentous injury (sprain or rupture)
|
Meniscal injury
|
Bone injury
|
Knee cysts and bursal injury
|
Other neurovascular and vascular injury
|
Adapted from English and Perret 2010.10
Examination
Standing posture revealed mild genu recurvatum bilaterally, with symmetrical pelvic alignment and observed equal weight distribution through bilateral lower extremities. Her gait was non‐antalgic and symmetrical bilaterally. Both lower extremities were determined to be neurovascularly intact, including normal capillary refill with unremarkable sensory (light touch) testing. She demonstrated full active and passive range of motion (ROM) of the lumbar spine, hips, knees, and ankles. Mobility testing was performed using the 90/90 SLR test,25 the Thomas Test,25,26 the Ober’s Test,25,27 and passive ROM of the ankles. All regions tested were found to be symmetrical, within normal limits, and pain free.
Manual muscle testing of the hips, knees, and ankles was performed as described by Kendall including the motions of hip flexion, extension, abduction, and adduction; knee flexion and extension; tibial internal and external rotation; and ankle plantar flexion, dorsiflexion, inversion, and eversion.28 All lower extremity musculature was measured as 5/5 bilaterally through manual muscle testing except for bilateral hip extension and abduction, which were measured as 4/5 bilaterally. Mild reproduction of a “pulling” sensation in the area of the right popliteal fossa was reported during muscle testing of the right ankle plantar flexors without the presence of weakness. Additionally, mild pain in the area of the right popliteus (rated as 2/10) was reported with resisted active tibial internal rotation testing.28
Functional testing was performed by having the subject perform a deep squat with arms at her sides.29 The subject was instructed to stand with feet facing forward, approximately shoulder width apart, descend as deeply as possible, then return to starting position. Her femurs were observed to descend below parallel to the floor, heels remained in contact with the floor, and her trunk was grossly in‐line with her tibiae with apparent symmetrical weight distribution between lower extremities throughout the entire descending movement. Upon return to the starting position, the subject weight‐shifted to the uninvolved lower extremity, indicating she may have been attempting to unload the involved extremity secondary to posterior right knee pain.
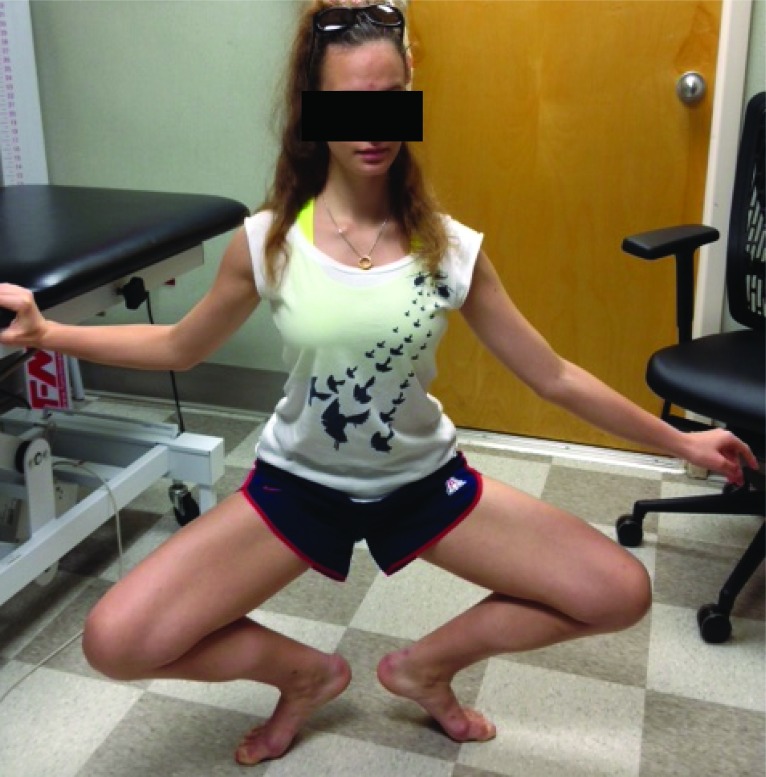
To further assess functional activities specific to her symptoms, she was asked to perform a grand plié. While descending into position, the subject was again observed weight‐shifting to the uninvolved lower extremity, which she attributed to increased posterior right knee pain. (Figure 1) She was also unable to complete a single leg squat on the right lower extremity secondary to posterior right knee pain. No gross deviations were observed, nor was pain experienced with full single leg squat on the left lower extremity.
Figure 1.
Pre‐treatment: Patient’s attempt at grand plie‐ noted weight shift to left lower extremity and inability to further descend without pain.
Palpation of the distal semitendinosis, semimembranosis, biceps femoris, gastrocnemius, and soleus muscles and tendons was unremarkable. Palpation of the popliteal space/distal popliteus and proximal medial head of the gastrocnemius was exquisitely tender and reproduced the subject’s primary pain. Multiple taut, ropey bands of muscle fiber throughout the proximal to distal medial head and mid to distal lateral head of the gastrocnemius and soleus were identified. Numerous hypersensitive points that reproduced the subject’s symptoms were also identified within these taut bands of muscle fiber, consistent with soft tissue (i.e., trigger point) dysfunction.13‐16 MTP locations and associated pain referral patterns are depicted in Figure 2.
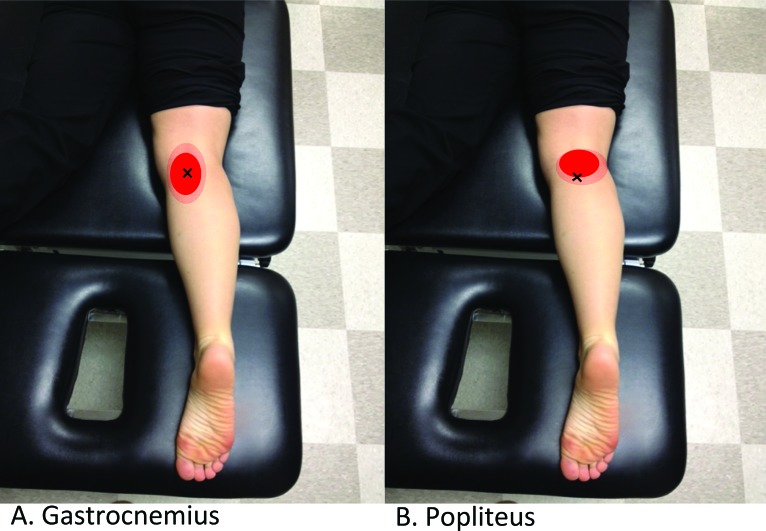
Figure 2.
Common MTP locations and corresponding pain referral patterns of the Gastrocnemius muscle (A) and the popliteus muscle (B). Adapted from Travell and Simons 1993.
Self‐report outcomes measures included the NRS and the Global Rating of Change (GROC). The NRS is an 11‐point scale intended to quantify self‐reported pain intensity, with a score of 0 indicating “pain free” and a score of 10 being the “worst pain imaginable”.30 Von Baeyer and colleagues showed the NRS to be a valid and reliable pain scale in children ages 8 to 17.31 A change of two points is considered the minimal clinically important difference (MCID).32 The GROC is a 15‐point scale designed to track subject perceived change in status. The scale ranges from ‐7 indicating “a very great deal worse” to +7 indicating “a very great deal better”. The MCID has been reported by Jaeschke and colleagues as a change in three points from baseline.33
Impression 2
Based on the subject’s otherwise normal physical and subjective exam findings, soft tissue dysfunction consistent with MTPs of the popliteus, gastrocnemius, and soleus muscles was suspected. Physical examination confirmed the absence of effusion, ligamentous instability, and further reduced the concern for sinister causes of knee pain described in Table 2.
Intervention
DN was selected as an adjunct intervention with the intent to mechanically influence the dysfunctional tissue to promote range of motion and pain inhibition.34‐36 The patient did not have any contraindications to DN and following a review of the associated risks (Table 3) she and her mother consented to treatment. All DN intervention methods described were performed by a physical therapist with advanced training in DN.
Table 3.
Contraindications, Risks, and Complications of Dry Needling (DN)
| Precautions and relative contraindications with use of DN | Absolute contraindications with use of DN | Common risks and potential complications of DN | Rare and serious risks/complications |
|---|---|---|---|
|
|
|
|
The subject was treated with DN at two separate visits, with 48 hours between treatment sessions. In both sessions DN was performed to target the palpable areas consistent with MTPs in the right gastrocnemius, soleus, and distal popliteus at the attachment to the medial tibia (See Figure 3). Solid monofilament needles, 0.30 mm in diameter and 40 mm in length, were used. After being inserted into the skin, the needle was directed towards the target MTP and repeatedly “pistoned” (inserted and withdrawn from each MTP without being fully withdrawn from the skin), to elicit local twitch responses (LTR). The elicitation of LTRs, reflex‐like contraction responses from the treated muscle, is often associated with favorable treatment effects from DN.22,23 Treatment was repeated to produce several LTRs and continued until all identified areas of dysfunction had been addressed.
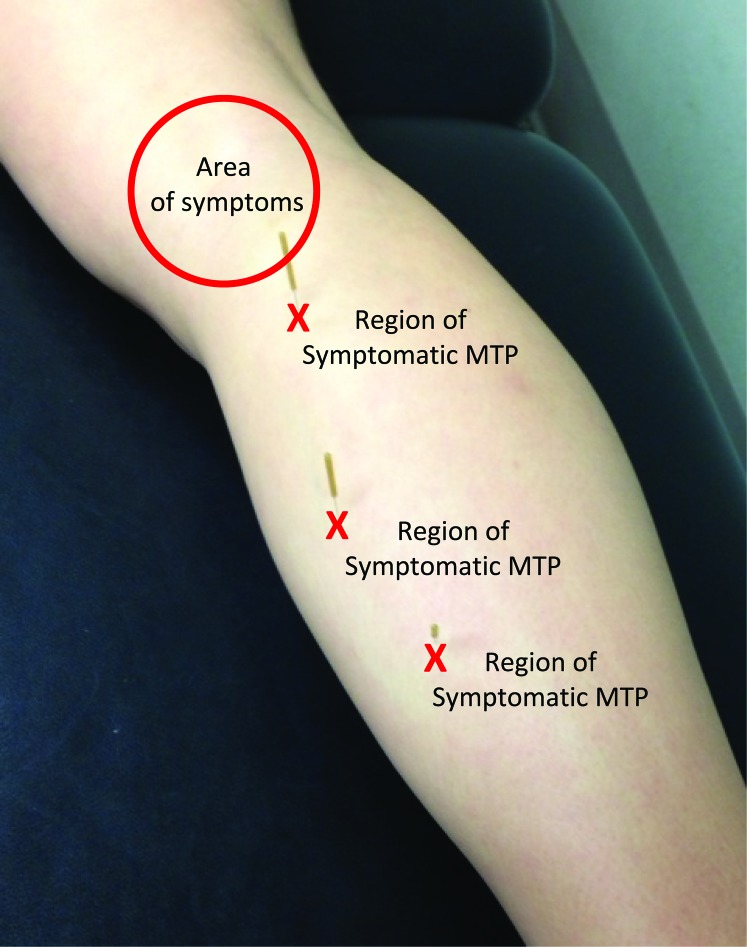
Figure 3.
Treatment areas and corresponding symptom locations.
Immediately following treatment, provocative testing was repeated to assess any immediate effects of intervention. The subject reported 0/10 pain on the NPS with full squat and grand plié. Symmetrical weight distribution between lower extremities was observed. (See Figure 4)
Figure 4.
Post‐treatment: Patient demonstrates full grand plie maneuver with symmetrical weightbearing and painfree movement.
At the end of the first visit, the subject was instructed to rest from dancing and other strenuous lower extremity exercise as well as to perform a home exercise program (HEP) designed to complement the treatment and progress towards established goals. Standing gastrocnemius and standing soleus stretches were instructed in and given to the patient. She was instructed to perform each stretch 2 to 3 times, statically holding for a minimum of 30 seconds, repeated 3 to 4 times daily.37
The first follow‐up appointment was 48 hours after initial treatment. The subject reported 0/10 pain at rest and her worst pain being 2/10 with squatting motions during the 48 hour period between visits. Overall symptoms were reported as generally improving; however, GROC was reported as ‐2 indicating “slightly worse”. The subject’s explanation for the perceived decrease in function was reported as a slight increase in pain in the area of primary symptoms and grossly to the gastocnemius/soleus region with initial steps first thing out of bed in the morning or after prolonged sitting. Otherwise, throughout the day the subject’s symptoms were reported as much improved, no longer having constant pain with fast walking or squatting maneuvers. At this visit, the subject was able to perform a full squat with symmetrical weight distribution and 0/10 on the NPS. A grand plié was performed with very mild pain, rating 2/10 on NPS, and mild weight‐shifting toward the contralateral lower extremity. Palpation revealed MTPs most notably in the medial head and mid muscle belly of the right gastrocnemius; however, the subject subjectively reported that deep palpation was significantly improved as compared to the initial visit. DN was repeated using the same technique and locations as the initial treatment. Following this treatment session the subject was able to perform a grand plié with 0/10 pain and symmetrical distribution of weight.
She was instructed to follow up in one week and continue to perform the same HEP three times daily. At her next follow up appointment, the subject reported being asymptomatic with all activity and reported a GROC score of +5, indicating “quite a bit better” improvement in perceived function. Full squat and grand plié were both symmetrical and pain free. Palpation of gastrocnemius, soleus, and distal popliteus was pain free without detection of focal soft tissue dysfunction. A summary of her objective measures is presented in Table 4. The HEP was progressed to include single leg squat with contralateral isometric hip abduction with instructions to progress to single leg squat focusing on pelvic stability and proper knee alignment and hip abduction with resisted side stepping using a resistance band. She was able to demonstrate good form and understanding of all activities prior to end of this session and was advised to perform the exercises at least 3 times per week for 30 repetitions each.
Table 4.
Objective Measures
| Objective Findings: | Initial Exam: (pre‐tx) | F/U day 2: | F/U day 9: | F/U 1 month: | F/U 3 months: |
|---|---|---|---|---|---|
| Deep Squat: | Pain limited during return to stand from full squat; weight shift to contralateral LE | Full symmetrical ROM, pain free through completion of movement | Full symmetrical ROM, pain free through completion of movement | Full symmetrical ROM, pain free through completion of movement | Full symmetrical ROM, pain free through completion of movement |
| Single Leg Squat: | Unable to perform due to pain | Not tested | Pain free, WNL ROM, observed increased femoral IR, knee valgus | Symmetrical and pain free | Symmetrical and pain free |
| Grand Plie: (see pictures 1 and 3) | Pain limited during return to stand; weight shift to contralateral LE | Mild pain with full symmetrical ROM | Full symmetrical ROM, pain free through completion of movement | Full symmetrical ROM, pain free through completion of movement | Full symmetrical ROM, pain free through completion of movement |
| Manual Muscle Test: | 5/5 all bilateral knee/ankle, gastroc/soleus with mod “pulling sensation,” mild pain with popliteus | 5/5 and pain free all bilateral knee and ankle | 5/5 and pain free all bilateral knee and ankle | 5/5 and pain free all bilateral LE | 5/5 and pain free all bilateral LE |
| Deep Palpation: | Severe TTP with MTPs proximal medial head of gastroc; severe to mod mid and distal popliteus, medial/lateral gastroc/soleus | Moderate TTP MTPs proximal to distal popliteus, gastroc/soleus, medial > lateral | No MTPs detected, pain free palpation | No MTPs detected, pain free palpation | No MTPs detected, pain free palpation |
KEY: f/u= follow up; gastroc= gastrocnemius muscle; LE= lower extremity(ies); mod= moderate; MTPs= myofascial trigger points; ROM= range of motion; TTP= tenderness to palpation; TX= treatment
Additional follow up appointments were planned for one and three months from the initial appointment. All subjective and objective measures were repeated as detailed on the initial evaluation. The subject reported +7 “a very great deal better” improvement on the GROC and 0‐0/10 best to worst pain with all activity, including dancing (Table 5). At the 1 month follow‐up appointment, the subject reported compliance with all HEP instructions since her last visit and gradually resumed her intensive dance training regimen, successfully progressing back to full training without recurrence of symptoms. At the three month follow‐up appointment, she reported being able to continue with her full training routine without recurrence of symptoms and she was formally discharged from physical therapy care.
Table 5.
Patient‐reported Outcome Measures
| Outcome | Initial Eval | F/U Day 2 | F/U Day 9 | F/U 1 Month | F/U 3 Months |
|---|---|---|---|---|---|
| NPS (rest‐activity/10) | 0‐6/10 | 0‐6/10 | 0/10 | 0/10 | 0/10 |
| GROC | N/A | −2 * | 5+ | 7+ | 7+ |
patient reported mild increased pain with first step out of bed or prolonged sitting for during 24 hour period following initial treatment; however, pain resolved after walking approximately 20ft. Overall, pain was reported as much improved throughout the day. Reported symptoms are possibly a result of post‐treatment soreness, a known side effect of treatment.
KEY: F/U = Follow Up; NPS = Numerical Pain Scale; GROC = Global Rating of Change
Conclusion/Discussion
Lower extremity injuries are a common occurrence among dancers of all genres, especially ballet dancers. Selecting effective treatments which may minimize time away from training and performing are important to dancers and health care providers alike. Although a full understanding of the exact physiological mechanism of action is not complete, there is evidence to suggest favorable local biomechanical changes may occur and the sensitivity of local muscle dysfunction can be altered in areas of muscle with MTPs.24,34,38 It has been postulated that DN may evoke a neurophysiological “reset,”20 and is commonly associated with improvements in mobility, local and referred pain, as well as MTP irritability21 which may account for the subject’s positive response to treatment.
Since DN has been reported as an effective adjunct to therapeutic intervention for a variety of musculoskeletal conditions,13,18,20,39 the therapist deemed it an appropriate adjuvant to consider for this patient. After completion of two treatments, the subject reported full resolution of symptoms. In addition, she was able to fully return to rigorous ballet training without relapse. This case suggests integrating DN in conjunction with a treatment plan of therapeutic exercises may be beneficial, however, it does not provide sufficient information to draw definitive cause and effect conclusions.
While there are limitations to a single subject design, the results of this case indicate that DN may be a useful therapeutic intervention, allowing this dancer to quickly return to activity. Prospective studies are needed to further determine the efficacy of this modality and to better determine what conditions would most likely benefit from needling techniques. Additional research is also needed to determine optimal parameters for duration and frequency of this treatment modality.
References
- 1.Gamboa JM, Roberts LA, Maring J, et al. Injury patterns in elite preprofessional ballet dancers and the utility of screening programs to identify risk characteristics. J Orthop Sports Phys Ther. 2008; 38: 126–136 [DOI] [PubMed] [Google Scholar]
- 2.Henderson J, MacIntyre D. A Descriptive Survey of Injury Patterns in Canadian Premier Highland Dancers. Physiother Canada. 2006; 58: 61–73 [Google Scholar]
- 3.Krasnow D, Mainwaring L, Kerr G. Injury, Stress, and Perfectionism in Young Dancers and Gymnasts. J Dance Med Sci. 1999; 3: 51–58 [Google Scholar]
- 4.Leanderson C, Leanderson J, Wykman A, et al. Musculoskeletal injuries in young ballet dancers. Knee Surg Sports Traumatol Arthrosc. 2011; 19: 1531–1535 [DOI] [PubMed] [Google Scholar]
- 5.Shnitser I, Attanasio A. The Point of Being “En Pointe”: Biomechanical Stresses and Injury in Clasically Trained Ballet Dancers. NYCPM Podiatric Medical Review. 2001; 20: 60–67 [Google Scholar]
- 6.Stretanski MF, Weber GJ. Medical and rehabilitation issues in classical ballet. Am J Phys Med Rehabil. 2002; 81: 383–391 [DOI] [PubMed] [Google Scholar]
- 7.Toledo SD, Akuthota V, Drake DF, et al. Sports and performing arts medicine: Issues relating to dancers. Arch Phys Med Rehabil. 2004; 85: S75–78 [DOI] [PubMed] [Google Scholar]
- 8.Miletic A, Kostic R, Bozanic A, et al. Pain Status Monitoring in Adolescent Dancers. Med Prob Perform Art. 2009; 24: 119 [Google Scholar]
- 9.Lin CW, Su FC, Lin CF. Influence of Ankle Injury on Muscle Activation and Postural Control During Ballet Grand‐plie. J Appl Biomech. 2013 [DOI] [PubMed] [Google Scholar]
- 10.English S, Perret D. Posterior knee pain. Curr Rev Musculoskelet Med. 2010; 3: 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohnmeiss DD, Vanharanta H, Ekholm J. Relation between pain location and disc pathology: a study of pain drawings and CT/discography. Clin J Pain. 1999; 15: 210–217 [DOI] [PubMed] [Google Scholar]
- 12.Simons DG, Travell JG, Simons LS. Travell and Simons’ Myofascial Pain and Dysfunction: the Trigger Point Manual; Volume 1 Upper Half of Body. Baltimore MD: Lippincott, Williams & Wilkins; 1999 [Google Scholar]
- 13.Dembowski SC, Westrick RB, Zylstra E, et al. Treatment of hamstring strain in a collegiate pole‐vaulter integrating dry needling with an eccentric training program: a resident’s case report. Int J Sports Phys Ther. 2013; 8: 328–339 [PMC free article] [PubMed] [Google Scholar]
- 14.Srbely JZ, Dickey JP, Lee D, et al. Dry needle stimulation of myofascial trigger points evokes segmental anti‐nociceptive effects. J Rehabil Med. 2010; 42: 463–468 [DOI] [PubMed] [Google Scholar]
- 15.Tekin L, Akarsu S, Durmus O, et al. The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double‐blinded placebo‐controlled trial. Clin Rheumatol. 2013; 32: 309–315 [DOI] [PubMed] [Google Scholar]
- 16.Westrick RB, Zylstra E, Issa T, et al. Evaluation and treatment of musculoskeletal chest wall pain in a military athlete. Int J Sports Phys Ther. 2012; 7: 323–332 [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007; 21: 427–445 [DOI] [PubMed] [Google Scholar]
- 18.Dommerholt J, Bron C, Franssen J. Myofascial Trigger Points: An Evidence‐Informed Review. J Manual Manipulative Ther. 2006; 14: 203–221 [Google Scholar]
- 19.Lucas K, Polus B, Rich P. Latent Myofascial Trigger Points: Their Effects on Muscle Activation and Movement Efficiency. J Bodyw Mov Ther. 2003; 8: 160–166 [Google Scholar]
- 20.Rainey CE. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. Int J Sports Phys Ther. 2013; 8: 145–161 [PMC free article] [PubMed] [Google Scholar]
- 21.APTA. Description of Dry Needling in Clinical Practice: An Educational Resource Paper. American Physical Therapy Association; 2013 [Google Scholar]
- 22.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil. 1994; 73: 256–263 [DOI] [PubMed] [Google Scholar]
- 23.Majlesi J, Unalan H. Effect of treatment on trigger points. Curr Pain Headache Rep. 2010; 14: 353–360 [DOI] [PubMed] [Google Scholar]
- 24.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008; 89: 16–23 [DOI] [PubMed] [Google Scholar]
- 25.Konen J, Wiksten D, Isear J, et al. Special Tests for Orthopedic Examination, 2nd Edition. Thorofare NJ: Slack Incorporated; 2002 [Google Scholar]
- 26.Harvey D. Assessment of the flexibility of elite athletes using the modified Thomas test. Br J Sports Med. 1998; 32: 68–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucas CA. Iliotibial band friction syndrome as exhibited in athletes. J Athl Train. 1992; 27: 250–252 [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall FP, McCreary EK, Provance PG, et al. Muscles: Testing and Function with Posture and Pain. 5th Baltimore MD: Lippincott, Williams & Wilkins; 2005 [Google Scholar]
- 29.Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc. 2001; 33: 127–141 [DOI] [PubMed] [Google Scholar]
- 30.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14: 798–804 [DOI] [PubMed] [Google Scholar]
- 31.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS‐11) for children’s self‐reports of pain intensity. Pain. 2009; 143: 223–227 [DOI] [PubMed] [Google Scholar]
- 32.Salaffi F, Stancati A, Silvestri CA, et al. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004; 8: 283–291 [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989; 10: 407–415 [DOI] [PubMed] [Google Scholar]
- 34.Dommerholt J, Fernandez de las Penas C. Trigger Point Dry Needling: An Evidence and Clinical‐based Approach. Churchill Livingstone; 2013 [Google Scholar]
- 35.Dommerholt J, Huijbregts P. Myofascial Trigger Points: Pathophysiology and Evidence‐Informed Diagnosis and Management. Sudbury MA: Jones and Bartlet; 2011 [Google Scholar]
- 36.Zylstra E. Functional Dry Needling: Level 1 Training Manual. KinetaCore; 2013 [Google Scholar]
- 37.Radford JA, Burns J, Buchbinder R, et al. Does stretching increase ankle dorsiflexion range of motion? A systematic review. Br J Sports Med. 2006; 40: 870–875; discussion 875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou LW, Kao MJ, Lin JG. Probable mechanisms of needling therapies for myofascial pain control. Evid Based Complement Alternat Med. 2012; 2012: 705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalichman L, Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010; 23: 640–646 [DOI] [PubMed] [Google Scholar]